Abstract
Alternative splicing of tau exon 10 generates tau with three or four microtubule-binding repeats (3R-tau or 4R-tau). The ratio of 3R-tau to 4R-tau is approximately 1:1 in the adult normal human brain. Disturbances in the ratio result in neurodegenerative tauopathies. Splicing factor SC35 acts on a SC35-like element located at the 5′ end of tau exon 10 and promotes tau exon 10 inclusion. Here, we report that protein kinase (PKA) was able to interact and phosphorylate SC35. Activation or overexpression of PKA catalytic subunits promoted SC35-mediated tau exon 10 inclusion. Four PKA catalytic subunits, α1, α2, β1, and β2, all enhanced SC35-promoted tau exon 10 inclusion. SC35 has four putative PKA phosphorylation sites, Ser121, Ser128, Ser130, and Ser171. Pseudophosphorylation (SC354E) and blockage (SC354A) of phosphorylation of SC35 at these four sites increased and decreased, respectively, SC35’s ability to promote tau exon 10 inclusion. Moreover, PKA catalytic subunits no longer further enhanced tau exon 10 inclusion when these four were mutated to either alanine or glutamate. These results suggest that PKA interacts with and phosphorylates SC35 and enhances SC35-promoted tau exon 10 inclusion. In Alzheimer’s brain, down-regulation of the PKA pathway could lead to dysregulation of tau exon 10, contributing to tau pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tau is a neuronal microtubule-associated protein. Its major biological function is to promote microtubule assembly and stabilize microtubules. Hyperphosphorylated tau aggregates to form neurofibrillary tangles in affected neurons, a hallmark pathology of Alzheimer’s disease (AD) and related neurodegenerative disorders called tauopathies [1–5]. There are six tau isoforms expressed in the central nervous system by alternative splicing of its exons 2, 3, and 10 from a single gene located on chromosome 17q21 in humans [6–11]. Tau exon 10 encodes the second microtubule-binding repeat [12]. Its alternative splicing generates tau isoforms with either three or four microtubule-binding repeats (3R-tau or 4R-tau) [12]. Alternative splicing of tau exon 10 undergoes developmental regulation. The fetal human brain only expresses 3R-tau, but the normal adult human brain expresses 3R-tau and 4R-tau approximately equally [12, 13]. Disruptions of the 3R-tau/4R-tau balance have been seen in several types of tauopathies, such as frontotemporal dementia with parkinsonism linked to chromosome 17, Pick’s disease, and supranuclear palsy [14–16], indicating that the balance between 3R-tau and 4R-tau must remain within a narrow range to ensure normal brain function. However, the mechanisms by which the imbalance causes or contributes to the development of tau pathology remain largely unknown.
Serine/arginine-rich (SR) proteins are one family of the splicing factors involved in alternative splicing. Among them, SC35 promotes tau exon 10 inclusion by acting on a SC35-like binding element of exonic splicing enhancer located at the 5′ end of tau exon 10 [17]. SC35 contains a single RNA recognition motif (RRM) and a carboxyl-terminal domain rich in arginine and serine residues (RS domain) [18, 19]. The function of SC35 is highly regulated by phosphorylation [17, 20–23]. Overexpression of Dyrk1A (dual-specificity tyrosine-phosphorylated and regulated kinase 1A) suppresses SC35-promoted tau exon 10 inclusion [17].
Cyclic AMP (cAMP)-dependent protein kinase (PKA) is a Ser/Thr protein kinase that is involved in many biological events. It is usually an inactive holoenzyme consisting of two catalytic (C) subunits bound to a regulatory (R) subunit dimer. There are three isoforms, α, β, and γ, of the PKA catalytic subunit [24]. PKA-Cα and PKA-Cβ are expressed ubiquitously, but PKA-Cγ is only expressed in the testis [25]. Both PKA-Cα and PKA-Cβ have two splicing variants, named PKA-Cα1, PKA-Cα2, PKA-Cβ1, and PKA-Cβ2 [24]. Upon binding of cAMP, the holoenzyme dissociates into a regulatory dimer and two active catalytic subunits; part of the latter translocates into the nucleus. PKA catalyzes tau phosphorylation in vitro and in vivo [26, 27]. Prephosphorylation of tau with PKA dramatically increases its subsequent phosphorylation by glycogen synthase kinase-3β (GSK-3β) at most of the AD-relevant phosphorylation sites in vitro [26]. PKA also phosphorylates Ser9 of GSK-3β, an inhibitory form of GSK-3β. Activation of PKA in vivo leads to tau hyperphosphorylation at both PKA and non-PKA phosphorylation sites [28]. In addition, we recently found that PKA phosphorylates the splicing factor ASF/SF2 and enhances its function in the promotion of tau exon 10 inclusion in a manner specific to its variant catalytic subunits [19]. However, the regulation of PKA on SC35 was not studied. In the present study, we report that PKA is able to interact with and phosphorylate SC35. Activation or overexpression of PKA promotes SC35-mediated tau splicing.
Materials and Methods
Plasmids, Antibodies, and Other Reagents
The cDNAs of human PKA-Cα1, PKA-Cα2, PKA-Cβ1, and PKA-Cβ2 were generated by reverse transcription PCR from RNA isolated from normal human neuronal progenitor cells and confirmed by DNA sequence analysis. All of the PKA isoforms tagged with HA at N-terminus were cloned into pCI-Neo vector via SalI and NotI sites. pCEP4/SC35 tagged with HA at N-terminus was a gift from Dr. Tarn of the Institute of Biomedical Sciences, Academia Sinica, Taiwan. pGEX-2T/SC35FL, pGEX-2T/SC3588–221, pGEX-2T/SC35108–221, pGEX-2T/SC351–191, and pGEX-2T/SC351–117 were constructed by PCR amplification from pCEP4/SC35-HA and subcloning into pGEX-2T to generate glutathione S-transferase (GST) fusion proteins of full-length SC35 and its deletion mutants. Tau mini-gene pCI/SI9–SI10 containing SI9/SI10, comprising tau exons 9, 10, and 11 and part of introns 9 and 10, was a gift from Dr. Zhou of the University of Massachusetts, Boston. PKA catalytic subunit and monoclonal anti-HA were bought from Sigma (St. Louis, MO, USA). Polyclonal anti-PKA-Cα and anti-PKA-Cβ were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Peroxidase-conjugated goat anti-mouse and anti-rabbit IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA); tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit IgG and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The ECL kit was from Thermo Fisher Scientific (Rockford, IL, USA), and [γ-32P]ATP was from MP Biomedicals (Irvine, CA, USA).
Cell Culture and Transfection
HEK-293FT cells and HeLa cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10 % fetal bovine serum (Invitrogen, Carlsbad, CA, USA) at 37 °C. All transfections were performed in triplicate with FuGENE HD (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s instructions.
In Vitro Phosphorylation of SC35 by PKA
GST-SC35, GST-SC35 mutants, or GST (0.2 mg/ml) was incubated with various concentrations of PKA catalytic subunit in a reaction buffer consisting of 50 mM HEPES (pH 6.8), 10 mM β-mercaptoethanol, 10 mM MgCl2, 1.0 mM EGTA, and 0.2 mM [γ-32P]ATP (500 cpm/pmol). After incubation at 30 °C for 30 min, the reaction was stopped by boiling with an equal volume of 2× Laemmli sample buffer. The reaction products were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Incorporation of 32P was detected by exposure of the dried gel to a phosphor imaging system.
GST Pull-Down Assay
GST, GST-SC35, and GST-SC35 deletion mutants were affinity-purified with glutathione-Sepharose but were not eluted from the beads. The beads coupled with GST, GST-SC35, and GST-SC35 deletion mutants were incubated with crude extract from rat brain homogenate in a buffer (50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 0.1 % Triton X-100, 2 mM EDTA, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml pepstatin). After 2 h of incubation at 4 °C, the beads were washed with washing buffer (50 mM Tris–HCl (pH 7.4), 150 mM NaCl, and 1 mM DTT) six times and the bound proteins were eluted by boiling in Laemmli sample buffer for analysis by Western blots.
Co-immunoprecipitation
HEK-293FT cells were transfected with pCEP4/SC35-HA for 40 h as described above and treated with 10 μM forskolin for 8 h, and then the cells were washed twice with PBS and lysed by sonication in lysate buffer (50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 2 mM EDTA, 1 mM PMSF, 2 μg/ml aprotinin, 2 μg/ml leupeptin, and 2 μg/ml pepstatin). The cell lysate was centrifuged at 16,000 × g for 10 min and incubated with anti-HA overnight at 4 °C, and then protein G beads were added. After 4 h of incubation at 4 °C, the beads were washed with lysate buffer twice and with TBS twice, and bound proteins were eluted by boiling in Laemmli sample buffer. The samples were subjected to Western blot analyses with the indicated primary antibodies.
Co-localization Study
HeLa cells were plated in 24-well plates onto cover slips 1 day before transfection at 50–60 % confluence and then transfected with pCEP4/SC35-HA as described above. After 40 h of transfection, the cells were treated with 10 μM forskolin for 30 min to activate PKA, and then the cells were washed with PBS and fixed with 4 % paraformaldehyde in PBS for 30 min at room temperature. After washing with PBS, the cells were blocked with 10 % goat serum in 0.2 % Triton X-100 and PBS for 2 h at 37 °C and incubated with rabbit anti-PKA-Cα/β (1:50) and mouse anti-HA (1:200) overnight at 4 °C. The cells were then washed and incubated with TRITC-conjugated goat anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG (1:200), and the cells were washed extensively with PBS and incubated with 5 mg/ml Hoechst 33342 for 5 min at room temperature. The cells were washed with PBS, mounted with Fluoromount-G, and visualized with a Leica TCSSP2 laser scanning confocal microscope.
Knockdown of SC35 or PKA Catalytic Subunits by Small Interfering RNA
For knockdown of SC35 or PKA, HEK-293FT cells were transfected with various amounts of small interfering RNA (siRNA) using Lipofectamine 2000. siRNA is a pool of three target-specific 20–25-nucleotide siRNAs used to knock down target gene expression (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Both strands of siRNAs had 3′-dTdT tails. The same amount of scramble siRNA was used as a control.
Quantitation of Tau Exon 10 Splicing by Reverse Transcription PCR
Total cellular RNA was isolated from cultured cells by using the RNeasy Mini Kit (Qiagen, GmbH, Germany). One microgram of total RNA was used for first-strand cDNA synthesis with oligo-(dT)15_18 by using the Omniscript Reverse Transcription kit (Qiagen, Valencia, CA, USA). PCR was performed by using PrimeSTARTM HS DNA Polymerase (Takara Bio Inc., Otsu, Shiga, Japan) with forward primer 5′-GGTGTCCACTCCCAGTTCAA-3′ and reverse primer 5′-CCCTGGTTTATGATGGATGTTGCCTAATGAG-3′ to measure alternative splicing products of tau exon 10. The PCR conditions were 94 °C for 5 min, 98 °C for 10 s, and 55 °C for 10 s for 30 cycles and then 72 °C for 7 min for extension. The PCR products were resolved on 1.5 % agarose gels and quantitated using the Molecular Imager system (Bio-Rad, Hercules, CA, USA).
DNA Site-Directed Mutagenesis
Two mutants, pCEP4/SC354A and pCEP4/SC354E, with Ser121/128/130/171 mutated to either alanine (A) or glutamate (E) were produced by site-directed mutagenesis using the KOD-Plus-Mutagenesis kit (TOYOBO, Dalian, China) and confirmed by sequencing.
Statistical Analysis
Data were presented as mean ± S.D. and analyzed by the unpaired two-tailed Student’s t test for two-population comparison or one-way ANOVA with Dunnett’s post hoc test for multiple comparisons.
Results
PKA Phosphorylates SC35 In Vitro
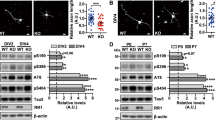
We previously reported that SC35 promotes tau exon 10 inclusion [17]. Thus, we determined whether PKA phosphorylates and regulates the activity of SC35 in affecting the alternative splicing of tau exon 10. We incubated GST-SC35 with 32P-ATP and various concentrations of PKA catalytic subunit in vitro for 30 min at 30 °C. The reaction mixtures were then separated by SDS-PAGE, and the radioactivity signal of the gels was captured by autoradiography. We observed that GST-SC35 was phosphorylated by PKA in an enzyme concentration-dependent manner (Fig. 1a and b). We previously showed that GST itself is not phosphorylated by PKA under similar conditions [29].
PKA phosphorylates SC35 and enhances its ability to promote tau exon 10 inclusion. a PKA phosphorylated SC35 in vitro. GST-SC35 (0.2 mg/ml) was incubated with various concentrations of PKA-C at 30 °C for 30 min, and the reaction products were separated by SDS-PAGE and visualized with Coomassie blue staining (lower panel). The 32P incorporated into SC35 in the dried gel was measured by using a phosphor imaging system (BAS-1500, Fuji) (upper panel). b Incorporation of 32P into GST-SC35 was plotted against PKA concentration. c, d Activation of PKA enhanced SC35-promoted tau exon 10 inclusion. HEK-293FT cells were transfected with pCEP4/SC35-HA (c) or siRNA of SC35 to knock down SC35 (d) for 40 h and then treated with 10 μM forskolin (Fors) for 8 h. Total RNA was extracted and subjected to RT-PCR to measure tau exon 10 splicing. The ratio of inclusion and exclusion of tau exon 10 was calculated after densitometric quantification of the PCR products. Results are presented as mean ± S.D. *Fors vs. Con; #SC35 or siSC35 vs. to Mock or Scramble; *, # p < 0.05; **, ## p < 0.01
Activation of PKA Enhances SC35-Promoted Tau Exon 10 Inclusion
Under non-stimulated conditions, PKA is present as an inactive heterotetramer consisting of two catalytic subunits and two regulatory subunits. Upon activation by cAMP, catalytic subunits of PKA are dissociated from regulatory subunits and are able to catalyze phosphorylation of the substrate proteins. To determine whether PKA modulates SC35 function in tau exon 10 splicing, we co-transfected the mini-tau gene pCI/SI9–SI10 (consisting of tau exons 9, 10, and 11, and partial introns 9 and 10) together with pCEP4/SC35 as a ratio of 1:5 into HEK-293FT cells for 40 h, and then treated the cells with 10 μM forskolin for 8 h to activate PKA [29]. The total RNA was extracted and splicing products of tau exon 10 were measured by RT-PCR. As reported previously [30], we observed that SC35 enhanced mini-tau mRNA expression and SC35 and forskolin both promoted tau exon 10 inclusion (Fig. 1c). Co-treatment of forskolin and SC35 promoted tau exon 10 inclusion synergistically (Fig. 1c). In addition, as reported previously, knockdown of SC35 by siRNA of SC35, siSC35, eliminated the effect of forskolin on tau exon 10 inclusion (Fig. 1d). These results suggest that PKA activation by forskolin enhances SC35-promoted tau exon 10 inclusion.
PKA Catalytic Subunits Interact with SC35
Interaction between PKA and SC35 is required in order for the former to catalyze the phosphorylation of the latter in a living system. Thus, we first examined whether the PKA catalytic subunit interacts with SC35 physically. We incubated GST-SC35-coupled beads with rat brain extract for 2 h at 4 °C. After extensive washing, the proteins pulled down with the beads were analyzed by Western blots. We found that PKA catalytic subunits were pulled down by GST-SC35, but not GST (Fig. 2a), suggesting that PKA catalytic subunits can interact with SC35.
SC35 interacts with PKA catalytic subunits. a PKA catalytic subunits (PKA-C) were pulled down by SC35. GST-SC35 or GST coupled onto glutathione-Sepharose was incubated with rat brain extract, and the bound proteins were analyzed by Western blots developed with anti-GST, anti-PKA-Cα, or anti-PKA-Cβ. b PKA catalytic subunits, α and β, were co-immunoprecipitated by SC35. SC35 tagged with HA was expressed in HEK-293FT cells for 40 h and treated with forskolin for 8 h. The cell extracts were then immunoprecipitated with anti-HA, and the immunoprecipitated complexes (IP) were subjected to Western blots developed with antibodies indicated at the right of each blots. c, d PKA catalytic subunits α (c) and β (d) of PKA were translocated to the nucleus and co-localized with SC35 after forskolin treatment. HeLa cells were transfected with SC35-HA and treated with forskolin (Fors) for 30 min, followed by triple immunofluorescence staining
The mammalians brain expresses both PKA-Cα and PKA-Cβ, but not PKA-Cγ. To determine isoform-specific interaction of PKA-C with SC35, we overexpressed SC35 tagged with HA at N-terminus in HEK-293FT cells and treated the cells with forskolin to activate PKA. The SC35 was immunoprecipitated with anti-HA, and co-immunoprecipitated proteins were analyzed with anti-PKA-Cα and PKA-Cβ. We found that both PKA-Cα and PKA-Cβ were co-immunoprecipitated by SC35 (Fig. 2b), supporting that both PKA catalytic subunits interact with SC35.
To determine the interaction between PKA catalytic subunits and SC35 in intact cells, we overexpressed HA-SC35 in HeLa cells and then activated PKA with forskolin treatment. The cells were double-immunostained with anti-HA and anti-PKA-Cα or PKA-Cβ. Confocal microscopic analysis revealed that SC35 was localized extensively in the nucleus (Fig. 2c), whereas PKA-Cα and PKA-Cβ were mainly located in the cytoplasm under basal conditions (Fig. 2c and d). Treatment of the cells with forskolin caused partial translocation of PKA-Cα and PKA-Cβ and co-localization with SC35 in the nucleus (Fig. 2c and d), suggesting that the activation of PKA leads to translocation of PKA-Cα and PKA-Cβ into the nucleus where they can interact with SC35.
To identify the regions of SC35 that are responsible for its interaction with PKA catalytic subunits, we used a series of deletion mutants of SC35 (Fig. 3a) fused with GST to perform GST pull-down assays of PKA catalytic subunits from rat brain extracts. We found that all SC35 mutants were able to pull down PKA-Cα, 51-kDa PKA-Cβ, and 43-kDa PKA-Cβ from rat brain extracts, but the nuclear retention signal (NRS) -deleted mutants had weaker ability to pull down PKA (Fig. 3b). These findings suggest that SC35 has more than one PKA-C-interacting regions, but C-terminal NRS is the major region that interacts probably with PKA-C.
Regional interaction of SC35 with PKA catalytic subunits. a Schematic of various SC35 deletion mutants employed for interaction with PKA-C. b PKA-C interacted with various domains of SC35. GST-SC35, its deletion mutants, or GST coupled onto glutathione-Sepharose was incubated with rat brain extract, and the bound proteins were analyzed by Western blots developed with anti-GST, anti-PKA-Cα, or anti-PKA-Cβ
PKA-Cα and PKA-Cβ Both Enhance SC35-Mediated Tau Exon 10 Inclusion
Both PKA-Cα and PKA-Cβ have two splicing variants (PKA-Cα1, PKA-Cα2, PKA-Cβ1, and PKA-Cβ2) with very high homology especially at their C-terminal portion (Fig. 4a). The human brain expresses these four forms of PKA-C by alternative splicing of pre-mRNAs of PKA-Cα and PKA-Cβ (Fig. 4b). To investigate the isoform-specific role of PKA catalytic subunits on SC35-mediated tau exon 10 splicing, we co-expressed PKA-Cα1, PKA-Cα2, PKA-Cβ1, and PKA-Cβ2 with pCEP4/SC35-HA in pCI/SI9–SI10-transfected HEK-293FT cells, and then measured exon 10 splicing products by RT-PCR 48 h after transfection. We observed that all four PKA-C variants promoted tau exon 10 inclusion significantly (Fig. 4c and d). Co-expression of SC35 with either PKA-Cα (Fig. 4c) or PKA-Cβ (Fig. 4d) further increased the SC35-promoted tau exon 10 inclusion, indicating that PKA-C enhances SC35-mediated tau exon 10 inclusion.
PKA catalytic subunits enhance SC35-promoted tau exon 10 inclusion. a Alignment of amino acid sequences of PKA catalytic subunits at protein level. b The human brain expresses PKA catalytic subunits of α and β. PKA catalytic subunits in brain homogenates of mouse, rat, and human were determined by Western blots developed with anti-PKA-Cα and anti-PKA-Cβ. c, d All isoforms of PKA catalytic subunits enhanced SC35-promoted tau exon 10 inclusion. HEK-293FT cells were co-transfected with SC35 and PKA-Cα (c) or PKA-Cβ (d) for 48 h. The splicing products of tau exon 10 were determined by RT-PCR, followed by agarose gel electrophoresis. The ratio of inclusion and exclusion of tau exon 10 was calculated after densitometric quantification. Results are presented as mean ± S.D. ##vs. Con; *vs. SC35; & vs. PKA-C. *, #, & p < 0.05; **, ##, && p < 0.01
To confirm the role of PKA-C on SC35-promoted tau exon 10 inclusion, we used siRNA to knock down PKA-Cα or PKA-Cβ specifically (Fig. 5a) and then treated SC35- and pCI/SI9–SI10-transfected HEK-293FT cells with forskolin. We found that SC35-promoted tau exon 10 inclusion was diminished by knockdown of either PKA-Cα or PKA-Cβ significantly (Fig. 5b). These results support that all PKA-Cα and PKA-Cβ isoforms can enhance SC35 function in promoting tau exon 10 inclusion.
Knockdown of PKA catalytic subunits reduces SC35-promoted exon 10 inclusion. a Down-regulation of PKA catalytic subunits by siRNA. HEK-293FT cells were transfected with siRNA of PKA-Cα or PKA-Cβ. After 48 h of transfection, the cell lysates were subjected to Western blots developed with the indicated antibodies. b Knockdown of PKA-Cα or PKA-Cβ by siRNA inhibited SC35-promoted exon 10 inclusion. Mini-tau gene was co-transfected into HEK-293FT cells with SC35 and PKA-Cα/β siRNA or control siRNA, and then tau exon 10 splicing was analyzed by RT-PCR 48 h after transfection. The ratio of inclusion and exclusion of tau exon 10 was calculated after densitometric quantification. Results are presented as mean ± S.D. *vs. Con; #vs. Con siRNA. *, # p < 0.05; **, ## p < 0.01
PKA Enhances SC35-Mediated Tau Exon 10 Inclusion Through Phosphorylation at Its RS Domain
PKA is a non-proline-directed serine/threonine protein kinase and phosphorylates the consensus motif R/KR/KX(X)(T/S) [31]. There are four putative PKA phosphorylation sites at the RS domain of SC35, including Ser121, Ser128, Ser130, and Ser171 (Fig. 6a). To learn whether these four putative PKA sites are indeed phosphorylated by PKA and mediate its enhancement of SC35-promoted tau exon10 inclusion, we mutated these four serines (Ser, S) into alanines (Ala, A), SC354A, to prevent phosphorylation or into glutamates (Glu, E), SC354E, to mimic constitutive phosphorylation, and then determined their effects on tau exon 10 splicing in pCI/SI9–SI10-transfected HEK-293FT cells. We found that overexpression of SC35WT (wild type of SC35), SC354A, and SC354E all increased tau exon 10 inclusion (Fig. 6b). However, SC354A was weaker than SC35WT and SC354E was much stronger than SC35WT in promoting tau exon 10 inclusion (Fig. 6b). These results suggest that these four putative sites of SC35 are involved in the regulation of SC35 on tau exon 10 splicing and that phosphorylation of SC35 at these four sites promotes tau exon 10 inclusion.
PKA enhances SC35-mediated tau exon 10 inclusion through phosphorylation at the RS domain. a Schematic of four putative PKA phosphorylation sites at the RS domain of SC35. b Pseudophosphorylation and blockage of phosphorylation of SC35 affected SC35’s ability to promote tau exon 10 inclusion. SC35WT, SC354A, or SC354E was transfected into HEK-293FT cells for 48 h. The splicing products of tau exon 10 were determined by RT-PCR. The ratio of inclusion and exclusion of tau exon 10 was calculated after densitometric quantification. Results are presented as the mean ± S.D. *vs. Con; ##vs. SC35WT. **, ## p < 0.01. c PKA catalytic subunits enhanced SC35WT- but not SC354A- or SC354E-mediated tau exon 10 inclusion. SC35WT, SC354A, or SC354E was co-transfected with PKA-Cα1, PKA-Cα2, PKA-Cβ1, or PKA-Cβ2 into HEK-293FT cells for 48 h, respectively. The ratio of inclusion and exclusion of tau exon 10 was calculated and presented as the mean ± S.D. *p < 0.05; **p < 0.01
To study the effect of PKA on SC354A-mediated tau exon 10 splicing, we co-transfected SC35WT or SC354A with PKA-Cα1, PKA-Cα2, PKA-Cβ1, and PKA-Cβ2 individually in the pCI/SI9–SI10-transfected HEK-293FT cells and then measured the tau exon 10 splicing products by RT-PCR. We found that all PKA-C isoforms promoted SC35WT-mediated tau exon 10 inclusion (Fig. 6c), but none of the PKA-Cs enhanced the function of SC354A in promoting tau exon 10 inclusion (Fig. 6c), suggesting the enhancement of SC35-mediated tau exon 10 inclusion by PKA through phosphorylation at these four sites, Ser121, Ser128, Ser130, and Ser171, and that these four sites are the main sites to mediate the regulation of SC35 by PKA.
To investigate whether PKA phosphorylates SC35 at additional sites than these four serines, we co-expressed SC354E with PKA-Cs in pCI/SI9–SI10-transfected HEK-293FT cells and then detected the splicing products of exon 10. We found that similar to SC354A, PKA-Cs did not further increase SC354E-mediated tau exon 10 inclusion (Fig. 6c). Actually, PKA-Cα2 was found to inhibit SC354D-promoted tau exon 10 splicing slightly (Fig. 6c). These results suggest that PKA promotes tau exon 10 inclusion through phosphorylation of SC35 at Ser121, Ser128, Ser130, and/or Ser171.
Discussion
Although the mechanisms underlying the onset and progression of tau pathology have not been fully elucidated, the altered ratio of 4R-tau to 3R-tau has been implicated in several tauopathies. In the normal adult brain, this ratio is approximately 1:1. In some tauopathies, the ratio is shifted in favor of either 3R-tau or 4R-tau [14–16]. Regardless of the direction of the shift and which isoform becomes predominant, neurodegeneration occurs [32]. Hence, further understanding of the molecular mechanisms by which the normal ratio is altered in some tauopathies can provide new insights into the mechanisms of these tauopathies.
In the present study, we found that PKA activation enhanced SC35-promoted tau exon 10 inclusion. SC35 interacted with catalytic subunits of PKA, and deletion of the RS domain of SC35 reduced its interaction with PKA catalytic subunits. PKA phosphorylated SC35 efficiently in vitro. Four isoforms of PKA catalytic subunits, PKA-Cα1, PKA-Cα2, PKA-Cβ1, and PKA-Cβ2, all enhanced SC35-promoted tau exon 10 inclusion. Pseudophosphorylation of the PKA putative phosphorylation sites of SC35 (Ser121, Ser128, Ser130, and Ser171) and Ala mutations at the same sites increased and decreased, respectively, SC35’s ability to promote tau exon 10 inclusion. These four putative phosphorylation sites are all located on the RS domain, which plays critical roles in interacting with other splicing factors. Enhanced SC35-mediated tau exon 10 splicing by PKA was dependent on the availability of these four putative Ser residues for its phosphorylation. Taken together, these findings suggest that PKA enhances SC35-promoted tau exon 10 inclusion via phosphorylating Ser121, Ser128, Ser130, and/or Ser171 of SC35.
SC35 is one of the SR proteins and is involved in constitutive and alternative splicing of pre-mRNA. In the previous study, we found that SC35 acts on the SC35-like exonic splicing enhancer of tau exon 10 to promote tau exon 10 inclusion [17] and enhances tau expression by increasing the stability of its mRNA [30]. As an SR protein, SC35 is rich in Ser residues that are potentially phosphorylated by several kinases [17, 33–35]. Phosphorylation of SC35 at different sites may have different impact on its function. We previously reported that Dyrk1A, a proline- and arginine-directed kinase, can phosphorylate SC35 and suppress its function in promoting tau exon 10 splicing [17]. In addition, it was reported that GSK-3β, another proline-directed kinase, also phosphorylates SC35 and suppresses tau exon 10 inclusion [33]. SC35 has 5 putative proline-directed phosphorylation sites and 42 non-proline-directed sites. PKA is a non-proline-directed protein kinase and prefers to phosphorylate the R/KR/KX(X)T/S motif. SC35 contains four such motifs. Mutations of Ser into Ala at these four motifs reduced SC35’s function to promote tau exon 10 inclusion. In contrast, pseudophosphorylation of these four residues increased SC35’s ability to promote tau exon 10 inclusion. Either mutation of these four Ser residues eliminated PKA’s activity to promote SC35-mediated tau exon 10 inclusion. Thus, phosphorylation of these putative PKA phosphorylation sites by PKA enhances SC35’s ability to promote tau exon 10 inclusion, which is different from proline-directed phosphorylation.
The human brain expresses high levels of PKA-Cα1 and PKA-Cβ2 and trace levels of PKA-Cα2 and PKA-Cβ1. The difference between these four isoforms is mainly at their N-terminus. In the present study, we found that SC35 interacted with both PKA-Cα and PKA-Cβ. Deletion of the N-terminal RRM domain of SC35 did not affect its interaction with PKA-C. However, deletion of the C-terminal RS domain significantly reduced its interaction with PKA. Thus, the RS domain appears to mediate the interaction of SC35 with PKA-C. PKA-C structurally has an acidic C-terminal tail, which interacts uniquely with functional motifs in the kinase core as well as with other proteins such as the activating kinase, PDK1, and Hsp 90 [36]. In light of the arginine-rich sequence at the RS domain of SC35, we speculate that SC35 probably interacts through its RS domain with the C-tail of PKA-Cs and it is phosphorylated at the RS domain by the kinase.
Earlier work from our group showed that PKA can also phosphorylate ASF/SF2 (SRSF1) and promote SRSF1-mediated tau exon 10 inclusion [19]. Unlike SC35, ASF/SF interacts only with PKA-Cα, but not PKA-Cβ through the RRM domain. Therefore, the role of PKA on SR protein-mediated alternative splicing may be isoform specific.
Previously, we demonstrated that in AD brain, overactivation of calpain I due to calcium dysregulation causes degradation of the regulatory subunit of PKA, PKA-RII [37]. A decrease in PKA-RII at basal conditions provides less protection to PKA-C from degradation. The catalytic subunits of PKA are thus also decreased in AD brain [37]. This leads us to postulate that the down-regulation of PKA may result in a decreased ratio of 4R-tau/3R-tau through suppressing the function of SC35 and SRSF1 in promotion of tau 10 exon inclusion. Down-regulation of the PKA pathway is indeed associated with the increased ratio of 3R-tau/4R-tau in AD brain [19]. In AD brain, isoform transition from four-repeat to three-repeat tau underlies dendrosomatic and regional progression of neurofibrillary pathology [38]. Severe pathology of AD is associated with abundant 3R-tau-positive tangles, suggesting that aggregation and deposition of 3R-tau may be associated with advanced stages of the disease [39]. Thus, the down-regulated PKA pathway might also contribute to tau pathogenesis in AD through dysregulation of tau exon 10 splicing.
In summary, the present study shows that PKA interacts with and phosphorylates the splicing factor SC35 at the RS domain and enhances SC35-mediated tau exon 10 splicing, resulting in an increased expression of 4R-tau. Down-regulation of the PKA pathway reported previously [37] can therefore lead to a reduction in the ratio of 4R-tau/3R-tau and tau pathogenesis.
References
Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K (2001) Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A 98:6923–6928
Ballatore C, Hyde E, Deiches RF, Lee VM, Trojanowski JQ, Huryn D, Smith AB 3rd (2007) Paclitaxel C-10 carbamates: potential candidates for the treatment of neurodegenerative tauopathies. Bioorg Med Chem Lett 17:3642–3646
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261:6084–6089
Montejo de Garcini E, Serrano L, Avila J (1986) Self assembly of microtubule associated protein tau into filaments resembling those found in Alzheimer disease. Biochem Biophys Res Commun 141:790–796
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A 83:4913–4917
Andreadis A, Brown WM, Kosik KS (1992) Structure and novel exons of the human tau gene. Biochemistry 31:10626–10633
Himmler A (1989) Structure of the bovine tau gene: alternatively spliced transcripts generate a protein family. Mol Cell Biol 9:1389–1396
Hardy J, Gwinn-Hardy K (1998) Genetic classification of primary neurodegenerative disease. Science 282:1075–1079
Himmler A, Drechsel D, Kirschner MW, Martin DW Jr (1989) Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol Cell Biol 9:1381–1388
Hasegawa M, Morishima-Kawashima M, Takio K, Suzuki M, Titani K, Ihara Y (1992) Protein sequence and mass spectrometric analyses of tau in the Alzheimer’s disease brain. J Biol Chem 267:17047–17054
Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA (1989) Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J 8:393–399
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA (1989) Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 3:519–526
Kosik KS, Orecchio LD, Bakalis S, Neve RL (1989) Developmentally regulated expression of specific tau sequences. Neuron 2:1389–1397
D’Souza I, Schellenberg GD (2005) Regulation of tau isoform expression and dementia. Biochim Biophys Acta 1739:104–115
Goedert M, Jakes R (2005) Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta 1739:240–250
Sergeant N, Delacourte A, Buee L (2005) Tau protein as a differential biomarker of tauopathies. Biochim Biophys Acta 1739:179–197
Qian W, Liang H, Shi J, Jin N, Grundke-Iqbal I, Iqbal K, Gong CX, Liu F (2011) Regulation of the alternative splicing of tau exon 10 by SC35 and Dyrk1A. Nucleic Acids Res 39:6161–6171
Birney E, Kumar S, Krainer AR (1993) Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res 21:5803–5816
Shi J, Qian W, Yin X, Iqbal K, Grundke-Iqbal I, Gu X, Ding F, Gong CX, Liu F (2011) Cyclic AMP-dependent protein kinase regulates the alternative splicing of tau exon 10: a mechanism involved in tau pathology of Alzheimer disease. J Biol Chem 286:14639–14648
Gui JF, Lane WS, Fu XD (1994) A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature 369:678–682
Wang HY, Lin W, Dyck JA, Yeakley JM, Songyang Z, Cantley LC, Fu XD (1998) SRPK2: a differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells. J Cell Biol 140:737–750
Colwill K, Pawson T, Andrews B, Prasad J, Manley JL, Bell JC, Duncan PI (1996) The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J 15:265–275
Rossi F, Labourier E, Forne T, Divita G, Derancourt J, Riou JF, Antoine E, Cathala G, Brunel C, Tazi J (1996) Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature 381:80–82
Doskeland SO, Maronde E, Gjertsen BT (1993) The genetic subtypes of cAMP-dependent protein kinase—functionally different or redundant? Biochim Biophys Acta 1178:249–258
Foss KB, Simard J, Berube D, Beebe SJ, Sandberg M, Grzeschik KH, Gagne R, Hansson V, Jahnsen T (1992) Localization of the catalytic subunit C gamma of the cAMP-dependent protein kinase gene (PRKACG) to human chromosome region 9q13. Cytogenet Cell Genet 60:22–25
Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX (2007) Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur J Neurosci 26:3429–3436
Liu F, Liang Z, Shi J, Yin D, El-Akkad E, Grundke-Iqbal I, Iqbal K, Gong CX (2006) PKA modulates GSK-3beta- and cdk5-catalyzed phosphorylation of tau in site- and kinase-specific manners. FEBS Lett 580:6269–6274
Liu SJ, Zhang JY, Li HL, Fang ZY, Wang Q, Deng HM, Gong CX, Grundke-Iqbal I, Iqbal K, Wang JZ (2004) Tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated by PKA in rat brain. J Biol Chem 279:50078–50088
Shi J, Zhang T, Zhou C, Chohan MO, Gu X, Wegiel J, Zhou J, Hwang YW, Iqbal K, Grundke-Iqbal I, Gong CX, Liu F (2008) Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of tau in Down syndrome. J Biol Chem 283:28660–28669
Qian W, Iqbal K, Grundke-Iqbal I, Gong CX, Liu F (2011) Splicing factor SC35 promotes tau expression through stabilization of its mRNA. FEBS Lett 585:875–880
Townsend RR, Lipniunas PH, Tulk BM, Verkman AS (1996) Identification of protein kinase A phosphorylation sites on NBD1 and R domains of CFTR using electrospray mass spectrometry with selective phosphate ion monitoring. Protein Sci 5:1865–1873
Gasparini L, Terni B, Spillantini MG (2007) Frontotemporal dementia with tau pathology. Neurodegener Dis 4:236–253
Hernandez F, Perez M, Lucas JJ, Mata AM, Bhat R, Avila J (2004) Glycogen synthase kinase-3 plays a crucial role in tau exon 10 splicing and intranuclear distribution of SC35. Implications for Alzheimer’s disease. J Biol Chem 279:3801–3806
Cataldi A, Zingariello M, Rapino M, Zara S, Daniele F, Di Giulio C, Antonucci A (2009) Effect of hypoxia and aging on PKC delta-mediated SC-35 phosphorylation in rat myocardial tissue. Anat Rec (Hoboken) 292:1135–1142
Jang SW, Liu X, Fu H, Rees H, Yepes M, Levey A, Ye K (2009) Interaction of Akt-phosphorylated SRPK2 with 14-3-3 mediates cell cycle and cell death in neurons. J Biol Chem 284:24512–24525
Taylor SS, Kim C, Cheng CY, Brown SH, Wu J, Kannan N (2008) Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim Biophys Acta 1784:16–26
Liang Z, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX (2007) Down-regulation of cAMP-dependent protein kinase by over-activated calpain in Alzheimer disease brain. J Neurochem 103:2462–2470
Hara M, Hirokawa K, Kamei S, Uchihara T (2013) Isoform transition from four-repeat to three-repeat tau underlies dendrosomatic and regional progression of neurofibrillary pathology. Acta Neuropathol 125:565–579
Espinoza M, de Silva R, Dickson DW, Davies P (2008) Differential incorporation of tau isoforms in Alzheimer’s disease. J Alzheimers Dis 14:1–16
Acknowledgments
We thank Ms. J. Murphy for secretarial assistance. This work was supported in part by Nantong University and the New York State Office for People with Developmental Disabilities, and grants from the National Natural Science Foundation of China (81030059 and 30973143 to L.F.), the U.S. Alzheimer’s Association (IIRG-10-173154 to L.F.), the Basic Research Program of Jiangsu Education Department (10KJA310040 to L.F.), and the Priority Academic Program Development of Jiangsu Higher Education institutions (PAPD).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, C., Jin, N., Qian, W. et al. Cyclic AMP-Dependent Protein Kinase Enhances SC35-Promoted Tau Exon 10 Inclusion. Mol Neurobiol 49, 615–624 (2014). https://doi.org/10.1007/s12035-013-8542-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8542-3