Abstract
Mutation of TAR DNA-binding protein-43 (TDP-43) was detected in familiar and sporadic amyotrophic lateral sclerosis, and pathological TDP-43 was identified in the frontotemporal lobar degeneration. The neuroprotective functions of curcumin derivatives were assessed in motor neurons transfected with mutant TDP-43. We found that curcumin derivatives reduced the levels of TDP-43 fragments. Furthermore, we evaluated these compounds on the cellular model that the cells were transfected with TDP-25. We found that the expression level and aggregate formation of TDP-25 were significantly reduced by monocarbonyl dimethoxycurcumin C (Compound C). To study on the neuroprotective functions of curcumin derivatives, the neuroblastoma-spinal cord-34 cells transfected with mutant TDP-43 were assessed by the level of lactate dehydrogenase (LDH) and malondialdehyde bisdimethyl acetal (MDA) that were involved in the oxidative stress. We found that Compound C ameliorated the damage of mutant TDP-43 by reducing the level of MDA and LDH. Furthermore, heme oxygenase-1 (HO-1) was induced by Compound C significantly higher than other compounds. Znpp, which is known an inhibitor of HO-1, dramatically interfered with the function of Compound C. In addition, Compound C was tested in vivo, and HO-1 was significantly upregulated at the hippocampus. These findings suggest that Compound C, which degrades TDP-43 fragment and strengthens the antioxidant ability by HO-1, is a promising agent for TDP-43 proteinopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
TAR DNA-binding protein-43 (TDP-43) is a constitutively and ubiquitously expressed DNA–RNA-binding protein. In 2006, TDP-43 was identified as the major protein in the ubiquitinated inclusion in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) [1, 2]. Furthermore, in a large study on ALS cases, including familial cases with and without SOD1 mutations, TDP-43 immunopositive inclusion was present in almost all cases of sporadic ALS, ALS with dementia, and SOD1-negative FALS [3]. In 2008, Sreedharan et al. [7] first identified TDP-43 Q331K and TDP-43M337V in ALS patient and found that mutant TDP-43 formed numerous fragments varying in molecular weight from ∼14 to ∼45 and induced apoptosis in chick embryos. The subcellular distribution or aggregation had no obvious differences in wild-type and mutant proteins. Subsequently, TDP-43 gene mutation was studied in a series of clinical research and was present in 6.5 % familial ALS and up to 4 % sporadic ALS [4]. However, the molecular mechanism by which mutant TDP-43 causes motor neuron degeneration remains unknown. Given that the toxicity of mutant TDP-43 may be related to increased oxidative damage [5, 6] and fragment formation [7], it is conceivable that these events may be potential drug targets in TDP-43-related diseases.
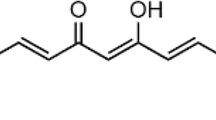
Curcumin is a polyphenolic compound with potent antioxidant and anti-inflammatory activities and is under development as a potential neuroprotective agent [8, 9]. However, the poor bioavailability and the rapid metabolism of this compound limited its applications. Phase I clinical studies on curcumin did not show any treatment-related toxicity even at 8 g/day, but its highest peak plasma concentration was only 1.8 μM, a subtherapeutic concentration [10]. The poor bioavailability could result from the β-diketone moiety in the structure of curcumin, which can be altered rapidly by a series of aldo-keto reductases in vivo [11]. Therefore, we designed several analogs without the β-diketone moiety, and as expected, these compounds showed improved stability in vitro and improved pharmacokinetic profiles in vivo [12].
To determine whether the new monocarbonyl curcumin derivatives had a neuroprotective activity, we tested these compounds in cell models of ALS that motor neuron-like cells (NSC-34) were transfected with mutant TDP-43. We found that the Compound C strongly protected the motor neuron-like cells against the toxicity of mutant TPD-43.
Results
Compound C Reduced the Level of TDP-43 Fragments
Curcumin is a well known antioxidative agent, but its bioavailability is low in vivo. Thus, we synthesized monocarbonyl dimethoxycurcumin B and C, which are more stable chemically than curcumin and dimethoxycurcumin (Fig. 1). We evaluated these compounds in an ALS cellular model that NSC-34 cells transfected with mutant TDP-43. NSC-34 is produced by fusion of motor neuron-enriched embryonic mouse spinal cord cells with mouse neuroblastoma and widely acknowledged as a validated model for studying on motor neuron toxicity. These cells express many properties of motor neurons, such as choline acetyltransferase; acetylcholine synthesis, storage, and release; and neurofilament triplet proteins [13]. We found that monocarbonyl dimethoxycurcumin significantly inhibited the formation of TDP-43 fragments, which were associated with the TDP-43 mutation (Fig. 2a–b). To eliminate the possibility that the protective effects from compounds are not artifacts of reduced TDP-43 transgene expression, TDP-43 mRNA was tested by quantitative real-time PCR. However, we found that the compounds did not have an obvious effect on the TDP-43 transcription (Fig. Sa). In addition, to test whether Compound C had an effect on mutant TDP-43 solubility/insolubility, the fractionation assay was performed according to the published papers [14–16]. We found that Compound C significantly reduced the insoluble TDP-43 fragments (Fig. Sb–e). Furthermore, we established a cellular model that NSC-34 transfected with TDP-25, which is involved in the process of motor neuron degeneration and prone to forming aggregates. We assessed the effect of curcumin, dimethoxycurcumin, and dimethoxycurcumin derivatives on the aggregates of TDP-25. Consistent with the results that monocarbonyl dimethoxycurcumin C reduced the generation of fragments in NSC-34 cells transfected with mutant TDP-43, Compound C decreased the expression level of TDP-25 by 56 % (p < 0.05), the number of cells expressing TDP-25 by 59 % (p < 0.05), and the size of TDP-25 aggregates, compared with the controls (Figs. 3a, b, 4a, b, and 5a, b). However, curcumin, dimethoxycurcumin, and dimethoxycurcumin derivative B could not reduce the expression of TDP-25 and number of cells expressing TDP-25. In addition, Compound C at 40 μM was more effective than at 10 μM (Fig. 3c–d). Subsequently, we evaluated the action of Compound C on the TDP-25 solubility/insolubility. The results showed that Compound C significantly reduced the expression of TDP-25 in soluble and insoluble fractions (Fig. 3e–f).
Compound C decreased fragment level of TDP-43. wt, wild-type TDP-43; q, Q331K TDP-43; empty, empty plasmid. a Western blot analysis of TDP-43 fragments in the treated and untreated group. b Quantitative assessment of the level of TDP-43 fragments from three independent experiments (mean ± SD, n = 3), *p < 0.05, compared with the untreated samples
Compound C decreased the expression of TDP-25. a–b NSC-34 cells transiently transfected with TDP-25 were treated with curcumin, DMC, Compound B, and Compound C at 10 μM for 24 h, respectively. Compound C significantly reduced the expression of TDP-25 (mean ± SD, n = 4). *p < 0.05, compared with the untreated samples. c–d Compound C decreased the expression of TDP-25 at 10 and 40 μM (mean ± SD, n = 4). *p < 0.05, compared with the untreated samples. e–f Compound C significantly reduced the expression of TDP-25 in the soluble and insoluble fraction (mean ± SD, n = 3). *p < 0.05, compared with the untreated samples
Compound C reduced the number of cells expressed with EGFP-TDP-25. a Confocal microscopy of NSC-34 cells transfected with EGFP fused to TDP-25 constructs. Representative images are showed. Scale bar = 200 μm. b Quantification of the number of cells with TDP-25 expression. Average number of cells expressing TDP-25 was collected from at least five random fields in three independent experiments (mean ± SD, n = 3), *p < 0.05, compared with the untreated samples
Compound C decreased the size of aggregates of TDP-25. a–b Cells were treated with 10 μM compound C for 24 h and then examined by confocal microscopy. The size of aggregates was evaluated by Image J software, and at least 20 cells expressing TDP-25 were analyzed (mean ± SD, n = 4). *p < 0.05. Nu, nuclei; Bf, bright field
Compound C Reduced Toxicity of Mutant TDP-43
Mutant TDP-43 was recently identified in ALS patients and induced motor neuron degeneration by increasing oxidative stress and generating fragments. We found that mutant TDP-43 significantly increased the level of lactate dehydrogenase (LDH) and malondialdehyde bisdimethyl acetal (MDA) in the NSC-34 cells. Monocarbonyl dimethoxycurcumin C significantly protected the cell against these events (Fig. 6a, b).
Compound C reduced the toxicity of mutant TDP-43. a Compound C reduced the level of LDH in the medium of cells transfected with mutant TDP-43. Average level of LDH from three independent experiments (mean ± SD, n = 3),*p < 0.05, compared with the untreated samples. b Compound C alleviates lipoperoxidation as determined by measuring MDA. Average level of MDA in from three independent experiments (means ± SD, n = 3), *p < 0.05, compared with the untreated samples
Compound C in Part Enhanced the Degradation of TDP-25 via Heme Oxygenase-1
In our previous studies, we found that heme oxygenase-1 (HO-1), known as an antioxidant enzyme, played an important role in ameliorating the toxicity of mutant TDP-43 [6]. The cells were treated by curcumin derivatives at the same dose, and we found that the expression of HO-1 in the cells treated by Compound C was 1.45–1.87 times higher than dimethoxycurcumin (DMC) and Compound B (Fig. 7a, b). Furthermore, the expression of HO-1 was induced by Compound C in time- and dose-dependent manner. Nrf2, which is associated with the transcription of HO-1, was also activated by Compound C (Fig. 8a–f). Subsequently, we asked whether HO-1 was involved in the degradation of TDP-43 fragment, and the cellular model was treated by Znpp, which is an inhibitor of HO-1 [17]. Znpp significantly increased the expression of TDP-25, and Znpp with Compound C reduced the level of TDP-25 by 34 %. These results suggest that the HO-1 could be involved into the degradation of TDP-25; however, it does not completely depend on HO-1 (Fig. 9a, b). Thus, Compound C enhanced the degradation of TDP-43 fragments via HO-1, partly.
Compound C dramatically increased HO-1 expression. wt, wild-type TDP-43; q, Q331K TDP-43; empty, empty plasmid. a–b Western blot analysis of HO-1 protein in the cellular model treated by curcumin derivatives. The average value of HO-1 protein in three different cultures (mean ± SD, n = 3). Each value marked by an asterisk is significantly different from the corresponding control value (*p < 0.05). c Western blot analysis of HO-1 protein in the cellular model treated by DMC, Compound B, and Compound C at 1 and 10 μM for 24 h
Compound C induced the expression of HO-1 in time- and dose-dependent manner and activated Nrf2. a–c The cells were treated by Compound C at 0, 5, 10, 20, 40, and 100 μM for 24 h. The analysis of HO-1 and Nrf2 were in three different cultures, respectively (mean ± SD, n = 3). d–f Forty micromolars of compound C-treated motor neuron-like cells at different times from 0, 3, 6, 12, 24 to 48 h. The expression of HO-1 and Nrf2 was evaluated by Western blot in three different cultures, respectively (mean ± SD, n = 3). Each value marked by an asterisk is significantly different from the corresponding control value (*p < 0.05)
Compound C degraded the TDP-43 fragment partly dependent on HO-1. a–b NSC-34 cells transiently transfected with TDP-25 were treated with Compound C and Znpp-9 at 10 μM for 24 h, respectively. Compound C significantly reduced the expression of TDP-25 (mean SD, n = 3). *p < 0.05, compared with the untreated samples. However, Znpp-9 significantly inhibited the degradation of Compound C
Compound C Induced HO-1 In Vivo
To investigate whether C could induce the expression of HO-1 in vivo, compound C was resolved into 38 % PEG400 and treated the mice by intravenous administration for 3 days. HO-1 in the lung (L), motor neuron cortex (Co), hippocampus (Hi), and spinal cord (Sp) was studied. We found that the level of the antioxidant enzyme HO-1 in Hi was significantly upregulated by Compound C (p < 0.05) (Fig. 10a, b). Immunochemistry of HO-1 in the hippocampus further confirmed the immunoblot results (Fig. 10c). Therefore, our results showed that compound C could increase the antioxidant defense ability in vivo via HO-1.
Compound C significantly induced the expression of HO-1 in the hippocampus. a–b Western blot analysis of HO-1 protein in different parts of the central nervous system (mean ± SD, n = 4). Each value marked by an asterisk is significantly different from the corresponding control value (*p < 0.05). c Immuno-chemistry of HO-1 in hippo-campus. Scale bar = 50 μm
Discussion
In this paper, we found that Compound C significantly protected the cells against the toxicity of mutant TDP-43 by reducing formation of its fragments, increasing antioxidant ability, and preventing the generation of the aggregates of TDP-25. TDP-43 is a DNA–RNA-binding protein located in the nucleus and plays a role in neurite outgrowth [18], RNA splicing [19], and cell proliferation [20] and is involved in the animal development [21]. TDP-43 gene mutation and TDP-43 fragments were identified in sporadic and familial ALS patients [2, 4, 7]. Mutant TDP-43 induced oxidative stress in the cell model, and mutant TDP-43 transgenic mice developed features of ALS and FTLD and presented fragmentation of TDP-43 at an early stage [6, 22]. Furthermore, expression of TDP-25 formed cytoplasmic inclusions that recapitulated the features of pathological TDP-43 [23, 24]. In addition, Caccamo et al. reported that transgenic mice expressing TDP-25 developed cognitive deficits [25]. These findings suggest that mutant TDP-43 is toxic and that formation of TDP-43 fragments is a key event in the pathogenesis of ALS and FTLD. In the present study, we found that Compound C not only reduced the level of oxidative stress caused by mutant TDP-43 but also significantly reduced the generation of TDP-25. In addition, Compound C dramatically induced the expression of HO-1 in the hippocampus. Hence, monocarbonyl dimethoxycurcumin C is a promising agent for the protection of the neurons in the hippocampus against the toxicity of pathological TDP-43. Recently, some papers supported our findings. Vaccaro et al. demonstrated that mutant TDP-43 induced oxidative stress in vivo with genetic animal models of TDP-43 toxicity [26], and chemical suppression of oxidative stress is protective in these models [27].
HO-1 was involved in the heme catabolism, iron ion homeostasis, and the process of antioxidative stress. The activity of HO-1 could be induced by its substrate heme and by various nonheme substances, such as oxidative stress, LPS, cytokines, hypoxia, and antioxidant compound [28–30]. HO-1 is regulated by Nrf2 that binds the antioxidant response elements in the promoter of HO-1 gene [31]. Besides Nrf2, HO-1 was also regulated by hypoxia-inducible factor (HIF)-1α that binds the hypoxia response element in the promotor. Data supported that the hypoxia induced the HO-1 expression dependent on the HIF-1α [32]. Yeligar et al. demonstrated that the ethanol-induced HO-1 expression was attenuated by HIF-1α siRNA. Therefore, HO-1 plays an important role in the conditions that the cell defends itself against some toxic factors and is regulated by different transcriptional factors. The neuroprotection of HO-1 was mentioned in many papers in which neurodegenerative diseases were studied. Till now, the mechanism of neurodegeneration is not clear, and multifactors could initiate the process of neuron death, such as oxidative stress, inflammatory factors, and aggregations. Interestingly, we found that Compound C degraded the TDP-43 fragment partly dependent on the HO-1. Recent data supported that TDP-25 degradation depended on autophagy and ubiquitin proteasome system [33]. Indeed, Unuma et al. demonstrated that HO-1 promotes autophagy and elimination of damaged mitochondria, thereby repressing oxidative stress [34]. These findings suggest that HO-1 could be involved in the process of TDP-25 degradation by autophagy.
Taken together, Compound C, which degrades TDP-43 fragment and increases the antioxidant ability by upregulating HO-1, is a promising agent for TDP-43 proteinopathy.
Experimental Procedures
Cell Lines and Transfection
NSC-34 is a hybrid cell line that retains the ability to proliferate and expresses several motor neuron characteristics [13]. NSC-34 was routinely maintained in DMEM (Invitrogen Corporation, CA, USA; cat. no. 21063-029) with 10 % heat-inactivated FBS (certified performance tested; Invitrogen Corporation, CA, USA; cat. no. 16000-044) and antibiotics (100 IU/mL penicillin and 100 μg/mL streptomycin). Following the manufacturer’s protocol, NSC-34 cells were transfected with the empty pCI-neo vector or the vector cloned with wild-type TDP-43, TDP-43 Q331K, and TDP-25cDNAs by using a Lipofectamine™ 2000 transfection reagent (Invitrogen Corporation, CA, USA; cat. no. 11668-019). NSC-34 cells were routinely maintained at 37 °C in a 5 % CO2 humidified atmosphere in 25-cm2 flasks (Corning Incorporated, Corning, NY, USA), changing the medium every 2–3 days. The derivatives of curcumin were donated by Dr. Jian Xiao. The compound was dissolved in DMSO. The final concentration of DMSO was 0.1 % in culture medium, and this amount did not have any effect on the cells in our culture (our previous data). To compare the action of the derivatives of curcumin, the cells were treated with a 10-μM compound including curcumin, DMC, compound B, and compound C, respectively. After transfection for 24 h, the compounds, such as curcumin, DMC, compound B, and compound C, were added to the medium for 24 h, respectively. Each experiment was repeated at least three times.
Lipoperoxidation
Levels of lipid peroxidation were quantified using the Thiobarbituric Acid-Reactive Substances (TBARS) Assay Kit (Jian Cheng Biological Engineering Institute, Nanjing, China). Lipid peroxidation products, including malondialdehyde and hydroperoxides, react with thiobarbituric acid to produce a product that can be sensitively measured spectroscopically. Samples were mixed with the reagents at room temperature, and the reaction mixtures were incubated at 95 °C for 40 min and then allowed to cool. Absorbance was read at 532 nm, and TBARS levels were calculated using a standard curve of MDA. Data are expressed as nanomoles of TBARS per milligram of protein. The protein concentration was calculated by Bradford’s method, using albumin as standard.
Measurement of LDH
The damage of NSC-34 cells as evaluated by measuring the release into medium of intracellular LDH. LDH activities were determined using a colorimetric LDH assay kit (JianCheng Technology, Nanjing, China). To more accurately evaluate LDH level, the ratio of medium LDH to tissue LDH was used to determine the damage of plasma membrane.
Western Blot
Cells were rinsed twice with PBS and then collected by centrifugation at 500×g for 5 min at 4 °C. The protein was extracted using Total Protein Extraction Kit (Applygen Technologies Inc., Beijing, China; cat. no. P1250) and cell lysis buffer for Western and immunoprecipitation (IP) (Beyotime Institute of Biotechnology, Nanjing, China; cat. no. P0013). Protein extracts were quantified using the Bradford method. Twenty micrograms of extracts was denatured at 95 °C for 5 min, loaded onto a 10 % sodium dodecyl sulfate (SDS)–polyacrylamide gel, and electrophoresed. After electrophoresis, the proteins in the gel were transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The membrane was then incubated overnight at 4 °C with the following primary antibodies: rabbit anti-TDP-43 (1:1,200; Protein Tech Group, Tucson, AZ, USA; cat. no. 12892-1-AP), rabbit anti-hemagglutinin (HA) (1:1,000; Sigma Chemical Co., St. Louis, MO, USA; cat. no. H6908), rabbit anti-HO-1 (1:500; Stressgen, MI, USA; cat. no. SPA-895), rabbit anti-Nrf2 (1:200; Santa Cruz Biotechnology, CA, USA; cat. no. sc-722), mouse anti-GAPDH (1:10,000; KangChen Biotech, Shanghai, China; cat. no. KC-5G4), and mouse anti-β-actin (1:500; Santa Cruz Biotechnology, CA, USA; cat. no. sc-47778). Membranes were then incubated for 1 h at 22–24 °C with a fluorescence-conjugated secondary antibody (1:3,000). The bands of interest on the membrane were detected using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA). The usual green or red color bands were converted to black and white colors for data presentation.
To study on the fragments of mutant TDP-43, NSC-34 cells were transfected with TDP-43 WT and TDP-43Q331K that have a HA-tag at the C-terminal. And then, HA antibody was used to identify human TDP-43 and the fragments of TDP-43 C-terminal. To evaluate the expression of TDP-25, the TDP-43 antibody was used for Western blot.
Fluorescence Study
For evaluating the effects of curcumin and its derivatives on the TDP-25 degradation by confocal microscopy, NSC-34 cells were transfected with EGFP-TDP-25 for 24 h and then treated with curcumin and its derivatives for 24 h, respectively. After the treatment, the cells were fixed by 4 % paraformaldehyde for 30 min at room temperature, then stained with Hoechst 33342 for 40 min, and finally washed twice with PBS. The cells were observed using confocal microscopy. Positive cells with EGFP were counted in five random view fields in three independent experiments.
The effect of Compound C on the degradation of TDP-25 was examined in cells transfected with EGFP-TDP-25 plasmids for 24 h and then treated by the Compound C for 24 h. Following the treatment, nuclei were stained with Hoechst 33342 for 40 min, and then the cells were washed twice with PBS. The medium was changed to DMEM without phenol red, and the cells were observed by confocal microscopy.
The area of the aggregates was measured by Image J software. The area of the largest aggregate in the cell was measured.
Mice and Curcumin Derivative Administration
Adult (7 weeks old) male C57BL/6 mice were maintained under temperature- and light-controlled conditions (20–23 °C, 12-h light/12-h dark cycle). Eight mice were randomly assigned to a vehicle-treated group (n = 4) and compound C-treated group (n = 4). Compound C was resolved in the 38 % PEG400. The compound C-PEG400 resolution was administrated intravenously at a dose of 1.5 mg/kg body weight, once daily for 3 days. The 38 % PEG400 was used in the vehicle-treated group in the same manner as the Compound C-PEG400-treated mice.
Immunochemistry
Animals were anesthetized with 0.2 mL 10 % chloralhydrate, perfused with 4 % paraformaldehyde in phosphate-buffered saline (PBS), and the brain was dissected. The samples were blocked for 30 min in 10 % horse serum and incubated overnight with primary antibodies against HO-1 (diluted 1:200) at 4 °C. The sections were washed three times with 0.01 M PBS containing 0.3 % TritonX-100 and incubated with a biotinylated secondary antibody for 1 h at room temperature. Then, the samples were further washed and incubated with horseradish peroxidase-conjugated ABC staining solution (Vector Laboratories, Burlingame, CA, USA). The negative control was used by replacing primary antibody with PBS.
Fractionation Assay
Briefly, cells were lysed in a cell lysis buffer for Western and IP including 20 mM Tris (pH 7.5), 150 mM NaCl, 1 % Triton X-100, sodium pyrophosphate, β-glycerophosphate, EDTA, Na3VO4, leupeptin, phosphatase inhibitor, protease inhibitor (Beyotime Institute of Biotechnology, Nanjing, China; cat. no. P0013). Following the manufacturer’s protocol, cells were centrifuged at 14,000×g at 4 °C for 10 min. Triton X-100-insoluble pellets were dissolved in the buffer plus 2 % SDS, protease inhibitor, and phosphatase inhibitor. The soluble and insoluble components were used for Western blot analysis.
Quantitative Real-Time PCR
Quantitative real-time PCR (qPCR) was used to verify the level of TDP-43 mRNA. RNA was isolated using RNA Pure Kit (BioTeke, Beijing, PR China; cat. no. RP1202), following the manufacturer’s instructions. RNA concentrations and qualities were determined by measuring absorbance at 260 and 280 nm. Two micrograms of RNA from each sample was used as template for Reverse Transcription System (Promega, Madison, USA; cat. no. A3500) with Oligo (dT), Reverse Transcription 10× Buffer, Recombinant RNasin® Ribonuclease Inhibitor, dNTP Mixture, and AMV Reverse Transcriptase at 42 °C for 60 min. Forward and reverse primers were 5′-gggaaacaatcaaggtag-3′ and 5′-cagccagaagacttagaatc-3′ for TDP-43, and 5′-tgttaccaactgggacgaca-3′ and 5′-ctctcagctgtggtggtgaa-3′ for mouse β-actin. TransStart® Top Green qPCR SuperMix (TransGen Biotech, Beijing, PR China; cat. no. AQ131) was used, and qPCR was performed, and then the product was detected by the strategene Mx3005p (Agilent, Santa Clara, USA). For the PCR, the amplification conditions included an initial activation step at 95 °C for 30 s, and 40 cycles at 95 °C for 5 s, 52 °C for 15 s, and 72 °C for 10 s. The fluorescence of the double-stranded products was monitored in real time. According to the standard curve, the concentrations of TDP-43 mRNA and β-actin mRNA were calculated, and the relative TDP-43 mRNA levels were normalized to the reference of housekeeping gene.
Statistical Analyses
All data are expressed as mean ± SD and analyzed by one-way ANOVA followed by a Student–Newman–Keuls multiple range test or Dunn’s t test. Results were considered significant when p < 0.05.
References
Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314:130–133
Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley JR, Kirby J, Siddique T, Shaw PJ, Lee VM, Trojanowski JQ (2007) Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol 61:427–434
Pratt AJ, Getzoff ED, Perry JJ (2012) Amyotrophic lateral sclerosis: update and new developments. Degener Neurol Neuromuscul Dis 2:1–14
Cleveland DW, Rothstein JD (2001) From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2:806–819
Duan W, Li X, Shi J, Guo Y, Li Z, Li C (2010) Mutant TDP-43 induces oxidative injury in motor neuron-like cell. Neuroscience 169:1621–1629
Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319:1668–1672
Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM (2001) The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 21:8370–8377
Chainani-Wu N (2003) Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa). J Altern Complement Med 9:161–168
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY (2001) Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 21:2895–900
Rosemond MJ, St John-Williams L, Yamaguchi T, Fujishita T, Walsh JS (2004) Enzymology of a carbonyl reduction clearance pathway for the HIV integrase inhibitor, S-1360: role of human liver cytosolic aldo-keto reductases. Chem Biol Interact 147:129–139
Liang G, Shao L, Wang Y, Zhao C, Chu Y, Xiao J, Zhao Y, Li X, Yang S (2009) Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorg Med Chem 17:2623–2631
Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP (1992) Neuroblastomaspinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn 194:209–221
Zhang YJ, Xu YF, Dickey CA et al (2007) Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci 27:10530–10534
Zhang YJ, Gendron TF, Xu YF et al (2010) Phosphorylation regulates proteasomal-mediated degradation and solubility of TAR DNA binding protein-43 C-terminal fragments. Mol Neurodegener 5:33
Kwiatkowski TJ Jr, Bosco DA, Leclerc AL et al (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323:1205120–8
Park SH, Jang JH, Li MH, Na HK, Cha YN, Surh YJ (2007) Nrf2-mediated heme oxygenase-1 induction confers adaptive survival response to tetrahydropapaveroline-induced oxidative PC12 cell death. Antioxid Redox Signal 9:2075–2086
Iguchi Y, Katsuno M, Niwa J, Yamada S, Sone J, Waza M, Adachi H, Tanaka F, Nagata K, Arimura N, Watanabe T, Kaibuchi K, Sobue G (2009) TDP-43 depletion induces neuronal cell damage through dysregulation of Rho family GTPases. J Biol Chem 284:22059–22066
Bose JK, Wang IF, Hung L, Tarn WY, Shen CK (2008) TDP-43 overexpression enhances exon 7 inclusion during the survival of motor neuron pre-mRNA splicing. J Biol Chem 283:28852–28859
Ayala YM, Misteli T, Baralle FE (2008) TDP-43 regulates retinoblastoma protein phosphorylation through the repression of cyclin-dependent kinase 6 expression. Proc Natl Acad Sci U S A 105:3785–3789
Wu LS, Cheng WC, Hou SC, Yan YT, Jiang ST, Shen CK (2010) TDP-43, a neuro-pathosignature factor, is essential for early mouse embryogenesis. Genesis 48:56–62
Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH (2009) TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci U S A 106:18809–18814
Igaz LM, Kwong LK, Chen-Plotkin A, Winton MJ, Unger TL, Xu Y, Neumann M, Trojanowski JQ, Lee VM (2009) Expression of TDP-43 C-terminal fragments in vitro recapitulates pathological features of TDP-43 proteinopathies. J Biol Chem 284:8516–8524
Zhang YJ, Xu YF, Cook C, Gendron TF, Roettges P, Link CD, Lin WL, Tong J, Castanedes-Casey M, Ash P, Gass J, Rangachari V, Buratti E, Baralle F, Golde TE, Dickson DW, Petrucelli L (2009) Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A 106:7607–7612
Caccamo A, Majumder S, Oddo S (2012) Cognitive decline typical of frontotemporal lobar degeneration in transgenic mice expressing the 25-kDa C-terminal fragment of TDP-43. Am J Pathol 180:293–302
Vaccaro A, Patten SA, Ciura S, Maios C, Therrien M, Drapeau P, Kabashi E, Parker JA (2012) Methylene blue protects against TDP-43 and FUS neuronal toxicity in C. elegans and D. rerio. PLoS One 7:e42117
Vaccaro A, Patten SA, Aggad D, Julien C, Maios C, Kabashi E, Drapeau P, Parker JA (2013) Pharmacological reduction of ER stress protects against TDP-43 neuronal toxicity in vivo. Neurobiol Dis 55:64–75
Xie W, Wang H, Wang L, Yao C, Yuan R, Wu Q (2013) Resolvin D1 reduces deterioration of tight junction proteins by upregulating HO-1 in LPS-induced mice. Lab Invest. doi:10.1038/labinvest.2013.80
Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S (1999) Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103:129–135
Yang C, Zhang X, Fan H, Liu Y (2009) Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res 1282:133–141
Zhang Y, Gordon GB (2004) A strategy for cancer prevention: stimulation of the Nrf2-ARE signaling pathway. Mol Cancer Ther 3:885–893
Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM (1997) Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem 272:5375–5381
Wang X, Fan H, Ying Z, Li B, Wang H, Wang G (2010) Degradation of TDP-43 and its pathogenic form by autophagy and the ubiquitin-proteasome system. Neurosci Lett 469:112–116
Unuma K, Aki T, Matsuda S, Funakoshi T, Yoshida K, Uemura K (2013) Inducer of heme oxygenase-1 cobalt protoporphyrin accelerates autophagy and suppresses oxidative damages during lipopolysaccharide treatment in rat liver. Hepatol Res 43:91–96
Acknowledgments
We are grateful to Dr. Jemeen Sreedharan and Dr. Christopher E. Shaw at Department of Clinical Neuroscience, King’s College London; Medical Research Council Centre for Neurodegeneration Research; and Institute of Psychiatry in UK for kind gifts of the plasmids expressing wild-type and mutant TDP-43.
Conflict of Interest
The authors declare that they have no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S
(a) RT-PCR analysis of TDP-43 mRNA in the curcumin derivative-treated and untreated group. The average value of TDP-43 mRNA/actin in three different cultures (mean±S.D, n=3). (b-e) Fractionation assay of TDP-43 in the Compound C-treated and untreated group. The average value of TDP-43 or TDP-43 fragments in three different cultures (mean±S.D, n=3). Each value marked by an asterisk is significantly different from the corresponding control value (* p<0.05) (JPEG 310 kb)
Rights and permissions
About this article
Cite this article
Duan, W., Guo, Y., Xiao, J. et al. Neuroprotection by Monocarbonyl Dimethoxycurcumin C: Ameliorating the Toxicity of Mutant TDP-43 via HO-1. Mol Neurobiol 49, 368–379 (2014). https://doi.org/10.1007/s12035-013-8525-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8525-4