Abstract
Curcumin protects neuronal cells exposed to β amyloid (Aβ); the mechanism, however, is still obscure. The aim of this study is to determine whether the type 2 superoxide dismutase (SOD2) mediates curcumin-induced protective effects in Aβ-treated neuronal cells. In this study, the HT22 neuronal cells were exposed to Aβ to imitate neuronal injury in Alzheimer’s disease (AD). After 24-h treatment, 10 μM Aβ decreased cell viability and mitochondrial functions, including mitochondrial complex activities and mitochondrial membrane potential (MMP), and also downregulated anti-oxidants SOD2, glutathione (GSH), and catalase (CAT) levels (P < 0.05), meanwhile, increased lactic dehydrogenase (LDH) release, apoptosis level, intracellular reactive oxygen species (ROS) and mitochondrial superoxide accumulation (P < 0.05). And, co-administration of 1 μM curcumin significantly reduced the Aβ-induced cell injury and oxidative damage above (P < 0.05). Downregulating SOD2 by using small interfering RNA (siRNA), however, significantly abolished the curcumin-induced protective and anti-oxidative effects in HT22 cells (P < 0.05); the scramble (SC)-siRNA did not cause marked effects on the curcumin-induced protective effects (P > 0.05). These findings showed that curcumin can alleviate Aβ-induced injury in neuronal cells, and SOD2 protein may mediate the neuroprotective effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia is a major global health problem. The number of patients living with dementia is about 46.8 million in the world (Anstey et al. 2017), and the number is predicted to be 131.5 million by the end of 2050 (Quinn et al. 2018). Alzheimer’s disease (AD) is the most common type of dementia, characterized as progressive decline of learning and cognitive functions (Bature et al. 2017). In 2010, China had more cases of AD than any other country in the world (Chan et al. 2013). Therefore, the Chinese should pay more attention to AD than the people of other countries. A great number of investigations showed that β amyloid (Aβ) accumulation in brain tissue contributes to the progression of AD (Bateman et al. 2012; Egan et al. 2018). High level of Aβ in brain can damage neurons and even induce neuron death (Wu et al. 2015; Ginanneschi et al. 2014). In addition, Aβ accumulation can also activate microglia, and the activated microglial cells may release inflammatory factors, such as tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and IL-6, to injure neurons (Yeo et al. 2018). Long-term exposure to the inflammatory factors can undermine neurons and induce neuronal apoptosis (Chuang et al. 2017). For this reason, reducing the neurotoxicity of Aβ is considered to be an effective method to prevent and treat AD.
Many epidemiological studies showed that the people in India and some Southeast Asian countries have a low incidence of AD as their diet is rich in curry (Pari et al. 2008; Lee et al. 2013). Curcumin is an extract from herbal Carcuma longa Linn (turmeric) and seasoning curry (Joshi et al. 2017; Douglass and Clouatre 2015). Some recent studies indicated that curcumin can induce anti-inflammation, anti-tumor, anti-oxidatition, and neuroprotection (Reddy et al. 2018; Zou et al. 2018). And, some researchers also found that curcumin decreased Aβ-induced neurotoxicity in neurons and microglial cells (Reddy et al. 2018). The curcumin-induced neuroprotective mechanism, however, is still obscure.
Type 2 superoxide dismutase (SOD2) is an anti-oxidant, expressed in mitochondria, which can protect mitochondria against oxidative stress injury (Li et al. 2016). In the present study, we used Aβ to treat HT22 hippocampal neuronal cells to mimic neuronal injury in AD, and explore whether SOD2 is involved in curcumin-induced protection against Aβ in HT22 cells.
Materials and Methods
Cells and Reagents
HT22 cells were obtained as a gift from the Affiliated Hospital of Xuzhou Medical University. Curcumin, β amyloid 1–42 peptide (Aβ), DMEM cell culture medium, fetal bovine serum (FBS), and methyl thiazolyl tetrazolium (MTT) were purchased from the Sigma-Aldrich (St. Louis, MO, USA). The anti-SOD2, anti-cleaved caspase-3, and anti-β-actin primary anti-bodies were obtained from the Abcam (Cambridge, UK). Lactic dehydrogenase (LDH), glutathione (GSH), and catalase (CAT) reagent kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Cell Culture and Treatments
The HT22 cells were cultured in DMEM medium containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. The air of the cell incubator contained 95% air and 5% CO2, and the temperature was 37 °C. The medium was changed every 2–3 days. The cells were passaged three times/week with a 1:4 split ratio.
To determine suitable curcumin concentration, the cells were divided into five groups, including control: cells were cultured in normal medium; Aβ: cells were exposed to 10 μM Aβ; and three curcumin treatment groups: cells were exposed to the medium containing 0.1 μM, 1 μM, or 5 μM curcumin plus 10 μM Aβ. After 24-h treatment, the cells were tested to evaluate cell injury level and mitochondrial functions.
Then, to explore the role of SOD2 in curcumin-induced protection against Aβ injury, the HT22 cells were divided into five groups, including control: cells cultured in normal medium; Aβ: cells were exposed to 10 μM Aβ; Cur+Aβ: cells were exposed to the medium containing 1 μM curcumin and 10 μM Aβ; SOD2-siRNA+Cur+Aβ: cells were exposed to SOD2-siRNA for 24 h to downregulate SOD2 expression, and then exposed to the medium containing 1 μM curcumin and 10 μM Aβ; SC-siRNA+Cur+Aβ: cells were exposed to scrambled (SC) siRNA for 24 h, and then exposed to the medium containing 1 μM curcumin and 10 μM Aβ, after 24-h treatment, the cells were tested to evaluate cell injury level, mitochondrial functions, and intracellular oxidants/anti-oxidant levels.
MTT Analysis
The cells were planted into 96-well cell culture plate at a density of 1 × 105 cells/well; 24 h later, the cells were treated with different drugs. After the treatments, 20 μl MTT solution (MTT powder dissolved in phosphate buffered saline (PBS) at a concentration of 5 μg/ml) was added into each well; after 4-h incubation at 37 °C, the medium in the culture plate was removed. And then, 150 μl dimethyl sulphoxide (DMSO) was added into each well. After 15-min shaking, as the formazan was dissolved completely, the absorbance was measured by using a spectrophotometer (TECAN, CH).
LDH Release Measurement
The cells were planted into a 24-well cell culture plate at a density of 5 × 105 cells/well. After the treatments, the supernatants of the wells were collected to evaluate the LDH activity in the medium as we previously described (Jia et al. 2014).
Western Blot Analysis
The cells were seeded into a 6-well cell culture plate at a density of 1 × 106 cells/well. After the treatments, the medium was removed and the total protein in the cells was determined by using the Bradford method as we previously described (Jia et al. 2014). Anti-SOD2 polyclonal anti-body (catalog number ab13533, 1:1000) and anti-cleaved caspase-3 anti-body (catalog number ab179517, 1:50) were used. Anti-β-actin anti-body (catalog number ab8226, 1:10000) was also used. The antigens were detected by using the chemiluminescence technique (Amersham Pharmacia Biotech Piscataway, NJ, USA). Image analysis was measured with the assistance of computerized analysis software (Bio-Rad, Hercules, CH).
Mitochondrial Function Measurement
The mitochondria of the HT22 cells were isolated according to the manufacturer’s instructions (Mitochondria isolation kit, Qiagen, Hilden, Germany). Then, the mitochondrial complex I and II activities were measured at 30 °C as a study previously described (Han et al. 2007).
Mitochondrial membrane potential (MMP) was measured by using the JC-1 (Sigma-Aldrich, St. Louis, MO, USA). Mitochondrial samples were exposed to JC-1 staining buffer according to the manufacturer’s instruction. At the end of the experiments, valinomycin was added to be the negative control. The fluorescence intensity was evaluated by using a fluorescence spectrophotometer at 37 °C (TECAN, CH). The ratio of aggregates (red, 590 nm) to monomer (green, 525 nm) was calculated as the MMP indicator.
Small RNA Interference
The SOD2-siRNA and SC-siRNA were purchased from Qiangen (Germany). Briefly, the SOD2-siRNA and SC-siRNA oligomers were diluted in serum-free DMEM medium and incubated for 5 min at room temperature. The oligomers were then combined with diluted Lipofectamine 2000 and incubated for 20 min. After the incubation, the cell culture medium was removed from the plate and the cells were washed twice with PBS at 37 °C. The siRNA-Lipofectamine complexes were added into the plate, and the cells were incubated for 24 h at 37 °C. Then, the cells were washed with PBS. The SOD2 expression was evaluated by using western blot analysis.
Apoptosis Evaluation
The apoptotic rate of cells was calculated by using a flow cytometry (BD, USA). The cells were seeded into a 6-well cell culture plate at a density of 5 × 105 cells/well. After the treatments, the cells were harvested by centrifugation for 5 min at 1000 rpm. Then, the cells were washed twice with ice-cold PBS, and the apoptotic rate was calculated as we described in a study (Jia et al. 2014).
Intracellular Glutathione and Catalase Measurements
The cells were planted into a 6-well plate at a density of 5 × 105 cells/well. After the treatments, the supernatants were removed and cells were harvested. The GSH and CAT levels were measured by using the corresponding reagent kit according to the manufacturer’s instruction as we previously described (Jia et al. 2014).
Reactive Oxygen Species Evaluation
Intracellular reactive oxygen species (ROS) level was detected by using a ROS reagent kit (Beyotime Biotechnology, China) as we previously described (Jia et al. 2014). Briefly, the treated cells were incubated with 10 μM 2′7′-dichlorofluorescin diacetate (DCFH-DA) at 37 °C for 10 min, and then washed twice with PBS at room temperature. And, the ROS level was evaluated by using a Leica DMI6000B fluorescence microscope (Leica, Germany). The excitation wavelength was 488 nm, and the emission wavelength was 525 nm. The images were analyzed using Image Pro-plus software (IPP 6.0, Media Cybernetics, Silver Spring, MD, USA).
Mitochondrial Superoxide Measurement
MitoSOX™ reagent kit was used to assess the mitochondrial superoxide level in the treated HT22 cells in this study. Briefly, the MitoSOX™ can target the mitochondrial selectively after permeating the cell membrane and reacting with the superoxide immediately. After the treatments, the cells were incubated with 5 μM MitoSOX™ red at 37 °C for 20 min. After the incubation, 100 μl DAPI staining solution was added into each well to mark the nuclei. After 10-min incubation at room temperature in darkness, the cells were observed with a co-focal microscope (Olympus, Japan). Fluorescence photos, including the mitochondrial superoxide (red, 510 nm at excitation and 580 nm at emission), the nuclei (blue, 340 nm at excitation and 488 nm at emission), and the merged photos, were taken randomly. The fluorescence intensity was quantified by using pro-plus software (IPP 6.0, Media Cybernetics, Silver Spring, MD, USA).
Statistical Analysis
SPSS 13.0 for windows (SPSS Inc., Chicago, IL) was used to conduct statistical analysis. All the values of this study were expressed as means ± standard deviation (SD) from at least three separate experiments, and no data were excluded. And, one-way ANOVA was taken to treat the data. The differences between groups were compared by using the Tukey’s Multiple Comparisons. P < 0.05 indicated statistical significance.
Results
Curcumin Reduced Aβ-Induced Injury in Neuronal Cells Dose-Dependently
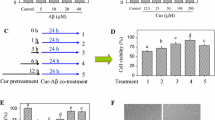
To find a suitable curcumin (Cur) concentration, HT22 cells were divided into five groups, including normal cultured control group, 10 μM Aβ treatment group, and three concentrations of curcumin (0.1 μM, 1 μM, and 5 μM, respectively) plus 10 μM Aβ treatment groups. After 24-h incubation, MTT and LDH releases were used to determine the cell injury level (Fig. 1). Compared with the control, 10 μM Aβ reduced cell viability (MTT) and increased LDH level in the medium (P < 0.05), and 1 μM and 5 μM curcumin obviously restored cell viability and reduced LDH release from the cells exposed to Aβ (P < 0.05); however, 0.1 μM curcumin did not induce significant protection against Aβ in the cells (P > 0.05). Curcumin of 1 μM was used in the subsequent experiments.
Curcumin restored cell viability and reduced LDH release in HT22 cells exposed to Aβ. HT22 cells were divided into five groups, including control: cells were cultured in normal medium; Aβ: cells were exposed to 10 μM Aβ; and three curcumin (Cur) treatment groups: cells were exposed to the medium containing 0.1 μM, 1 μM, or 5 μM curcumin plus 10 μM Aβ. After 24-h treatment, MTT and LDH releases were used to evaluate cell injury level. a Curcumin restored cell viability (n = 8). b Curcumin reduced LDH release (n = 8). *P < 0.05; NS no significance
Curcumin Ameliorated Mitochondrial Functions in Neuronal Cells Exposed to Aβ
To observer curcumin-induced effects on SOD2 expression in HT22 cells, we used western blot to measure SOD2 expression level (Fig. 2a) and also evaluated mitochondrial functions (Fig. 2b, d). The cells were divided into five groups as mentioned above, including control, Aβ, and three curcumin treatment groups (0.1 μM, 1 μM, and 5 μM curcumin plus 10 μM Aβ, respectively). After 24-h treatment, compared with the control, Aβ exposure decreased SOD2 expression and neuronal mitochondria functions significantly (P < 0.05), including MMP and mitochondrial complex I and II activities. Similarly, 1 μM and 5 μM curcumin markedly restored SOD2 level, neuronal MMP, and mitochondrial complex activities. These findings above indicated that SOD2 expression upregulation and mitochondrial function ameliorations may be involved in curcumin-induced protections in HT22 cells exposed to Aβ.
Curcumin restored SOD2 expression and mitochondrial functions in HT22 cells exposed to Aβ. HT22 cells were divided into five groups, including control: cells were cultured in normal medium; Aβ: cells were exposed to 10 μM Aβ; and three curcumin (Cur) treatment groups: cells were exposed to the medium containing 0.1 μM, 1 μM, or 5 μM curcumin plus 10 μM Aβ. After 24-h treatment, SOD2 expression, mitochondrial membrane potential (MMP), and mitochondrial complex activities were measured. a Curcumin restored SOD2 expression (n = 4). b Curcumin increased MMP level (n = 8). c Curcumin increased mitochondrial complex I activity (n = 8). d Curcumin restored mitochondrial complex II activity (n = 8). *P < 0.05; NS no significance
SOD2-siRNA Abolished Curcumin-Induced Protective Effects Against Aβ
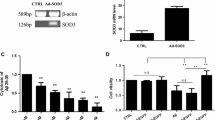
To further observe the role of SOD2 in curcumin-induced cytoprotection in HT22 cells exposed to Aβ, we used SOD2-siRNA to downregulate SOD2 expression in HT22 cells. Compared with the control, SOD2-siRNA (Fig. 3a), but not the SC-siRNA, significantly inhibited SOD2 expression in HT22 cells (P < 0.05), indicating the SOD2-siRNA used in this study was effective. Then, the cells were divided into five groups, including control, 10 μM Aβ treatment group, 1 μM curcumin plus 10 μM Aβ treatment group (Cur+Aβ), SOD2-siRNA treatment group (SOD2-siRNA+Cur+Aβ), and scramble siRNA group (SC-siRNA+Cur+Aβ). After the treatments, we used MTT and LDH release to test cell injury level (Fig. 3b, c), and took flow cytometry and western blot to evaluate cell apoptosis rate and cleaved caspase-3 expression (Fig. 3d–f), an apoptosis-associated protein, respectively. After 24-h treatment, Aβ exposure reduced cell viability and increased LDH release (P < 0.05), compared with the control, and curcumin restored cell viability and inhibited LDH release in the cells exposed to Aβ (P < 0.05). However, SOD2-siRNA (P < 0.05), but not the SC-siRNA (P > 0.05), significantly reversed the curcumin-induced neuroprotection.
SOD2-siRNA reversed curcumin-induced cytoprotection and anti-apoptosis effects in HT22 cells exposed to Aβ. The HT22 cells were divided into three groups, including normal cultured control, SOD2-siRNA group, and scrambled (SC)-siRNA group; after 24-h treatment, western blot was taken to evaluate SOD2 protein expression. Then, the HT22 cells were divided into five groups, including the control, Aβ: cells were exposed to 10 μM Aβ; Cur+Aβ: cells were exposed to the medium containing 1 μM curcumin and 10 μM Aβ; SOD2-siRNA+Cur+Aβ: cells were exposed to SOD2-siRNA for 24 h, and then exposed to the medium containing 1 μM curcumin and 10 μM Aβ; SC-siRNA+Cur+Aβ: cells were exposed to SC-siRNA for 24 h, and then exposed to the medium containing 1 μM curcumin and 10 μM Aβ; after 24-h treatment, MTT, LDH release, and apoptosis were evaluated. a SOD2-siRNA reduced SOD2 expression (n = 3). b SOD2-siRNA abolished curcumin-induced enhancement on cell viability (n = 8). c SOD2-siRNA abolished curcumin-induced inhibition on LDH release (n = 8). d Flow cytometry results of curcumin-induced anti-apoptotic effects. e SOD2-siRNA abolished curcumin-induced effects on apoptosis rate (n = 6). e SOD2-siRNA reversed curcumin-induced decrease on cleaved caspase-3 expression (n = 4). *P < 0.05; NS no significance
Similarly, compared with the control, Aβ exposure enhanced cell apoptosis rate and cleaved caspase-3 expression, and curcumin reduced the apoptosis and cleaved caspase-3 levels (P < 0.05). The SOD2-siRNA obviously abolished the curcumin-induced anti-apoptotic effects above (P < 0.05). Nevertheless, the SC-siRNA did not cause significant influence on the curcumin-induced anti-apoptosis effects (P > 0.05). These findings showed that SOD2 protein may mediate curcumin-induced neuroprotection and anti-apoptosis effects in Aβ-treated neuronal cells.
SOD2-siRNA Reversed Curcumin-Induced Mitochondrial Function Ameliorations in Neuronal Cells
To further determine the role of SOD2 in curcumin-induced neuroprotection, we tested mitochondrial functions, including mitochondrial complex I and II activities, MMP (Fig. 4a, b, e) and mitochondrial superoxide level (Fig. 4c, d). The cells were divided into five groups, including control, Aβ, Cur+Aβ, SOD2-siRNA+Cur+Aβ, and SC-siRNA+Cur+Aβ groups. After 24-h treatments, 1 μM curcumin significantly reversed 10 μM Aβ-induced decrease of mitochondrial complex I and II activities and MMP level, and inhibited the accumulation of mitochondrial superoxide in the cells (P < 0.05). SOD2-siRNA, but not the SC-siRNA (P > 0.05), obviously abolished curcumin-induced effects on mitochondrial functions and superoxide level (P < 0.05). These findings indicated SOD2 protein may mediate curcumin-induced ameliorations of mitochondrial functions in Aβ-treated neuronal cells.
SOD2-siRNA reversed curcumin-induced ameliorations of mitochondrial functions in HT22 cells. HT22 cells were divided into five groups, including the normal cultured control; Aβ: cells were exposed to 10 μM Aβ; Cur+Aβ: cells were exposed to the medium containing 1 μM curcumin and 10 μM Aβ; SOD2-siRNA+Cur+Aβ: cells were exposed to SOD2-siRNA for 24 h, and then exposed to the medium containing 1 μM curcumin and 10 μM Aβ; and SC-siRNA+Cur+Aβ: cells were exposed to scrambled (SC) siRNA for 24 h, and then exposed to the medium containing 1 μM curcumin and 10 μM Aβ; after 24-h treatment, mitochondrial complex I and II activities, membrane potential (MMP), and superoxide were evaluated. a SOD2-siRNA abolished curcumin-induced amelioration of mitochondrial complex I activity (n = 8). b SOD2-siRNA abolished curcumin-induced amelioration of mitochondrial complex II activity (n = 8). c Staining results of mitochondrial superoxide (red). d SOD2-siRNA abolished curcumin-induced inhibition of mitochondrial superoxide accumulation (n = 8). e SOD2-siRNA reversed curcumin-induced amelioration of MMP (n = 8). *P < 0.05; NS no significance; bar = 10 μm
SOD2-siRNA Abolished Curcumin-Induced Elevations of Neuronal Anti-Oxidants
Maintaining intracellular anti-oxidant levels is of great importance for cells to reduce oxidative injury. In this study, we evaluated intracellular oxidant ROS level, and anti-oxidants GSH, and CAT to assess the anti-oxidative ability induced by curcumin. The cells were divided into five groups as mentioned above. After 24-h treatments, compared with control group, 10 μM Aβ increased intracellular ROS level (Fig. 5a, b) and decreased intracellular GSH and CAT levels (P < 0.05), 1 μM curcumin reduced intracellular ROS level and restored GSH and CAT activities (P < 0.05). The SOD2-siRNA (P < 0.05), but not the SC-siRNA (P > 0.05), significantly reversed the curcumin-induced effects on intracellular ROS, GSH, and CAT levels. These observations showed that curcumin can reduce intracellular ROS and maintain the redox status in the HT22 cells exposed to Aβ, and the effects may be mediated by SOD2 protein (Fig. 6).
SOD2-siRNA reversed curcumin-induced effects on intracellular ROS, GSH, and CAT in HT22 cells. HT22 cells were divided into five groups, including the normal cultured control; Aβ: cells were exposed to 10 μM Aβ; Cur+Aβ: cells were exposed to the medium containing 1 μM curcumin and 10 μM Aβ; SOD2-siRNA+Cur+Aβ: cells were exposed to SOD2-siRNA for 24 h, and then exposed to the medium containing 1 μM curcumin and 10 μM Aβ; and SC-siRNA+Cur+Aβ: cells were exposed to scrambled (SC) siRNA for 24 h, and then exposed to the medium containing 1 μM curcumin and 10 μM Aβ. After 24-h treatment, intracellular reactive oxygen species (ROS), glutathione (GSH), and catalase (CAT) were evaluated. a Fluorescence staining results of ROS (green). b SOD2-siRNA abolished curcumin-induced intracellular ROS reduction (n = 8). c SOD2-siRNA abolished curcumin-induced intracellular GSH elevation (n = 8). d SOD2-siRNA abolished curcumin-induced intracellular CAT elevation (n = 8). *P < 0.05; NS no significance; bar = 20 μm
SOD2 mediates curcumin-induced protective effects on HT22 cells exposed to Aβ. Aβ exposure can induce oxidative stress in neuronal cells, then cause cell injury and mitochondrial dysfunctions, leading to neuronal injury ultimately. Curcumin can increase mitochondrial SOD2 level in neuronal cells, and reduce oxidative stress via upregulating SOD2 expression in the cells, then decrease cell injury and ameliorate mitochondrial functions, bringing about neuroprotection
Discussion
In the present study, we found Aβ exposure injured HT22 neuronal cells and induced mitochondrial dysfunction in the cells, and curcumin treatment alleviated the Aβ-induced cell injury and mitochondrial dysfunction. Downregulating SOD2 protein by using siRNA, however, significantly reversed the neuroprotective effects induced by curcumin. These results showed that SOD2 protein may mediate the curcumin-induced protection against Aβ in neuronal cells (Fig. 6).
AD is a chronic neurodegenerative disorder, characterized by selective and progressive neuron loss and dysfunction, leading to memory loss and cognitive impairments ultimately (Bature et al. 2017). With the development of population aging, an increasing number of people will suffer from the disease, bringing about heavy economic and medical lord for the world (Quinn et al. 2018). Unfortunately, at least today, AD could not be completely cured, and the severity of the disease can just be alleviated. Some recent investigations showed that cerebral over-accumulation of Aβ, especially the Aβ1–42 peptide, is considered to be the key factor, inducing neuron loss in the brain of the AD patients (Bateman et al. 2012; Egan et al. 2018). Therefore, reducing Aβ neurotoxicity is believed to be an effective therapy in treating AD. In the present study, we took Aβ1–42 peptide to damage HT22 neuronal cells, a mouse hippocampal neuronal cell-line, to mimic Aβ-induced neurotoxicity in AD. In fact, Aβ induces neuronal injury by two main mechanisms. Firstly, over-accumulation of Aβ in brain can activate glial cells, and the activated glial cells can release pro-inflammatory cytokines to damage neurons, leading to neuronal inflammatory injury and death. Secondly, Aβ can damage neuron directly, causing neuron injury, death, and neurobiological dysfunction. Many studies showed that Aβ can bring about oxidative injury to neurons (Xu et al. 2016). High-level of Aβ can consume intracellular anti-oxidants, including GSH, CAT, SOD2, and GSH reductase (Alberdi et al. 2018). Meanwhile, cell membrane, DNA, and cell organs, including mitochondria, can be damaged. For this reason, modulating intracellular anti-oxidant level is an effective method to reduce Aβ neurotoxicity.
Curcumin is a bioactive substance, derived from seasoning curry and herbal Carcuma longa Linn. Pure curcumin is solid powder with anti-tumor, anti-inflammation, anti-oxidative injury, and reducing blood cholesterol abilities (Reddy et al. 2018; Zou et al. 2018). In this study, we observed that curcumin treatment protected Aβ-induced injury to neuronal cells, and restored SOD2 expression, intracellular GSH, and CAT levels in the Aβ-treated neuronal cells. In addition, curcumin also ameliorated mitochondrial functions in the cells. These results showed that curcumin-induced neuroprotection may be via increasing intracellular anti-oxidants levels and ameliorating mitochondrial functions. As SOD2 is mainly expressed in mitochondria, and curcumin also increases mitochondrial functions; we tested the role of SOD2 in curcumin-induced protective effects of this study. In fact, the SOD family includes SOD1–3, except for SOD2; SOD1 is observed in the cytoplasm, and SOD3 is found in extracellular matrix (Nguyen et al. 2018). Mitochondrial functions were improved in the presence of curcumin in this study; therefore, we investigated SOD2, but not SOD1 nor SOD3. We found that SOD2-siRNA downregulated SOD2 expression, and reversed curcumin-induced neuroprotection and amelioration of mitochondrial functions, indicating that the curcumin-induced effects above may probably been mediated by SOD2 protein. In the pathogenesis of AD, mitochondrial dysfunction is also observed. High-Aβ accumulation can inhibit mitochondrial biogenesis and reduce the mitochondrial number in neuronal cells, so the energy generated by the mitochondria may be decreased (Reddy et al. 2018). Mitochondria are an energy factory of cells, which can generate ATP for cellular activities. In fact, moderate MMP level and mitochondrial complex activities are vital indicators, reflecting mitochondrial ability of producing enough energy (Ma et al. 2018). In the present study, we found that curcumin treatment restored MMP, mitochondrial complex I and II activities, indicating curcumin can restore mitochondrial functions in Aβ-treated neuronal cells.
In an in vitro investigation of astrocyte, curcumin exposure can increase SOD2 protein expression via the adenosine monophosphate–activated protein kinase (AMPK) signaling pathway (Qin et al. 2018). Another investigation showed that curcumin can increase SOD2 protein expression and improve mitochondrial function in human hepatoma cells via sirtuin 3 (Gounden and Chuturgoon 2017). And, it is also reported that curcumin can increase SOD2 transcription level and activity via peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) in hepatic stellate cells in vitro (Zhai et al. 2015). By increasing intracellular anti-oxidants, cells can maintain the intracellular redox status, reduce oxidative stress injury, and protect cell organs, such as mitochondria. However, it has not been reported that SOD2 mediates curcumin-induced protection against Aβ in neuronal cells, which was discovered in the present study. In addition, several investigations showed that curcumin can promote the cerebral Aβ disaggregation and prevent aggregation of new amyloid (Yang et al. 2005). And, another study showed that curcumin can enhance the uptake of Aβ by macrophages in AD patients to reduce cerebral Aβ level (Fiala et al. 2007). These observations indicated that curcumin-induced protection against Aβ may include several mechanisms, not just reducing the Aβ neurotoxicity in neuronal cells. Our findings of this study explained, to some extent, the neuroprotective mechanism of curcumin in Aβ-treated neuronal cells. However, there are still some limitations in this study. First, this study was in vitro; therefore, the findings of this study should be verified in vivo and in clinical trials. Second, we just investigated SOD2 in curcumin-induced protection against Aβ in neuronal cells, whether SOD1 or SOD3 plays a role in curcumin-induced bioactivities against AD is still unknown.
In conclusion, in the present study, we found that curcumin can reduce Aβ-induced oxidative injury in HT22 cells, and SOD2 may mediate the neuroprotection.
References
Alberdi E, Sánchez-Gómez MV, Ruiz A, Cavaliere F, Ortiz-Sanz C, Quintela-López T, Capetillo-Zarate E, Solé-Domènech S, Matute C (2018) Mangiferin and morin attenuate oxidative stress, mitochondrial dysfunction, and neurocytotoxicity, induced by amyloid beta oligomers. Oxidative Med Cell Longev 2018:2856063. https://doi.org/10.1155/2018/2856063
Anstey KJ, Peters R, Clare L, Lautenschlager NT, Dodge HH, Barnes DE, Shahar S, Brodaty H, Rees G (2017) Joining forces to prevent dementia: the international research network on dementia prevention (IRNDP). Int Psychogeriatr 29:1757–1760. https://doi.org/10.1017/S1041610217001685
Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC, Dominantly Inherited Alzheimer Network (2012) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 367(9):795–804. https://doi.org/10.1056/NEJMoa1202753
Bature F, Guinn BA, Pang D, Pappas Y (2017) Signs and symptoms preceding the diagnosis of Alzheimer’s disease: a systematic scoping review of literature from 1937 to 2016. BMJ Open 7:e015746. https://doi.org/10.1136/bmjopen-2016-015746
Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, Middleton L, Russ TC, Deary IJ, Campbell H, Wang W, Rudan I, Global Health Epidemiology Reference Group (GHERG) (2013) Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet 381:2016–2023. https://doi.org/10.1016/S0140-6736(13)60221-4
Chuang KA, Li MH, Lin NH, Chang CH, Lu IH, Pan IH, Takahashi T, Perng MD, Wen SF (2017) Rhinacanthin C alleviates amyloid-β fibrils’ toxicity on neurons and attenuates neuroinflammation triggered by LPS, amyloid-β, and interferon-γ in glial cells. Oxidative Med Cell Longev 2017:5414297. https://doi.org/10.1155/2017/5414297
Douglass BJ, Clouatre DL (2015) Beyond yellow curry: assessing commercial curcumin absorption technologies. J Am Coll Nutr 34:347–358. https://doi.org/10.1080/07315724.2014.950392
Egan MF, Kost J, Tariot PN, Aisen PS, Cummings JL, Vellas B, Sur C, Mukai Y, Voss T, Furtek C, Mahoney E, Harper Mozley L, Vandenberghe R, Mo Y, Michelson D (2018) Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 378:1691–1703. https://doi.org/10.1056/NEJMoa1706441
Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, Sayre J, Zhang L, Zaghi J, Dejbakhsh S, Chiang B, Hui J, Mahanian M, Baghaee A, Hong P, Cashman J (2007) Innate immunity and transcription of MGAT-III and toll-like receptors in Alzheimer's disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci U S A 104:12849–11254. https://doi.org/10.1073/pnas.0701267104
Ginanneschi F, Filippou G, Bonifazi M, Frediani B, Rossi A (2014) Effects of local corticosteroid injection on electrical properties of aβ-fibers in carpal tunnel syndrome. J Mol Neurosci 52:525–530. https://doi.org/10.1007/s12031-013-0107-4
Gounden S, Chuturgoon A (2017) Curcumin upregulates antioxidant defense, lon protease, and heat-shock protein 70 under hyperglycemic conditions in human hepatoma cells. J Med Food 20:465–473. https://doi.org/10.1089/jmf.2016.0146
Han Z, Chen YR, Jones CI 3rd, Meenakshisundaram G, Zweier JL, Alevriadou BR (2007) Shear-induced reactive nitrogen species inhibit mitochondrial respiratory complex activities in cultured vascular endothelial cells. Am J Physiol Cell Physiol 292:C1103–C1112. https://doi.org/10.1152/ajpcell.00389.2006
Jia J, Ma L, Wu M, Zhang L, Zhang X, Zhai Q, Jiang T, Wang Q, Xiong L (2014) Anandamide protects HT22 cells exposed to hydrogen peroxide by inhibiting CB1 receptor-mediated type 2 NADPH oxidase. Oxidative Med Cell Longev 2014:893516. https://doi.org/10.1155/2014/893516
Joshi D, Mittal DK, Shukla S, Srivastav SK, Dixit VA (2017) Curcuma longa Linn. Extract and curcumin protect CYP 2E1 enzymatic activity against mercuric chloride-induced hepatotoxicity and oxidative stress: a protective approach. Exp Toxicol Pathol 69:373–382. https://doi.org/10.1016/j.etp.2017.02.009
Lee WH, Loo CY, Bebawy M, Luk F, Mason RS, Rohanizadeh R (2013) Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the 21st century. Curr Neuropharmacol 11:338–378. https://doi.org/10.2174/1570159X11311040002
Li L, Xiao L, Hou Y, He Q, Zhu J, Li Y, Wu J, Zhao J, Yu S, Zhao Y (2016) Sestrin2 silencing exacerbates cerebral ischemia/reperfusion injury by decreasing mitochondrial biogenesis through the AMPK/PGC-1α pathway in rats. Sci Rep 6:30272. https://doi.org/10.1038/srep30272
Ma L, Niu W, Yang S, Tian J, Luan H, Cao M, Xi W, Tu W, Jia J, Lv J (2018) Inhibition of mitochondrial permeability transition pore opening contributes to cannabinoid type 1 receptor agonist ACEA-induced neuroprotection. Neuropharmacology 135:211–222. https://doi.org/10.1016/j.neuropharm.2018.03.024
Nguyen CT, Sah SK, Zouboulis CC, Kim TY (2018) Inhibitory effects of superoxide dismutase 3 on Propionibacterium acnes-induced skin inflammation. Sci Rep 8:4024. https://doi.org/10.1038/s41598-018-22132-z
Pari L, Tewas D, Eckel J (2008) Role of curcumin in health and disease. Arch Physiol Biochem 114:127–149. https://doi.org/10.1080/13813450802033958
Qin X, Qiao H, Wu S, Cheng J, Wan Q, Liu R (2018) Curcumin inhibits monocyte chemoattractant protein-1 expression in TNF-α induced astrocytes through AMPK pathway. Neurochem Res 43:775–784. https://doi.org/10.1007/s11064-018-2479-x
Quinn JP, Corbett NJ, Kellett KAB, Hooper NM (2018) Tau proteolysis in the pathogenesis of tauopathies: neurotoxic fragments and novel biomarkers. J Alzheimers Dis 63:13–33. https://doi.org/10.3233/JAD-170959
Reddy PH, Manczak M, Yin X, Grady MC, Mitchell A, Tonk S, Kuruva CS, Bhatti JS, Kandimalla R, Vijayan M, Kumar S, Wang R, Pradeepkiran JA, Ogunmokun G, Thamarai K, Quesada K, Boles A, Reddy AP (2018) Protective effects of Indian spice curcumin against amyloid-β in Alzheimer’s disease. J Alzheimers Dis 61:843–866. https://doi.org/10.3233/JAD-170512
Wu M, Jia J, Lei C, Ji L, Chen X, Sang H, Xiong L (2015) Cannabinoid receptor CB1 is involved in nicotine-induced protection against Aβ1-42 neurotoxicity in HT22 cells. J Mol Neurosci 55:778–787. https://doi.org/10.1007/s12031-014-0422-4
Xu P, Wang H, Li Z, Yang Z (2016) Triptolide attenuated injury via inhibiting oxidative stress in amyloid-Beta25-35-treated differentiated PC12 cells. Life Sci 145:19–26. https://doi.org/10.1016/j.lfs.2015.12.018
Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM (2005) Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280:5892–5901. https://doi.org/10.1074/jbc.M404751200
Yeo ETY, Wong KWL, See ML, Wong KY, Gan SY, Chan EWL (2018) Piper sarmentosum Roxb. Confers neuroprotection on beta-amyloid (Aβ)-induced microglia-mediated neuroinflammation and attenuates tau hyperphosphorylation in SH-SY5Y cells. J Ethnopharmacol 217:187–194. https://doi.org/10.1016/j.jep.2018.02.025
Zhai X, Qiao H, Guan W, Li Z, Cheng Y, Jia X, Zhou Y (2015) Curcumin regulates peroxisome proliferator-activated receptor-γ coactivator-1α expression by AMPK pathway in hepatic stellate cells in vitro. Eur J Pharmacol 746:56–62. https://doi.org/10.1016/j.ejphar.2014.10.055
Zou J, Zhang S, Li P, Zheng X, Feng D (2018) Supplementation with curcumin inhibits intestinal cholesterol absorption and prevents atherosclerosis in high-fat diet-fed apolipoprotein E knockout mice. Nutr Res 56:32–40. https://doi.org/10.1016/j.nutres.2018.04.017
Funding
This work was supported by the National Nature Science Foundation of China (61773130), Natural Science Foundation of Guangdong Province, China (2016A030313613), and the Science and Technology Program of Guangzhou, China (201707010027).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
All procedures were approved by the Ethics Committee of the General Hospital of Southern Theatre of PLA.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Du, S., Zhang, Y., Yang, J. et al. Curcumin Alleviates β Amyloid-Induced Neurotoxicity in HT22 Cells via Upregulating SOD2. J Mol Neurosci 67, 540–549 (2019). https://doi.org/10.1007/s12031-019-01267-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01267-2