Abstract
The role of Toll-like receptor 4 (TLR4) in the activation of innate immunity has been extensively studied in the past several years. Here, we are the first to report that myeloid-related protein 8 (MRP8), an endogenous TLR4 ligand, is involved in the epileptogenesis of mesial temporal lobe epilepsy (MTLE). We find that the expression of MRP8, TLR4, and interleukin 1-β (IL-1β) was upregulated in a MTLE model during both acute and chronic disease stages. We next investigated the possible roles played by astrocytes, which have been shown to be the major source of IL-1β during epilepsy. Stimulation via MRP8 led to the induction of IL-1β in astrocytes in vitro, accompanied by the activation of Nuclear Factor-κB, while knockdown of TLR4 or inhibition of NF-κB in astrocytes prevented this IL-1β induction. Thus, MRP8 may potentiate the perpetuation of MTLE by activating the NF-κB pathway in astrocytes, and could be a new target for anticonvulsant therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a chronic neurological disorder characterized by recurrent spontaneous seizures. This disease affects nearly 50 million people worldwide [1]. Seizure susceptibility reaches its peak during the intense period of rapid brain growth and synaptogenesis that occurs in the immature brain [2], with 4–10 % of children having at least one attack of seizures in the first 16 years of life [3].
Mesial temporal lobe epilepsy (MTLE) is one of the most common and intractable forms of epilepsy [4]. Clinical and neuropathological evidence strongly supports the involvement of inflammation in MTLE pathogenesis [5–10], particularly in the generation of seizures and the transformation of a normal neuronal network into a seizure-generating one [11]. In the developing brain, it has been shown that neuro-inflammation increases the induction and severity of seizures, and increases seizure susceptibility [12].
Chronic brain inflammation was first reported in patients with Rasmussen encephalitis [13]. Later studies found that the immune system is activated in some patients with seizure disorders, reviewed in [14]. Central nervous system (CNS) inflammatory response can be induced in the absence of CNS infection, as observed in cases of febrile seizures (FS) [15]. FS is considered to be the most common form of seizure disorder that exclusively occurs in children [16] and may predispose children to the development of epilepsies, including temporal lobe epilepsy (TLE) [17]. Inflammation is now considered to be a key player in MTLE pathogenesis, but little is known about the initial triggering of the inflammatory process.
Toll-like receptors (TLRs) are a family of innate immune system receptors that respond to pathogen-derived and tissue damage-related ligands. Toll-like receptor 4 (TLR4), the first member of the TLR family expressed in different brain cells [18], participates in a chain of events that eventually leads to the precipitation and recurrence of seizures [19]. Damage-associated molecular patterns (DAMPs), such as heat shock protein (HSP) and myeloid-related protein 8 (MRP8), and pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), are the major kinds of inflammatory substances involved [20, 21]. DAMPs-related activation of TLR4 prompts the endogenous inflammatory reaction [22].
MRP8 is a member of the S100 family and contains two calcium-binding sites per molecule. It is expressed in the activated granulocytes and macrophages in the inflammatory process and has emerged as a pro-inflammatory maker protein in many inflammation-related disorders [23–25]. MRP8 is also abundantly expressed in different brain lesions, including cerebral ischemia [26] and traumatic brain injury [27]. MRP8 has recently been characterized as an endogenous TLR4 agonist and may mediate TLR4 activation in endotoxin-induced shock [20], acute coronary syndromes [28], and systemic-onset juvenile idiopathic arthritis [29].
Nuclear factor-kappa B (NF-κB), a key regulator of immune and inflammatory response, is present in cytosol as an inactive form due to the presence of inhibitory kappa B (IκB), an inhibitory subunit linked to the active subunits p50 and p65. Emerging evidence suggests that NF-κB is activated by the neuropathological processes of seizure and epilepsy [30–34]. As one of the most important downstream molecules in the TLR4 signaling pathway, NF-κB is a transcriptional factor required for the gene expression of many inflammatory mediators, including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) [35, 36].
Pro-inflammatory IL-1β shows high levels of expression in the hippocampus of epileptic patients and in a rat model [37–40], and is thought to play an important role in the development of seizures and epilepsy [41, 42]. IL-1β is induced in reactive astrocytes in the spike-and-wave discharges model [38]; this is followed by a cascade of downstream inflammatory events recruiting cells of the adaptive immune system [43].
The identification of signaling molecules expressed during epileptogenesis may help us understand the processes involved in brain damage. In this study, we found that MRP8 expression is increased in the hippocampi of an MTLE rat model as well as patients, leading to NF-κB activation and subsequent IL-1β induction in astrocytes via TLR4, which may potentiate the perpetuation of MTLE.
Materials and Methods
Experimental Animals
All animal procedures were approved by the Animal Research Committee at the Central South University (Animal permit number SCXK 2006-0002), and all procedures conformed to the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. Twenty-five-day-old male Sprague–Dawley (SD) rats (PN25, n = 105) were randomly divided into an experimental group (E, n = 75) and a control group (C, n = 30). Following arrival, animals were housed in a room with a controlled light–dark cycle (lights on from 0700 to 1900) and a constant temperature (20 ± 2 °C) and humidity (50–60 %).
Epilepsy Induction
On PN25, the E rats (n = 75) were injected with lithium hydrochloride (127 mg/kg, i.p.; Sigma-Aldrich Chemie, Deisenhofen, Germany), 18–20 h before pilocarpine treatment. Methylscopolamine (1 mg/kg, i.p.) was administered 30 min before pilocarpine to reduce the peripheral effects of the convulsant agent and thus enhance survival. Pilocarpine hydrochloride (50 mg/kg, i.p.; Boehringer Mannheim,Indianapolis, IN, USA) was then injected to induce status epilepticus (SE). Seizures were observed within 20–40 min. Diazepam (10 mg/kg, i.p.; Sigma-Aldrich) was used to terminate the seizure activity 90 min after the onset of SE. Severity of the convulsions was evaluated by Racine’s classification [44]. Animals classified as less than Racine’s stage 4 (n = 27) were removed from the experiment. The control rats (C, n = 30) were injected with the same dose of normal saline instead of pilocarpine. We observed spontaneous seizures occurring 3 weeks after SE induction. Chronic seizures occurred at 8 weeks post-pilocarpine administration with a frequency of 5–12 seizures in 24 h. The time between the last spontaneous seizure and analysis was usually <24 h.
Based on the epilepsy development stages, the sample size was divided randomly into six groups: (a) acute control group, AC (control rats, 2 h after saline administration; n = 10); (b) acute seizure group, AS (induced rats, 2 h after pilocarpine administration; n = 10); (c) latent control group, LC (control rats, 3 weeks after saline administration; n = 10); (d) latent seizure group, LS (induced rats, 3 weeks after pilocarpine administration; n = 10); (e) chronic control group, CC (control rats, 8 weeks after saline administration; n = 10); and (f) chronic seizure group, CS (induced rats that showed spontaneous seizures 8 weeks after pilocarpine administration; n = 12). The rats that failed to develop SE after pilocarpine treatment were euthanized and were rejected from the experiment.
MTLE Children and Controls
Specimens were obtained at surgery from five children undergoing unilateral selective amygdalohippocampectomy for drug-resistant MTLE with typical imaging features and pathological confirmation of hippocampal sclerosis. Samples were obtained mainly from the hippocampal forepart and caudomedial parts. The decision for surgery was based on convergent evidence of clinical and electroencephalography (EEG) recordings during prolonged video-EEG monitoring, high-resolution magnetic resonance imaging (MRI) indicating mesial temporal lobe seizure onset, and invasive electroencephalography recordings. Surgical specimens were examined by routine pathology. As control tissue, five normal whole hippocampal samples were obtained at autopsy from children, and the corresponding parts to patients’ sample were used (postmortem delay: maximum of 12 h) with no history of any brain disease. Neuropathological studies confirmed that control tissues were normal. Clinical information on children with MTLE and controls are presented in Table 1. This study was approved by the Institutional Ethics Committee of Central South University and written informed consent was obtained from the parents of all patients before analysis.
Nissl Staining to Detect the Extent of Neuron Loss
For Nissl staining, 5 μm paraffin sections were stained with 0.2 % crystal violet (Sigma-Aldrich, Saint Louis, USA) for 10 min. After a quick rinse in distilled water, the sections were differentiated in 95 % ethyl alcohol for 5 sec. After dehydration, the slices were mounted and observed under a light microscope. The Nissl positive neurons were counted. Under 40× magnification, 6 visual fields were randomly selected for each section, and the neurons were counted. For Nissl staining, four sections were examined for each rat before the means were obtained for statistical analysis.
Timm Staining to Detect Mossy Fiber Sprouting (MFS)
For the Timm stain, the anesthetic rats were perfused by 250 ml of sodium chloride and 200 ml of 0.4 % sodium sulfide solution for more than 30 min, followed by fixation for 15 min with 4 % (v/v) formaldehyde solution. Frozen sections of brain were obtained at 20 μm. After being washed with 0.1 M phosphate buffer, the cultures were dehydrated with 70 %, 90 %, 95 %, and 100 % ethanol, and dried. To perform the sulfide silver staining, they were submerged in the physical developer according to the method of Sloviter [46] and were then incubated in a dark room for 90 min at room temperature (RT). The slices were washed with distilled water at the end of the reaction.
Immunohistochemistry (IHC)
Paraffin sections from hippocampi were stained by indirect IHC. Briefly, slides were incubated with 0.03 % H2O2 in Phosphate Buffered Saline (PBS) for 10 min at RT to block endogenous peroxidase activity, then incubated in a humidified chamber for 15 min with 5 % normal goat serum in PBS to block non-specific binding. Sections were then incubated with different primary antibodies: MRP8 (1:150), TLR4 (1:200), and NF-κB p65 (1:1,000) in 5 % bovine albumin serum in PBS at 4 °C overnight. Immunoreactivity was tested with the avidinbiotin–peroxidase technique, using 3,3′-diaminobenzidine (DAB) as the chromogen, and counterstained with Mayer’s hematoxylin. Sections were viewed using an Olympus BH-2 optical microscope and Cell F Imaging Software (Olympus, Hamburg, Germany).
Primary Astrocyte Culture
Astrocytes were obtained from the cerebral cortices of male neonatal SD rats at less than 2 day postnatal age. The animals were decapitated and the brains were fetched immediately and placed in PBS at 4 °C. After removing the meninges and blood vessels, the tissues were minced, washed, centrifuged, and incubated in 0.25 % (w/v) trypsin and 1 mg/ml DNase for 15–20 min. High-glucose of Dulbecco’s Modified Eagle Medium (DMEM) (Highclone, USA) containing 10 % (v/v) Fetal Bovine Serum (FBS) was added to complete the trypsinization. Cells were cultured in DMEM supplemented with 10 % FBS, 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin and were maintained at 37 °C under >90 % humidity and 5 % CO2. The culture medium was renewed twice a week until cells reached confluence. Then the cells were purified by repeated trypsinization and inoculated at a density of 2.5 × 104 cells/cm2 at least 4 times. More than 95 % of the cultured cells were astrocytes as identified by immunofluorescent staining for Glial Fibrillary Acidic Protein (GFAP). Experiments were carried out 21 days after culturing of the astrocytes.
Cell Transfection of shRNA
The TLR4 gene targeting (Rat, GenBank accession NM 019178) duplex components were obtained from Shanghai GeneChem Co., Ltd (China). The shRNA was transfected into astrocytes by Lipofectamine 2000 solution (Invitrogen, Carlsbad, CA, USA) as recommended by the manufacturer. Briefly, the astrocytes were inoculated in a 6-well plate at 5 × 105 cells/well and incubated at 37 °C for 24 h. In serum-free medium, 10 μl of 40 μM shRNA duplex in a total volume of 250 μl of Opti-MEMI was mixed with 10 μl of Lipofectamine 2000 for 20 min at RT. Lipofectamine–shRNA complex was added to each well of astrocytes and the solution was mixed gently by rocking the plate for 6 h at 37 °C in a humidified 5 % CO2 incubator. After 24 h further incubation of serum-free medium at 37 °C in a humidified 5 % CO2 atmosphere, further treatment was performed.
Cell Treatments
After transfection, the cells were divided into six groups: (a) control group (C), (b) MRP8 group (M), (c) PDTC + MRP8 group (P + M), (d) shRNA-TLR4 control group (shRNA-C), (e) shRNA-TLR4 MRP8 group (shRNA-M), and (f) shRNA-TLR4-PDTC + MRP8 group (shRNA P + M). The cells were pretreated with PDTC (100 nM) for 1 h and removed. Next, MRP8 (0.5 ug/mL) was introduced to intervene with the cells for 24 h at 37 °C under >90 % humidity and 5 % CO2.
RNA Isolation
For RNA isolation, hippocampal tissues and cells were homogenized in 1 ml Trizol Reagent (Invitrogen, Carlsbad, CA, USA). After adding 0.2 ml of chloroform, the aqueous phase was isolated using Phase Lock tubes (Eppendorf, Hamburg, Germany). RNA was precipitated with 0.5 ml isopropyl alcohol, washed twice with 75 % ethanol, and dissolved in nuclease-free water. The concentration and purity of RNA were determined at 260/280 nm using a nanodrop spectrophotometer (Ocean Optics, Dunedin, FL, USA).
MRP8 Expression by qPCR
cDNA was generated using a Prime Script RT Reagent Kit (TAKARA, Dalian, China) according to the manufacturer’s instructions. MRP8 expression was analyzed using SYBR® Premix ExTaq™ II (TAKARA) which was run on the Bio-Rad CFX384 Touch Real-Time PCR detection system according to the manufacturer’s instructions. Data analysis was performed with the software provided by the manufacturer, using the 2-(ÄÄCt) method to determine the relative-quantitative level of MRP8, and expressed as a fold-difference to the relevant control.
Western Blotting
The hippocampi and astrocytes were added to an NP-40 lysis solution on ice to extract the total protein, and the homogenate was centrifuged at 12,000 rpm for 30 min at 4 °C. The supernate was collected and the protein concentration detected. The protein concentration was determined by a Bicinchoninic Acid (BCA) protein assay kit. Equal amounts of protein (20 μg) were loaded on a 10 % sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, and blotted onto a polyvinylidene difluoride (PVDF) membrane. The membrane was equilibrated in Tris–buffered saline and Tween (TBST) for 10 min, blocked with TBST plus 5 % fat-free dry milk in TBST for 1 h at RT, and the proteins were detected with specific antibodies [TLR4 (1:320), IL-1β (1:200), and β-actin (1:1,000)] overnight with gentle checking at 4 °C. Rabbit and mouse anti-mouse/rabbit IgG (1:10,000) peroxidase reactions were used for immunostaining. The membranes were developed using an enhanced chemiluminescence (ECL) kit (Applygen Technologies, Beijing, China) and exposed to X-ray film. Image J 1.41o software (National Institutes of Health, Bethesda, MD, USA) was used to quantitatively analyze protein expression levels.
Electrophoretic Mobility Shift Assay (EMSA) to Detect NF-κB
Hippocampal tissue samples and transfected astrocytes were homogenized on ice and the protocol of nuclear protein extraction was performed according to the manufacturer’s instructions for the cytosolic-nuclear extraction kit (Pierce). 32P-NF-κB double-stranded consensus oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′, Promega Corp Madison, WI) and nuclear extracts (5 μg) were used. DNA-protein complexes were separated by electrophoresis through a 6 % nondenaturing acrylamide–bis-acrylamide (37.5:1) gel in 0.5 × Tris–borate/EDTA (TBE) for 2 h at 150 V. The membrane was blocked with 15 ml blocking buffer for 30 min, developed using an ECL kit (Applygen Technologies, Beijing, China), and exposed to X-ray film. Image J 1.41o software (National Institutes of Health, Bethesda, MD, USA) was used to estimate the NF-κB activity in hippocampal and astrocyte nuclear protein extracts.
Enzyme-Linked Immunosorbent Assay (ELISA) to Detect the Expression of IL-1β
Total proteins of the rat model’s hippocampal tissues were extracted and cell culture media collected for ELISA tests. IL-1β level was determined by ELISA assay (R&D Systems) following the manufacturer’s instructions.
Double Immunostaining
Astrocytes were seeded onto cover slips in 12-well tissue culture plates. After treatment, astroctyes were fixed by 4 % paraformaldehyde for 15 min at RT, then used for immunofluorescence analysis. For cytoplasmic staining, astrocytes were incubated by 0.25 % Trixton for 10 min and blocked with 5 % BSA in PBS, then incubated with primary antibodies TLR4 (1:100) and NF-κB (1:200) together overnight at 4 °C, then incubated with goat anti-rabbit and anti-mouse IgG (FITC)-conjugated secondary antibodies (1:500). Cellular DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI, 1.5 μg/ml). After antibody labeling, cells were viewed with an Olympus BX51 laser confocal microscope and Cell F Imaging Software (Olympus, Hamburg, Germany). Co-localization studies were carried out to identify TLR4 and NF-κB expression in astrocytes.
Statistical Analysis
The results are the means ± standard deviation (SD) of at least three independent experiments. Statistical analyses were carried out with Student’s t test when comparing between two variables and one-way ANOVA when comparing among multiple variables. Values were considered significant when p < 0.05.
Results
Neuron Loss and MFS in an MTLE Rat Model
Hippocampal cytoarchitecture was evaluated using Nissl staining in different stages (acute, latent, chronic) after pilocarpine (seizure rat) or saline (control rat) intraperitoneal injection. In this study, the number of neurons in the CA1, CA3, and DG areas were counted and compared with the corresponding control group in the three stages of MTLE development (Fig. 1a). A dramatic pilocarpine-induced degeneration of neurons in the three hippocampal areas occurred mainly in the chronic stage of MTLE development in our rat model (Fig. 1b, c, d). This significant decrease in neuronal density suggests that recurrent seizures and epilepsy result in the loss of neurons in the CA1, CA3, and DG areas (*p < 0.05).
Cytoarchitecture visualization and neuron density in the hippocampus of the MTLE rat model. a AC, LC, and CC shows the neurons in the hippocampal regions of the CA1, CA3, and DG areas from control rats. AS, LS, and CS shows the same areas from seizure rats. b Histogram shows neuron density in the CA1, CA3, and DG areas in acute stage of MTLE, with significant neuronal loss only in the CA1 area in the AS group. c Histogram shows neuron density in the CA1, CA3, and DG areas in latent stage of MTLE, with no statistical differences between seizure and control rats. d Histogram shows neuron density in the CA1, CA3, and DG areas, with significant neuronal loss in all hippocampal regions in the CS group (*p < 0.05; ◇p > 0.05)
Mossy fibers from granule cells in the CA3 and DG areas undergo reorganization of their terminal projections during the chronic stage of MTLE in our animal model. As demonstrated by Timm’s method, aberrant MFS selectively labeled synaptic terminals of mossy fibers due to their high zinc content compared to the control group (*p < 0.05). In seizure rats, Timm’s staining showed obvious MFS that extended into the dentate supragranular layer (Fig. 2 CS). On the contrary, in the control group (Fig. 2 CC), supragranular MFS was less intense and more dispersed. These data support the idea that seizure and epilepsy can result in aberrant MFS in an MTLE rat model.
Distribution patterns of Timm granules in the hippocampus of the MTLE rat model. a CC shows the Timm granules in the hippocampal regions of the CA3 and DG areas from control rats. CS shows the same spots from seizure rats. b Histograms show significant increase of Timm granules in the CA3 and DG areas in the CS group (*p < 0.05, vs CC)
Dynamic Expression of MRP8, TLR4, and IL-1β in MTLE Rat Hippocampi
The fact that MRP8’s expression is highly increased in different brain lesions, including cerebral ischemia, suggests that MRP8 may play important roles in MTLE. Real-time PCR results show upregulation of MRP8 gene expression in all hippocampal tissues in all (acute, latent, chronic) stages, with significant upregulation during the acute and chronic stages (Figs. 3a). These results were confirmed by immunostaining, which revealed a similar pattern of MRP8 expression in the three stages of MTLE development in the CA1, CA3, and DG regions (Fig. 4a, b). We then detected the expression of TLR4. WB showed that the expression of the TLR4 protein in hippocampal tissues was significantly upregulated during the acute and chronic stages of MTLE development compared to the control group (Fig. 3b, c). These results were further confirmed by IHC of TLR4 in the CA1, CA3, and DG regions (Fig. 4c, d).
Dynamic expression of the MRP8 gene and TLR4, NF-κB, and IL-1β proteins in the MTLE rat model. a The expression of the MRP8 gene in the MTLE rat model. Significant upregulation of MRP8 occurred in the acute and chronic stages (*p < 0.05, ◊p > 0.05). In the latent stage of disease in the MTLE rat model, expression increased only slightly, without statistical significance, compared to the control group. b Gray value rate of TLR4, IL-1β, and β-actin (by WB), and NF-κB (by EMSA) expression in the three stages of MTLE development compared with control groups. c, d, e TLR4, NF-κB, and IL-1β significantly upregulated in the acute and chronic stages. In the latent stage of MTLE, expression increased only slightly, without statistical significance, compared to the control group (*p < 0.05, ◊p > 0.05)
MRP8, TLR4, and NF-κB immunostaining in the hippocampal CA1, CA3, and DG regions. a Cytoarchitecture visualization of MRP8 immunostaining in the three stages of MTLE development in seizure (AS, LS, CS) and control (AC, LC, CC) rats in the CA1, CA3, and DG areas of hippocampus. c TLR4 immunostaining in the three stages of disease in seizure and control rats in the CA1, CA3, and DG area of the hippocampus. e NF-κB immunostaining in the three stages of MTLE development in seizure and control rats in the CA1, CA3, and DG areas of the hippocampus. b, d, f MRP8, TLR4, and NF-κB-positive cells significantly increased in the CA1, CA3, and DG areas in seizure rats, especially in the acute and chronic stages (*p < 0.05, vs control rat). In the latent stage, positive cells were also observed in the three areas, but with no statistical significance compared to the control rats (◊p > 0.05)
IL-1β dynamic expression patterns in the three stages of MTLE development in the rat model determined by ELISA. IL-1β showed significant upregulation in the acute and chronic stages of disease in the seizure rat compared to the control rat (*p < 0.05). In the latent stage of MTLE, expression increased only slightly compared to the control group (◊p > 0.05)
Since IL-1β is the major cytokine reported to be involved in epilepsy, we studied the expression of IL-1β in hippocampal tissues with WB and ELISA. As expected, IL-1β was significantly upregulated in the acute and chronic stages, but only slightly increased in the latent stage compared with the control group (Figs. 3b, e and 5).
Expressions of MRP8, TLR4, and IL-1β Are Increased in Hippocampi from Children with MTLE
For further confirmation of our above results, we examined the surgically removed hippocampi of five children undergoing unilateral selective amygdalohippocampectomy for drug-resistant MTLE. Real-time PCR results showed significant upregulation of the MRP8 gene in the hippocampal tissues of children with MTLE compared with controls (Fig. 6a). As indicated in Fig. 6b, c, e, TLR4 and IL-1β expression in the hippocampal tissues of children with MTLE also increased.
Expression of MRP8, TLR4, NF-κB, and IL-1β in children with MTLE. a Real-time PCR showed significant upregulation of MRP8 in children with MTLE (n = 5/group, *p < 0.05). b TLR4, IL-1β, and β-actin (by WB), and NF-κB (by EMSA) showed significant upregulation in children with MTLE compared to controls. c, d, e Significant upregulation of TLR4, NF-κB, and IL-1β occurred in the MTLE children compared to normal controls (n = 5/group, *p < 0.05)
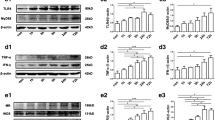
MRP8 leads to IL-1β Induction in Astrocytes Via the TLR4/NF-κB Pathway
It has been reported that IL-1β plays a key role in the development of seizures and epilepsy, and that astrocytes sustain inflammation during epileptogenesis [47]. Thus, we hypothesized that MRP8 executed its convulsant effects via astrocytes. Primary astrocytes isolated from rat hippocampi were stimulated with MRP8 for 24 h, and an abundant amount of IL-1β was induced, accompanied by an increase in TLR4 expression and NF-κB activation. Knockdown of TLR4 in astrocytes with shRNA ameliorated IL-1β induction (Figs. 7a, d and 8), indicating that MRP8 functions in a TLR4-dependent way. When the cells were pretreated with an NF-κB inhibitor (PDTC) for 30 min, this IL-1β induction was significantly reduced (Figs. 7a, d and 8), suggesting that the MRP8/TLR4 axis induces IL-1β production in astrocytes in an NF-κB-dependent manner.
MRP8 induces TLR4/NF-κB-dependent IL-1β expression in vitro. a The expression of TLR4, NF-κB, IL-1β, and β-actin in astrocytes after preincubation for 30 min with 100 nM PDTC (inhibitor of NF-κB) or transfection of shRNA-TLR4 (lipo2000), followed by 24 h stimulation with MRP8 (0.5 ug/ml). b The expression of TLR4 upregulated after pretreatment with MRP8 (*p < 0.05, vs control group). PDTC could not inhibit the upregulation of TLR4 induced by MRP8 ( p > 0.05, vs MRP8 group). After transfection of shRNA-TLR4, the expression of TLR4 significantly downregulated (▼
p < 0.05, vs control group) and MRP8 could not increase the expression of TLR4. c The expression of NF-κB upregulated after pretreatment with MRP8 (*p < 0.05, vs control group). PDTC could inhibit the upregulation of NF-κB induced by MRP8 (#
p < 0.05, vs MRP8 group). After TLR4 was blocked by transfection of shRNA-TLR4, MRP8 could not upregulate the expression of NF-κB (△
p < 0.05, vs MRP8 group). d MRP8 increased the expression of IL-1β (*p < 0.05, vs control group). PDTC partly inhibited the upregulation of IL-1β induced by MRP8 (#
p < 0.05, vs MRP8 group). After blocking TLR4 by transfection of shRNA-TLR4, the expression of IL-1β was not obviously changed by pretreatment with MRP8 (△
p < 0.05, vs MRP8 group)
p > 0.05, vs MRP8 group). After transfection of shRNA-TLR4, the expression of TLR4 significantly downregulated (▼
p < 0.05, vs control group) and MRP8 could not increase the expression of TLR4. c The expression of NF-κB upregulated after pretreatment with MRP8 (*p < 0.05, vs control group). PDTC could inhibit the upregulation of NF-κB induced by MRP8 (#
p < 0.05, vs MRP8 group). After TLR4 was blocked by transfection of shRNA-TLR4, MRP8 could not upregulate the expression of NF-κB (△
p < 0.05, vs MRP8 group). d MRP8 increased the expression of IL-1β (*p < 0.05, vs control group). PDTC partly inhibited the upregulation of IL-1β induced by MRP8 (#
p < 0.05, vs MRP8 group). After blocking TLR4 by transfection of shRNA-TLR4, the expression of IL-1β was not obviously changed by pretreatment with MRP8 (△
p < 0.05, vs MRP8 group)
IL-1β changes in the supernate of astrocytes in vitro. MRP8 increased the expression of IL-1β in the supernate of astrocytes (*p < 0.05, vs control group). After pretreatment with PDTC, the upregulation of IL-1β induced by MRP8 was partially inhibited (*p < 0.05, vs control group; # p < 0.05, vs MRP8 group). After blocking TLR4 by transfecting shRNA-TLR4, the upregulation of IL-1 induced by MRP8 was completely inhibited (△ p < 0.05, vs MRP8 group)
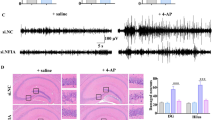
To further confirm the involvement of NF-κB in MTLE induction, EMSA was used to detect the expression of NF-κB in the hippocampus of the MTLE rat model. The results showed significant upregulation of NF-κB expression in hippocampal tissues in the acute and chronic stages, but only a small increase in the latent stage compared to the control group (Fig. 3b, d). We used IHC to detect the NF-κB-positive cells, which increased in the acute and chronic stages, consistent with the WB results (Fig. 4e, f). In addition, the migration of NF-κB from cytoplasm to nucleus was observed (Fig. 4e) in AS and CS. We also observed significant upregulation of DNA-binded NF-κB in the hippocampal tissues of children with MTLE compared to the controls (Fig. 6b, d). At the same time, knockdown of TLR4 in astrocytes reduced the MRP8-induced NF-κB activation (Fig. 9).
TLR4 and NF-κB changes in astrocytes in vitro. a C control group. TLR4 distributed in the area surrounding the cytoplasm of astrocytes (white arrows) and NF-κB distributed in the astrocyte peri-nuclear area (red arrows). b M MRP8 stimulated group. After pretreatment with MRP8, TLR4 expression increased and NF-κB transferred into the nucleus. c P + M PDTC + MRP8 group. After preincubation with PDTC, followed by stimulation with MRP8, the expression of TLR4 increased, but the change in NF-κB was limited. d shRNA-C transfection of shRNA-TLR4 group. e shRNA-M transfection of shRNA-TLR4 + MRP8 group. f shRNA-M + P transfection of shRNA-TLR4 + PDTC + MRP8 group. d, e, f After transfection of shRNA-TLR4, the expression of TLR4 decreased the expression of NF-κB uniformly, and migration of NF-κB was not observed
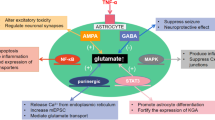
Next we determined the sub-cellular distribution of TLR4 and NF-κB in astrocytes under MRP8 stimulation. Immunofluorescence results showed that TLR4 mainly distributed in the cytomembrane in astrocytes, and NF-κB distributed in the area near the nucleus of cytoplasm in astrocytes in the control group (Fig. 9a). After pretreatment by MRP8, expression of TLR4 and NF-κB increased significantly, and NF-κB transferred into the nucleus (Fig. 9b). PDTC inhibited the increase of NF-κB stimulated by MRP8, but did not limit the expression of TLR4 (Fig. 9c). After transfection of shRNA-TLR4, the expression of TLR4 decreased, while the expression of NF-κB remained uniform, and no migration of NF-κB was observed. (Fig. 9d, e, f). Double-immunostaining obtained the same results as WB and EMSA (Fig. 7a, b, c). In conclusion, these data support the hypothesis that the MRP8/TLR4 axis activates the NF-κB pathway, leading to the production of IL-1β, which ultimately potentiates the perpetuation of MTLE.
Discussion
MTLE is a debilitating disease with a high percentage of patients that do not respond to conventional anti-epileptic drugs. Current anti-epileptic drug treatment is limited to suppressing seizure activity, and does not affect the actual disease process [48, 49].
Clinical and experimental evidence highlights the fact that brain inflammation is one key factor contributing to the epileptogenic process [50]. Brain inflammation promotes increased neuronal excitability, decreases the seizure threshold, and is likely to be involved in the molecular, structural, and synaptic changes characterizing epileptogenesis [51], enhancing disease severity in the immature brain [50]. Targeting inflammation with anti-inflammatory drugs has been shown to be useful in blocking the epileptogenic process and preventing seizures [53–55]. Cerebral injury caused by PAMPs inflammation has been clearly identified as promoting the procession of epilepsy [52, 56, 57]. Meanwhile, a large number of clinical data have shown that seizures are frequently followed by brain disorder without bacterial infection [58]. The specific relationship between inflammation and epilepsy pathogenesis in the absence of bacterial infection signifies the role of DAMPs in the inflammatory process associated with epilepsy. Seizure and inflammation are considered to be the cause and effect of brain injury in epilepsy pathogenesis. But there are minimal data about the relationship between epileptology and the DAMPs-inflammatory mechanism.
As one of the DAMPs, MRP8 (which is widely used as a marker protein for activated or recruited phagocytes) has a modulatory role in inflammatory response [59]. The expression of MRP8 in the human nervous system has been detected in normal brain tissue, the expression of MRP8 is very low, but MRP8-positive cells have been detected in multiple brain diseases [24]. When macrophage/microglia are activated, MRP8 upregulates [26, 60]. S100 proteins, including MRP8, have been found to mediate inflammatory responses and to be involved in the recruitment of inflammatory cells to sites of injury [61, 62]. Moreover, MRP8 complexes induce a variety of inflammatory reactions, and the extent of MRP8 expression correlates with disease activity in several inflammatory disorders [62, 63]. In other inflammatory diseases, MRP8 is secreted by activated phagocytes and has pro-inflammatory effects [61, 64]. However, there is no available data about the role of MRP8 as a pro-inflammatory marker in epileptogenesis. In this experiment we attempted to explore the functional role of MRP8 in the inflammatory process in MTLE development in a rat model. We started our experiment by detecting the dynamic expression pattern of MRP8 in the three stages of MTLE. MRP8 was found to be significantly upregulated in the seizure-associated (acute and chronic) stages: highest in the acute stage, and only a slight increase over the control group in the latent stage. MRP8 was also found to be upregulated in the human tissues of MTLE patients. Knowledge about MRP8 expression in epilepsy is highly limited. Our results verify that high expression of MRP8 exists during the whole process of MTLE development, which indicates that the DAMPs-inflammatory response may play an important role in MTLE pathogenesis. Understanding the role of MRP8 in the progression of MTLE may open a new avenue of research into the inflammatory mechanism of MTLE.
TLR4, the most important ligand of MRP8, has recently been identified as a new contributor to the inflammatory process during seizures. It is involved in seizure generation and recurrence. Antagonists of TLR4 reduce both acute and chronic recurrent seizures. Interestingly, mice that lack TLR4 are intrinsically resistant to seizures [19]. In vivo TLRs are expressed at low levels in brain cells under physiological conditions, but these receptors can be rapidly upregulated under various pathological conditions, including cell injury and seizures [5, 19, 65, 66]. Transcription factor NF-κB, which coordinates the induction of many genes that encode inflammatory mediators [67], plays a crucial role in many acute inflammatory reactions. TLRs recruit downstream signaling proteins and result in the transcription of genes encoding inflammation-associated molecules and cytokines. When combined with a specific ligand, the activation of TLR4 induces the phosphorylation of IκB and dissociates IκB from NF-κB. Then, NF-κB translocates into the nucleus and activates and regulates the transcription of genes related to inflammatory responses [68, 69]. A large amount of research has explored the direct relationship between TLR4 and NF-κB [70–72]. The TLR4/NF-κB signaling pathway has been verified as playing a critical role in the induction of immunological/inflammatory responses, and has been implicated in several CNS diseases, such as subarachnoid hemorrhage, traumatic brain injury, and ischemic brain injury [73–75]. In our work, we found that TLR4 expression followed the same dynamic pattern as MRP8: upregulation in the acute and chronic stages, and only a small increase compared to the control in the latent stage. TLR4 also showed enhanced expression in human tissues with MTLE. In line with our results, Maroso et al. [19] observed activation of TLR4 in the brain after administration of convulsant drugs. We also observed that NF-κB (p65) showed the same pattern of dynamic changes in the acute, latent, and chronic stages of MTLE development, just like MRP8 and TLR4, and was also significantly upregulated in human tissues. In experimental limbic SE in rat, NF-κB over-expression was reported in neurons and reactive astrocytes, concomitant with microglial activation [76]. These reactions were still obvious 2 weeks after SE, suggesting that inflammatory processes participate in post-ictal events involved in secondary epileptogenesis. In line with our results, TLR4 and NF-κB were found to be significantly and persistently upregulated in all hippocampal foci of patients with chronic MTLE and typical sclerosis [30]. The overexpressed MRP8, TLR4, and NF-κB in the MTLE patients and MTLE rat model suggest that MRP8 acts as an endogenous TLR4 ligand, activating TLR4-mediated NF-κB signaling and initiating inflammatory responses in MTLE. IL-1β is one of the pro-inflammatory cytokines that can induce acute inflammation in the host response cascade. It has multiple functions in CNS, such as increasing neuronal excitability and the risk of excitotoxity. The first evidence of an active role for IL-1β in seizures was provided by Vezzani et al. [77, 78], and showed that the intracerebral application of IL-1β increases seizure activity. In addition, the expression of IL-1β measured in human epileptogenic tissue positively correlates with the frequency of seizures in the same patients before surgery, highlighting a possible link between the brain level of this cytokine and seizure recurrence [79]. Exploring the role of IL-1β in epileptogenesis has been the target for many investigators in the last decades, seeking to define whether IL-1β is related to increased seizure susceptibility and plays a key role in epileptogenesis, or if it is merely a biochemical epiphenomenon of a seizure. Cerebrospinal fluid (CSF) studies in children and animal models with FS have revealed significant release of endogenous cytokines, especially IL-1β, suggesting a key role for IL-1β in FS generation and development of epilepsy after FS [80]. In confirmation of our previous work [40], we found that IL-1β in a rat model of MTLE has the same dynamic changes, with significant upregulation in the acute and chronic stages, and nearly equal levels of upregulation in the latent stage compared to the controls. We found the same results in tissue samples from children with MTLE as well. Seizures induce a wave of inflammation in brain endothelial cells, including upregulation of IL-1β and its receptor IL-1R1 [7]. IL-1β activation during MTLE development has been linked to neurodegenerative changes, including MFS and neuronal cell loss [80]. Collectively, the high expression of IL-1β and its relation to hippocampal pathology observed in the MTLE animal model indicate that IL-1β plays an important role in inflammation and autoimmunity during MTLE development, and increases depending on the stage of disease and the development of recurrent spontaneous seizures. Despite the extensive work on IL-1β in epilepsy research and its role in the inflammatory pathogenesis of MTLE development, the initial triggering for its release and potentiating of MTLE endogenous inflammation is still a matter of controversy that needs more exploration. Interestingly, we found for the first time that MRP8 showed the same dynamics of expression pattern changes for TLR4, NF-κB, and IL-1β in the three stages of MTLE development in an immature rat model, and in children. Detecting the relationship among these cytokines may uncover the mechanism of epileptogenesis associated with inflammation and seizure. Astrocytes assumed to be immune cells in the CNS and a major source of inflammatory mediators in multiple brain diseases are now considered to have dual protagonist and assistant roles in the puzzle of epilepsy and inflammation [43, 81, 82]. The role of astrocytes as players in epileptogenesis was initially proposed over 20 years ago, but has only recently been suggested as having a direct role in the generation of epileptiform activities [83–85]. IL-1β is upregulated during epileptogenesis in astrocytes, but not in microglia, suggesting that astrocytes play a dominant role in sustaining chronic inflammation before the onset of spontaneous seizures [7]. In consideration of the astrocytes’ multiple actions in the CNS and the close relations to both inflammation and epilepsy, we decided to use primary astrocyte cultures in vitro to further confirm our hypotheses about the relationship among MRP8-TLR4, NF-κB, and IL-1β. In our experiment, MRP8, as a ligand of TLR4 and a member of DAMPs, markedly increased the expression of TLR4 and NF-κB in astrocytes in vitro. These results show for the first time that the astrocyte endogenous inflammatory response triggered by MRP8 actively participates in TLR4 and NF-κB production. Activated astrocytes triggered the synthesis of cytokines, including IL-1β, IL-6, and transforming growth factor-β (TGF-β) [86]. Our results show that MRP8 stimulated astrocytes also associated with significant upregulation of IL-1β. IL-1β-induced NF-κB activation in primary culture of mouse astrocytes is mediated by the interaction of this cytokine with the IL-1 type I receptor/IL-1 receptor accessory protein complex [87] while the inhibition of NF-κB strongly decreases production and release of TNF-α and IL-1β, and limits apoptosis by b-amyloid (Ab) peptides [88]. Using astrocytes transfected by shRNA-TLR4 and inhibition of NF-κB in vitro, the IL-1β upregulation triggered by MRP8 is inhibited. Therefore, MRP8 might be a new inflammatory component that potentiates astrocyte activation via ligands with TLR4 to induce IL-1β secretion through the NF-κB pathway during MTLE pathogenesis. In conclusion, our observations demonstrate the upregulation of MRP8, TLR4, NF-κB, and IL-1β with prominent expression in hippocampus during epileptogenesis in a rat MTLE model as well as in children with MTLE. Further experiments prove that MRP8 stimulates activation of astrocytes and secretion of IL-1β by the TLR4/NF-κB signaling pathway. These novel experimental findings on the astrocyte level and the same dynamic changes for MRP8/TLR4/NF-κB/IL-1β in the three stages of MTLE development in both rat and children, strongly support the hypothesis that these pro-inflammatory signaling pathways bind to DAMPs and play a critical role in MTLE pathogenesis. Activation of these molecular markers in seizure-related disease stages may mediate the proconvulsant actions of this signaling cascade, providing a target for new biological therapies for MTLE.
References
Sander JW (2003) The epidemiology of epilepsy revisited. Curr Opin Neurol 16:165–170
Rakhade SN, Jensen FE (2009) Epileptogenesis in the immature brain: emerging mechanisms. Nat Rev Neurol 5:380–391
Friedman MJ, Sharieff GQ (2006) Seizures in children. Pediatr Clin North Am 53:257–277
Cascino GD (2008) When drugs and surgery don't work. Epilepsia 49:79–84
Turrin NP, Rivest S (2004) Innate immune reaction in response to seizures: implications for the neuropathology associated with epilepsy. Neurobiol Dis 16:321–334
Vezzani A, Granata T (2005) Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46:1724–1743
Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A (2008) Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis 29:142–160
Choi J, Nordli DR Jr, Alden TD, DiPatri A Jr, Laux L, Kelley K, Rosenow J, Schuele SU, Rajaram V, Koh S (2009) Cellular injury and neuroinflammation in children with chronic intractable epilepsy. J Neuroinflammation 6:38
Peng J, Omran A, Ashhab MU, Kong H, Gan N, He F, Yin F (2013) Expression Patterns of miR-124, miR-134, miR-132, and miR-21 in an Immature Rat Model and Children with Mesial Temporal Lobe Epilepsy. J Mol Neurosci doi:10.1007/s12031-013-9953-3.
Ashhab MU, Omran A, Kong H, Gan N, He F, Peng J, Yin F (2013) Expressions of Tumor Necrosis Factor-Alpha and MicroRNA-155 in Immature Rat Model of Status Epilepticus and Children with Mesial Temporal Lobe Epilepsy. J Mol Neurosci [Epub ahead of print]. doi:10.1007/s12031-013-0013-9
Pitkanen A, Sutula TP (2002) Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol 1:173–181
Galic MA, Riazi K, Henderson AK, Tsutsui S, Pittman QJ (2009) Viral-like brain inflammation during development causes increased seizure susceptibility in adult rats. Neurobiol Dis 36:343–351
Rasmussen T, Olszewski J, Lloyd-Smith D (1958) Focal seizures due to chronic localized encephalitis. Neurology 8:435–445
Cojocaru IM, Cojocaru M (2010) Reactions of the immune system in epilepsy. Maedica (Buchar) 3:201–206
Choi J, Min HJ, Shin JS (2011) Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J Neuroinflammation 8:135
Berg AT, Shinnar S (1996) Unprovoked seizures in children with febrile seizures: short-term outcome. Neurology 47:562–568
Shinnar S (2003) Febrile seizures and mesial temporal sclerosis. Epilepsy Curr 3:115–118
Bsibsi M, Ravid R, Gveric D, van Noort JM (2002) Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol 61:1013–1021
Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A (2010) Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16:413–419
Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J (2007) Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med 13:1042–1049
Hayashi T, Nakamura T, Takaoka A (2011) Pattern recognition receptors. Nihon Rinsho Meneki Gakkai Kaishi 34:329–345
Mencin A, Kluwe J, Schwabe RF (2009) Toll-like receptors as targets in chronic liver diseases. Gut 58:704–720
Burkhardt K, Schwarz S, Pan C, Stelter F, Kotliar K, Von Eynatten M, Sollinger D, Lanzl I, Heemann U, Baumann M (2009) Myeloid-related protein 8/14 complex describes microcirculatory alterations in patients with type 2 diabetes and nephropathy. Cardiovasc Diabetol 8:10
Achouiti A, Vogl T, Urban CF, Röhm M, Hommes TJ, van Zoelen MA, Florquin S, Roth J, van 't Veer C, de Vos AF, van der Poll T (2012) Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS Pathog 8:e1002987
Maiseyeu A, Badgeley MA, Kampfrath T, Mihai G, Deiuliis JA, Liu C, Sun Q, Parthasarathy S, Simon DI, Croce K, Rajagopalan S (2012) In vivo targeting of inflammation-associated myeloid-related protein 8/14 via gadolinium immunonanoparticles. Arterioscler Thromb Vasc Biol 32:962–970
Ziegler G, Prinz V, Albrecht MW, Harhausen D, Khojasteh U, Nacken W, Endres M, Dirnagl U, Nietfeld W, Trendelenburg G (2009) Mrp-8 and −14 mediate CNS injury in focal cerebral ischemia. Biochim Biophys Acta 1792:1198–1204
Engel S, Schluesener H, Mittelbronn M, Seid K, Adjodah D, Wehner HD, Meyermann R (2000) Dynamics of microglial activation after human traumatic brain injury are revealed by delayed expression of macrophage-related proteins MRP8 and MRP14. Acta Neuropathol 100:313–322
Yonekawa K, Neidhart M, Altwegg LA, Wyss CA, Corti R, Vogl T, Grigorian M, Gay S, Lüscher TF, Maier W (2011) Myeloid related proteins activate Toll-like receptor 4 in human acute coronary syndromes. Atherosclerosis 218:486–492
Holzinger D, Frosch M, Kastrup A, Prince FH, Otten MH, Van Suijlekom-Smit LW, ten Cate R, Hoppenreijs EP, Hansmann S, Moncrieffe H, Ursu S, Wedderburn LR, Roth J, Foell D, Wittkowski H (2012) The Toll-like receptor 4 agonist MRP8/14 protein complex is a sensitive indicator for disease activity and predicts relapses in systemic-onset juvenile idiopathic arthritis. Ann Rheum Dis 71:974–980
Crespel A, Coubes P, Rousset MC, Brana C, Rougier A, Rondouin G, Bockaert J, Baldy-Moulinier M, Lerner-Natoli M (2002) Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res 952:159–169
Voutsinos-Porche B, Koning E, Kaplan H, Ferrandon A, Guenounou M, Nehlig A, Motte J (2004) Temporal patterns of the cerebral inflammatory response in the rat lithium-pilocarpine model of temporal lobe epilepsy. Neurobiol Dis 17:385–402
Lubin FD, Ren Y, Xu X, Anderson AE (2007) Nuclear factor-kB regulates seizure threshold and gene transcription following convulsant stimulation. J Neurochem 103:1381–1395
Chuang YC, Chen SD, Lin TK, Chang WN, Lu CH, Liou CW, Chan SH, Chang AY (2010) Transcriptional upregulation of nitric oxide synthase II by nuclear factor-kappaB promotes apoptotic neuronal cell death in the hippocampus following experimental status epilepticus. J Neurosci Res 88:1898–1907
Yu N, Di Q, Liu H, Hu Y, Jiang Y, Yan YK, Zhang YF, Zhang YD (2011) Nuclear factor-kappa B activity regulates brain expression of P-glycoprotein in the kainic acid-induced seizure rats. Mediators Inflamm : 670613
Takeda K, Akira S (2004) TLR signaling pathways. Semin Immunol 16:3–9
Okun E, Griffioen KJ, Mattson MP (2011) Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci 34:269–281
Sayyah M, Beheshti S, Shokrgozar MA, Eslami-far A, Deljoo Z, Khabiri AR, Haeri Rohani A (2005) Antiepileptogenic and nticonvulsant activity of interleukin-1 beta in amygdala-kindled rats. Exp Neurol 191:145–153
Akin D, Ravizza T, Maroso M, Carcak N, Eryigit T, Vanzulli I, Aker RG, Vezzani A, Onat FY (2011) IL-1β is induced in reactive astrocytes in the somatosensory cortex of rats with genetic absence epilepsy at the onset of spike-and-wave discharges, and contributes to their occurrence. Neurobiol Dis 44:259–269
Järvelä JT, Lopez-Picon FR, Plysjuk A, Ruohonen S, Holopainen IE (2011) Temporal profiles of age-dependent changes in cytokine mRNA expression and glial cell activation after status epilepticus in postnatal rat hippocampus. J Neuroinflammation 8:29
Omran A, Peng J, Zhang C, Xiang QL, Xue J, Gan N, Kong H, Yin F (2012) Interleukin-1β and microRNA-146a in an immature rat model and children with mesial temporal lobe epilepsy. Epilepsia 53:1215–1224
Rizzi M, Perego C, Aliprandi M, Richichi C, Ravizza T, Colella D, Velískŏvá J, Moshé SL, De Simoni MG, Vezzani A (2003) Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol Dis 14:494–503
Vezzani A, Baram TZ (2007) New roles for interleukin-1 Beta in the mechanisms of epilepsy. Epilepsy Curr 7:45–50
Nguyen MD, Julien JP, Rivest S (2002) Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci 3:216–227
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Blümcke I, Pauli E, Clusmann H, Schramm J, Becker A, Elger C, Merschhemke M, Meencke HJ, Lehmann T, von Deimling A, Scheiwe C, Zentner J, Volk B, Romstöck J, Stefan H, Hildebrandt M (2007) A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol 113:235–244
Sloviter RS (1982) A simplified Timm stain procedure compatible with formaldehyde fixation and routine paraffin embedding of rat brain. Brain Res Bull 8:771–774
Choi J, Koh S (2008) Role of Brain Inflammation in Epileptogenesis. Yonsei Med J 49:1–18
Loscher W, Schmidt D (2006) New horizons in the development of antiepileptic drugs: innovative strategies. Epilepsy Res 69:183–272
Stafstrom CE (2010) Mechanisms of action of antiepileptic drugs: the search for synergy. Curr Opin Neurol 23:157–163
Vezzani A, French J, Bartfai T, Baram TZ (2011) The role of inflammation in epilepsy. Nat Rev Neurol 7(31–40):51
Ravizza T, Balosso S, Vezzani A (2011) Inflammation and prevention of epileptogenesis. Neurosci Lett 497:223–230
Auvin S, Mazarati A, Shin D, Sankar R (2010) Inflammation enhances epileptogenesis in the developing rat brain. Neurobiol Dis 40:303–310
Marchi N, Fan Q, Ghosh C, Fazio V, Bertolini F, Betto G, Batra A, Carlton E, Najm I, Granata T, Janigro D (2009) Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol Dis 33:171–181
Abraham J, Fox PD, Condello C, Bartolini A, Koh S (2012) Minocycline attenuates microglia activation and blocks the long-term epileptogenic effects of early-life seizures. Neurobiol Dis 46:425–430
Wang DD, Englot DJ, Garcia PA, Lawton MT, Young WL (2012) Minocycline- and tetracycline-class antibiotics are protective against partial seizures in vivo. Epilepsy Behav 24:314–318
Jaworska-Adamu J, Dmowska M, Cybulska R, Krawczyk A, Pawlikowska-Pawlęga B (2011) Investigations of hippocampal astrocytes in lipopolysaccharide-preconditioned rats in the pilocarpine model ofepilepsy. Folia Histochem Cytobiol 49:219–224
Lee SH, Kim BJ, Kim YB, Chung PW, Moon HS, Suh BC, Yoon WT, Jin DK, Park YS, Lee YT, Park KY (2012) IL-1β induction and IL-6 suppression are associated with aggravated neuronal damage in a lipopolysaccharide-pretreated kainic acid-induced rat pup seizure model. Neuroimmunomodulation 19:319–325
Granata T, Cross H, Theodore W, Avanzini G (2011) Immune-mediated epilepsies. Epilepsia 52:5–11
Kerkhoff C, Eue I, Sorg C (1999) The regulatory role of MRP8 (S100A8) and MRP14 (S100A9) in the transendothelial migration of human leukocytes. Pathobiology 67:230–232
Meeuwsen S, Bsibsi M, Persoon-Deen C, Ravid R, van Noort JM (2005) Cultured human adult microglia from different donors display stable cytokine, chemokine and growth factor gene profiles but respond differently to a pro-inflammatory stimulus. Neuroimmunomodulation 12:235–245
Roth J, Vogl T, Sorg C, Sunderkotter C (2003) Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol 24:155–158
Foell D, Wittkowski H, Vogl T, Roth J (2007) S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 81:28–37
Foell D, Roth J (2004) Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum 50:3762–3771
Frosch M, Strey A, Vogl T, Wulffraat NM, Kuis W, Sunderkötter C, Harms E, Sorg C, Roth J (2000) Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum 43:628–637
Peltier DC, Simms A, Farmer JR, Miller DJ (2010) Human neuronal cells possess functional cytoplasmic and TLR-mediated innate immune pathways influenced by phosphatidylinositol-3 kinase signaling. J Immunol 184:7010–7021
Zurolo E, Iyer A, Maroso M, Carbonell C, Anink JJ, Ravizza T, Fluiter K, Spliet WG, van Rijen PC, Vezzani A, Aronica E (2011) Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain 134:10151032
Gorina R, Font-Nieves M, Márquez-Kisinousky L, Santalucia T, Planas AM (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. GLIA 59:242–255
Anderson KV (2000) Toll signaling pathways in the innate immune response. Curr Opin Immunol 12:13–19
Akira S, Takeda K, Kaisho T (2001) Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2:675–680
Yan Q, Carmody RJ, Qu Z, Ruan Q, Jager J, Mullican SE, Lazar MA, Chen YH (2012) Nuclear factor-κB binding motifs specify Toll-like receptor-induced gene repression through an inducible repressosome. Proc Natl Acad Sci U S A 109:14140–14145
Ge Y, Xu Y, Sun W, Man Z, Zhu L, Xia X, Zhao L, Zhao Y, Wang X (2012) The molecular mechanisms of the effect of Dexamethasone and Cyclosporin A on TLR4 /NF-κB signaling pathway activation in oral lichen planus. Gene 508:157–164
Wang PP, Xie DY, Liang XJ, Peng L, Zhang GL, Ye YN, Xie C, Gao ZL (2012) HGF and direct mesenchymal stem cells contact synergize to inhibit hepatic stellate cells activation through TLR4/NF-kB pathway. PLoS One 7:e43408
Ma CX, Yin WN, Cai BW, Wu J, Wang JY, He M, Sun H, Ding JL, You C (2009) Toll-like receptor 4/nuclear factor-kappa B signaling detected in brain after early subarachnoid hemorrhage. Chin Med J (Engl) 122:1575–1581
Dong XQ, Yu WH, Hu YY, Zhang ZY, Huang M (2011) Oxymatrine reduces neuronal cell apoptosis by inhibiting Toll-like receptor 4/nuclear factor kappa-B-dependent inflammatory responses in traumatic rat brain injury. Inflamm Res 60:533–539
Lan L, Tao J, Chen A, Xie G, Huang J, Lin J, Peng J, Chen L (2013) Electroacupuncture exerts anti-inflammatory effects in cerebral ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB pathway. Int J Mol Med 31:75–80
Lerner-Natoli M, Montpied P, Rousset MC, Bockaert J, Rondouin G (2000) Sequential expression of surface antigens and transcription factor NF-kappaB by hippocampal cells in excitotoxicity and experimental epilepsy. Epilepsy Res 41:141–154
Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni MG (1999) Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci 19:5054–5065
Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, De Simoni MG, Sperk G, Andell-Jonsson S, Lundkvist J, Iverfeldt K, Bartfai T (2000) Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A 97:11534–11539
Ravizza T, Boer K, Redeker S, Spliet WG, van Rijen PC, Troost D, Vezzani A, Aronica E (2006) The IL-1beta system in epilepsy-associated malformations of cortical development. Neurobiol Dis 24:128–143
Xie C, Sun J, Qiao W, Lu D, Wei L, Na M, Song Y, Hou X, Lin Z (2011) Administration of simvastatin after kainic acid-induced status epilepticus restrains chronic temporal lobe epilepsy. PLoS One 6:e24966
Allan SM, Tyrrell PJ, Rothwell NJ (2005) Interleukin-1 and neuronal injury. Nat Rev Immunol 5:629–640
Appel SH, Beers DR, Henkel JS (2009) T cell-microglial dialog in Parkinson’s disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol 31:7–17
Volman V, Bazhenov M, Sejnowski TJ (2012) Computational models of neuron-astrocyte interaction in epilepsy. Front Comput Neurosci 6:58
Iyer A, Zurolo E, Prabowo A, Fluiter K, Spliet WG, van Rijen PC, Gorter JA, Aronica E (2012) MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PLoS One 7:e44789
Witcher MR, Ellis TL (2012) Astroglial networks and implications for therapeutic neuromodulation of epilepsy. Front Comput Neurosci 6:61
Ridet JL, Malhotra SK, Privat A, Gage FH (1997) Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 20:570–577
Pousset F, Dantzer R, Kelley KW, Parnet P (2000) Interleukin-1 signaling in mouse astrocytes involves Akt: a study with interleukin-4 and IL-10. Eur Cytokine Netw 11:427–434
Couturier J, Paccalin M, Morel M, Terro F, Milin S, Pontcharraud R, Fauconneau B, Page G (2011) Prevention of the β-amyloid peptide-induced inflammatory process by inhibition of double-stranded RNA-dependent protein kinase in primary murine mixed co-cultures. J Neuroinflammation 8:72
Acknowledgments
We thank Dr. Zhiquan Yang (Department of Neurosurgery, Xiangya Hospital, China) for providing the experimental hippocampus of MTLE patients and Dr Hongzhuan Tan (Department of Public Health, Central South University, China) for revising the statistical analysis of our manuscript. This work was supported by the National Natural Science Foundation of China, 81171126 & 81100846, and the national 973 programs, 2012CB94460. Doctoral innovation project, hunan, China (CX2012B087).
Conflict of interest
The authors declare no actual or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gan, N., Yang, L., Omran, A. et al. Myoloid-Related Protein 8, an Endogenous Ligand of Toll-Like Receptor 4, Is Involved in Epileptogenesis of Mesial Temporal Lobe Epilepsy Via Activation of the Nuclear Factor-κB Pathway in Astrocytes. Mol Neurobiol 49, 337–351 (2014). https://doi.org/10.1007/s12035-013-8522-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8522-7