Abstract
The potential of exosomes to treat central nervous system (CNS) pathologies has been recently demonstrated. These studies make way for a complete new field that aims to exploit the natural characteristics of these vesicles, considered for a long time as side products of physiological cellular pathways. Recently, however, the biological significance of exosomes has been evaluated and exosomes can now be viewed upon as new relevant functional entities for development of novel therapeutic strategies. In this review, we aim to summarize the state-of-the-art role of exosomes in the CNS and to speculate about possible future therapeutic applications of exosomes. In particular, we will speculate about the use of these vesicles as a substitute of cell-based therapies for the treatment of brain damage and review the potential of exosomes as drug delivery vehicles for the CNS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Nature of Exosomes
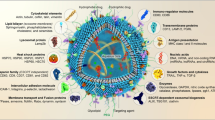
Paracrine secretion plays a fundamental role in cell–cell signaling. In eukaryotic cells, carrier vesicles bud from the donor cell membrane and after being secreted in the extracellular space, subsequently fuse with the intracellular compartment of acceptor cells [1]. A specific subtype of secreted vesicles is represented by exosomes: small spherical vesicles (~50 nm in diameter) limited by a lipid bilayer, enriched in lipids such as cholesterol, ceramide, and sphingolipids [2]. The content of exosomes varies ranging from numerous proteins and lipids to mRNAs and microRNAs [3]. Exosomes are derived from late endocytic compartments, known as multivesicular bodies (MVBs). The secretion of exosomes occurs when the MVBs fuse with the plasma membrane [4]. The vesicles diffuse into the intercellular fluids and reach the recipient cells. Here, the exosomal surface molecules will bind to membrane receptors including intercellular adhesion molecule 1, lymphocyte function-associated antigen 1, and TIM1 or TIM4 [4]. This interaction might result in the fusion of the exosomes with the recipient plasma membrane or in endocytosis of the exosomes into the recipient cell. In the former case, the exosomal content is directly released into the cytoplasm. Alternatively, endocytosed exosomes fuse with the limiting membrane of the endosome, leading to the release of exosomal content into the cytoplasm of the recipient cell [4]. The recipient cell will then respond to the change in the intracellular compartment depending of the composition of the exosomal content. Exosome secretion is constitutive in many cell types: Epstein–Barr virus-transformed B cells [5], dendritic cells (DCs) [6], macrophages [7], and most tumor cell lines. By contrast, reticulocytes [8], T cells [9, 10], mast cells [5], and resting B cells [11] increase the secretion of exosomes following activation of a relevant membrane receptor even though the basal level of exosomes is still detectable in resting conditions. Furthermore, a variety of conditions—such as cell activation, radiation, maturation stage of the cell, and senescence—can modulate the level of exosomal secretion in different cell types [4, 12–15]. The biological role of exosomes has been intensively studied during the last decades, especially regarding their function in the immune system [4, 16] and in cancer [17–20]. Remarkably, a growing body of evidence indicates how these vesicles are central players in cell–cell communication in other contexts, like in CNS.
Role of Exosomes in the CNS
The study on the role of exosomes in the CNS is a relatively new field: in 2006, the group of Faure et al. described for the first time the release of exosomes by cortical neurons in vitro [21]. Furthermore, Taylor et al. reported the secretion of exosomes by astrocytes [22].
Exosomes can also be found in the cerebral spinal fluid (CSF) in both adult [23] and embryonic animals [24]. The presence of exosomes in the embryonic CSF raises the possibility that they might also play a role in normal brain development.
Interestingly, it has been discovered that exosome-mediated interactions between neurons and glia induce neurite outgrowth and neuronal survival [25]. Concordantly, Xin et al. showed how exosomes secreted by mesenchymal stem cells (MSCs) are capable of transferring microRNA-133b into neurons, resulting in the induction of neurite outgrowth [26]. Morel et al. showed that neurons secrete microRNA-124a through exosomes: the vesicles are subsequently transported into astrocytes, thereby indirectly increasing GLT1 protein expression [27]. Taken together, these observations support the hypothesis that exosomes mediate cell–cell communication within the CNS.
Given this scenario, it is not surprising that exosomes play an important role in the pathophysiology of neurodegenerative diseases. The use of in vitro models allowed Rajendran et al. to describe the mechanism through which Aβ peptides (responsible for the formation of β-amyloid plaques in the brain of Alzheimer's disease (AD) patients) are secreted within exosomes [28]. Additionally, in vitro studies showed that several key members of the secretase family of proteases—involved in the progression of AD—are also found in exosomes [29, 30]. Furthermore, the cytoskeletal protein tau, another important player in AD, has been found in exosomes as well [31].
In 2012, Perez-Gonzalez et al. published the first in vivo evidence of exosome-mediated secretion of amyloid precursor protein carboxyl-terminal fragments from brain cells into the brain extracellular space, evidence that supports the concept of an important physiological role of exosomes in the brain and in AD [32].
The group of Emmanouilidou et al. discovered that also α-synuclein (a central player in the pathogenesis of Parkinson's disease) is secreted in a calcium-dependent manner by exosomes, suggesting a role of the vesicle also in this neurodegenerative disease [33].
Recently, a study reported that exosomes are implicated in brain malignancies: glioblastoma cells are capable of secreting exosomes loaded with mRNAs and microRNAs that are utilized by the recipient tumor cells to contribute to tumor-proliferation and angiogenesis [34]. Moreover, hypoxic conditions are capable of triggering a pro-angiogenic pathway that involves exosome secretion in glioblastoma [35].
We therefore propose that the role of exosomes is of great importance in the biology of CNS diseases, but the question remains whether we can exploit our knowledge about these vesicles to treat CNS diseases like AD, brain tumors, or brain damage.
Exosomes for Drug Delivery
Targeting drugs to specific cell types has been a major challenge for scientists during the last decades. The necessity for specific delivery vesicles arises from the need to obtain a selective effect of the drug on the tissue/cell-type of interest without altering the normal physiology of other cell types or tissues. Thus, for a proper application in vivo, the aim of pioneer research groups has been to develop transport systems that allow drugs to selectively pass biological barriers. With the boom of the small-interfering RNA (siRNA) technology that theoretically allows the modulation of the expression of almost any disease-related gene, the demand of a tailored delivery became even greater. But, despite the intensive research, the practical answers are still insufficient. The number of theoretical possibilities dramatically decreases when we consider the available drug delivery systems directed to the brain.
In fact, since the blood–brain barrier (BBB) isolates the CNS to a certain extent, creating an exclusive biochemical and immunological niche, the delivery of drugs to the brain is a troublesome challenge.
To date, there are a few validated non-invasive methods that allow therapeutic molecules (siRNA or more conventional drugs) to reach the CNS. The use of endogenous transport systems allows the therapeutic drug to cross the BBB through (direct or indirect) binding to specific carrier receptors. This can be achieved by conjugating receptor-directed antibodies e.g., a monoclonal antibody directed against the transferrin receptor [36] entire carrier proteins or chemical groups directly to the drug or to drug-loaded liposomes, small lipidic particles synthetically derived [37]. Although strong optimism has been demonstrated for the pre-clinical success of this approach, it has been shown that the use of liposomes leads to adverse immunogenic reactions [38].
In this field, craving for new solutions, the paper published in 2011 by Alvarez-Erviti et al. provides the proof-of-concept for the employment of exosomes to deliver siRNA to the mouse brain [39]. The authors exploited autologous murine dendritic cells to produce exosomes. In order to achieve specific targeting, they engineered the dendritic cells to express the exosomal membrane protein Lamp2b fused to the neuron-specific rabies viral glycoprotein peptide. With the use of electroporation, they loaded exosomes with glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-directed siRNA and injected the modified vesicles intravenously. The authors were able to observe a specific gene knockdown of GAPDH in neurons, microglia, and oligodendrocytes in the brain. To prove the therapeutic potential of the exosome-based delivery system, they loaded the vesicles with siRNA against beta-site APP cleaving enzyme 1 (BACE1), a protein with an important role in the pathology of AD, and achieved a specific and efficient down-regulation of the BACE1 protein. Last but not least, the authors provide an initial indication that engineered exosomes might exhibit a low immunogenicity, since the serum level of pro-inflammatory cytokines in vivo did not increase by the therapy. In conclusion, the work of Alvarez-Erviti et al. demonstrates not only that exosomes could be used as an efficient delivery tool that is capable of crossing the BBB via a non-invasive route; they also showed that these particles do not induce an immune response, an obstacle that dampened the development of other delivery vectors, such as liposomes[40].
In view of the fact that that siRNA can be transferred to exosomes to achieve a cell-specific gene knockdown, the next question is: are these vesicles capable of ferrying a different cargo, such as anti-inflammatory or anti-cancer drugs?
Sun et al. demonstrated that cancer cell-line derived-exosomes can encapsulate curcumin, a drug exhibiting anti-inflammatory activity [41]. Curcumin, due to its insolubility in aqueous solution and relatively low stability, has a low systemic bioavailability and this constitutes a barrier for its clinical application. Interestingly, Sun et al. showed that the incorporation of curcumin into exosomes increases the solubility, the stability, and the bioavailability of the drug. Subsequently, they showed through in vitro experiments how the curcumin loaded exosomes (Exo-cur) are taken up by RAW 264.7 cells, an immortalized macrophage cell line. Furthermore, to prove that Exo-cur can effectively exert the anti-inflammatory activity by accumulating in cellular targets, they provided in vitro evidence that Exo-cur is capable of inhibiting the inflammatory activity of RAW 264.7 cells upon stimulation with LPS. Consistently, they showed that in an LPS-induced septic shock murine model, Exo-cur is taken up by pro-inflammatory circulating macrophages to shift the immune response to an anti-inflammatory M2 phenotype [41].
Exosomes incorporate, stabilize, and deliver curcumin to target cells (macrophages), but is this concept applicable to the delivery of drugs to the CNS? The same group tried to solve the issue: Sun et al. showed how the Exo-cur can be rapidly transported to the mouse brain after intranasal administration, with a very low accumulation of the vesicles in stomach and intestine: the exosomal vesicles transport curcumin to the brain by entering the CNS along the olfactory route, thus allowing the drug to reach the brain in minutes. Sun et al. assessed that exosomes are taken up by microglial (60 %) as well as non-microglial cells (i.e., neuronal cells and astrocytes) (40 %) throughout the whole brain consistently with the data previously published by Sun et al. [41]. Therefore, we can conclude that exosomes cross the BBB, diffuse throughout the brain, and deliver the drugs locally. To verify the real therapeutic efficacy of this approach, Zhuang et al. continued their work showing the effectiveness of drug-loaded exosomes. They first showed how intranasally injected Exo-cur is capable of inhibiting brain inflammation and autoimmune responses in a model of experimental autoimmune encephalomyelitis. Moreover, they demonstrated how the intranasal delivered exosomes—loaded with the Stat3 inhibitor JSI-124—inhibit the growth of GL26 tumor cells in a xenograft model of glioblastoma [42].
If development of exosomes as carrier system will be pursued, it could generate a valuable strategy for treatment of CNS diseases that were previously considered as untreatable.
Exosomes and Brain Regeneration: An Alternative to Cell-Based Therapies?
MSC-based therapies are currently being developed to efficiently regenerate tissues damaged by various pathological conditions, such as renal fibrosis [43], lung injuries [44], nucleus pulposus degeneration [45], infarct-induced heart injury [46] and, interestingly, a number of brain pathologies. Promising pre-clinical studies, performed in murine models, show the efficacy of this approach both in the treatment of adult [47] and neonatal brain injuries [48, 49]. The capability of these cells to induce regeneration of the injured tissue makes them an attractive candidate for clinical trials: MSCs are not only an effective way to induce brain repair, but they can be collected in an autologous fashion from the bone marrow of the patient, reducing the risk of inducing an allogeneic immune response. Moreover, MSCs immunogenicity is intrinsically low since they do not express HLA-DR antigens. However, as every cell-based therapy, MSC treatments are still to be proven safe (i.e., risk of carcinogenesis).
The concept has recently emerged that MSCs exert their regenerative effect via exosomes. In favor of this theory, recent in vitro and in vivo studies have reported beneficial effects of MSC-derived exosomes (MSC-Exo). MSC-Exo has been shown to have a beneficial effect on damaged proximal tubular epithelial cell, via transfer of the mRNA encoding the insulin-like growth factor-1 receptor [50].
Lai et al. performed a study in a mouse model of myocardial ischemia/reperfusion injury, demonstrating how intravenous injections of MSC-Exo, isolated from human embryo-derived mesenchymal stem cells immortalized with c-Myc, significantly reduced the infarct size after ischemia/reperfusion [51]. In another study, Lee et al. demonstrated that MSC-Exo are capable of suppressing the hypoxic pulmonary influx of macrophages and the induction of a pro-inflammatory response in a murine model of hypoxic pulmonary hypertension [52].
Since the efficacy of MSCs in treating the most disparate and diverse diseases points at the existence of an underlying common mechanism such as tissue regeneration, these findings together suggest that MSC-Exo could as well represent the regenerative potential of MSCs to boost endogenous repair mechanisms in models of brain damage [48, 49].
Supporting this hypothesis, Xin et al. demonstrated that MSC-Exo secreted by MSCs that have been exposed to brain extracts of rats subjected to middle cerebral artery occlusion (MCAo) are capable to induce neurite outgrowth in neural cells. The authors continued by showing that the effect is mediated by the transfer, via exosomes, of microRNA-133b to neurons. Thus, this work suggests that transfer of exosomes from MSCs to neural cells could be one of the pathways on how MSCs regenerate the brain after damage. It is important to notice that the transfer of microRNA-133b from MSCs to neural cells is induced only after stimulation of MSCs with MCAo brain extracts [26].
Hence, the cargo of MSC-Exo may change after exposure of MSCs to specific factors. Upon stimulation, the exosome content may vary to include factors that induce a response in the acceptor cells (e.g., neurite outgrowth in the case of neural cells). This suggests that the regulatory state of MSCs at the moment of exosome collection may decide the differential biological activity of the vesicles.
Although Xin et al. focused on the MSC-Exo-mediated transfer of microRNA-133b, it is unlikely that this microRNA is the only molecule responsible for the effect these authors observed. Most probably, not one, but a battery of molecules is capable of triggering neurite outgrowth in the neural cells after the uptake of the vesicles. Here lies the potential of exosomes: with their naturally varying cargo, they can have a more powerful and adaptive effect than an artificial vesicle (i.e., liposomes) loaded with a given limited amount of factors. It is not clear yet if other brain cell types than neurons are effectively targeted by MSC-Exo, since the study took only neurons and astrocytes into account.
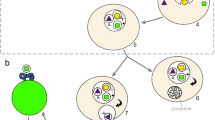
In the context of brain damage, it is unlikely that MSCs stimulate adult neurons in the CNS to induce regeneration, since these mature and differentiated cells can no longer proliferate and thus will not be capable of reconstituting the damaged area. Moreover, following a brain insult, affected neurons die in a short time: these cells do not survive long enough to be the potential recipients of MSC-Exo, since MSCs are administered days after the neuronal death occurs [48, 49]. For these reasons, it is more logical that MSCs, perhaps through exosomes, activate the endogenous neural stem cells (NSCs). NSCs are resident cells in the subventricular zone of the mammalian brain throughout life [53]. Not only do these cells have the potential to replace the damaged cells but also, it has been shown that they also participate in the endogenous regenerative response following brain damage [54, 55]. Hypothetically, MSCs-Exo could be delivered intranasally to mediate the repair of the damaged region [42]. If this hypothesis will be proven true, it would imply a revolution in the field of regenerative medicine. In fact, exosomes can be easily derived using established culturing protocols and isolated through high-throughput techniques in an industrial manner [56]. Due to lack of immunogenicity [42], exosomes could be used in an allogeneic way, consistently speeding up the treatment process, with a possible great improvement in clinical outcome. We propose that delivering exosomes through the intranasal route might be the most optimal route to administer the vesicles to treat cerebral pathologies as we have also shown for MSC in models of brain damage [48, 49] (Fig. 1).
Exosomes as therapeutics. a Exosomes are purified from the supernatant of cultured MSCs and administered via the intranasal route. Naive exosomes fuse with neural stem cell (NSC) thereby inducing differentiation of NSC possibly leading to neuroregeneration. b Exosomes purified from the supernatant of dendritic cells (DCs) or other cell types can be loaded with drugs or siRNAs via incubation or electroporation respectively. The modified exosomes can be administered intranasally to treat pathologies such as brain tumors, Alzheimer's disease (AD) or Parkinson's disease (PD)
Exosomes could be derived from immortalized human embryonic stem cells-derived mesenchymal stem cells, a possibility that has been already explored [51]. These cells can undergo more divisions than normal MSCs, allowing the production of exosomes on a larger scale [56]. However, in order to avoid ethical problems, the use of non-embryonic stem cells to obtain immortalized MSCs may be preferable, such as induced pluripotent stem cells-derived MSCs.
Moreover, MSCs could be stimulated with specific growth and differentiation factors to change the content of MSC-Exo in order to improve beneficial effect, although these factors have still to be identified.
Additionally, MSCs-Exo could be modified in order to enhance their native efficacy and to achieve a specific targeted delivery. For example, to boost white matter regeneration, MSCs could be engineered to express microRNAs that will trigger NSCs differentiation towards the oligodendrocytic lineage [57]. These microRNAs might be taken up by exosomes, enhancing their regenerative properties [26]. To achieve tailored delivery, one way could be to engineer the expression of specific proteins (e.g., antibodies, ligands) on the surface of the exosomes, recognized by receptors selectively expressed on the surface of the target cells. For example, a fusion protein obtained by cloning the gene of an exosomal surface protein in frame with a protein that binds a neuron-specific receptor could increase the affinity for the exosomes towards neurons.
Conclusion
The potential of exosomes in treating CNS diseases still has to be proven, but an increasing number of high-quality studies on the subject suggest that in the near future, these vesicles may represent a powerful tool. In fact, exosomes may be capable of surpassing the limits of the current conventional drugs to treat brain pathology. These vesicles could become a valuable tool for those conditions with limited treatment options, like neonatal encephalopathy or stroke, conditions that have already been proven to benefit from MSC treatment. Exosomes derived from allogeneic sources could be administered “from the shelf” via the intranasal route right away after the insult has been diagnosed. Additionally, the possibility to engineer exosomes suggests how their therapeutic strength could grow exponentially within a few years. However, the clinical safety of exosomes should be fully investigated before being translated to the clinic; the content of exosomes remains to be characterized to allow the inclusion/depletion of factors from exosomes in order to achieve the optimal therapeutic effect. Using proteomics combined with microRNA profiling of these exosomes can lead us to highlight which factors play a role in the therapeutic effect and which molecules could be potentially harmful (i.e., increasing the risk of malignancies).
The development of novel techniques to study these vesicles in relationship to the CNS could accelerate the evolution of exosomes as a clinical weapon. For example, an efficient system to track exosomes in vivo could help us to learn more about the final destination of these vesicles, thus providing insight on their cellular targets. Anyhow a lot can be done with the available tools, in particular, to answer a number of important questions: is the source of exosomes an important factor to consider (the use of cancer cell-line derived exosomes could be hazardous, since the vesicles may carry endogenous tumor molecules that may induce malignancies)? Which method should be used to obtain drug incorporation? Which diseases can be effectively treated with naïve or engineered exosomes?
Certainly, the escalating interest in these particles will lead the scientific community to boost the generation of knowledge about the basic mechanism and the therapeutic applications of these tiny powerful vesicles.
References
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579
Mittelbrunn M, Sanchez-Madrid F (2012) Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol 13:328–335
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659
Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593
Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C (1997) Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell 8:2631–2645
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4:594–600
Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS (2007) Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110:3234–3244
Pan BT, Teng K, Wu C, Adam M, Johnstone RM (1985) Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101:942–948
Peters PJ, Geuze HJ, Van der Donk HA, Slot JW, Griffith JM, Stam NJ, Clevers HC, Borst J (1989) Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. Eur J Immunol 19:1469–1475
Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C (2002) TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol 168:3235–3241
Rialland P, Lankar D, Raposo G, Bonnerot C, Hubert P (2006) BCR-bound antigen is targeted to exosomes in human follicular lymphoma B-cells. Biol Cell 98:491–501
Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1999) Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 147:599–610
Yu X, Harris SL, Levine AJ (2006) The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res 66:4795–4801
Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, Wang R, Terrian DM (2008) Senescence-associated exosome release from human prostate cancer cells. Cancer Res 68:7864–7871
Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, Vidal M, Amson R, Telerman A (2008) Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ 15:1723–1733
Bobrie A, Colombo M, Raposo G, Thery C (2011) Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 12:1659–1668
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della MP, Menard S, Filipazzi P, Rivoltini L, Tagliabue E, Pupa SM (2012) Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol 227:658–667
Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18(6):883–891
Garnier D, Magnus N, Lee TH, Bentley V, Meehan B, Milsom C, Montermini L, Kislinger T, Rak J (2012) Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem 287:43565–43572
Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL (2012) Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 151:1542–1556
Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R (2006) Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 31:642–648
Taylor DD, Gercel-Taylor C (2011) Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol 33:441–454
Vella LJ, Greenwood DL, Cappai R, Scheerlinck JP, Hill AF (2008) Enrichment of prion protein in exosomes derived from ovine cerebral spinal fluid. Vet Immunol Immunopathol 124:385–393
Bachy I, Kozyraki R, Wassef M (2008) The particles of the embryonic cerebrospinal fluid: how could they influence brain development? Brain Res Bull 75:289–294
Wang S, Cesca F, Loers G, Schweizer M, Buck F, Benfenati F, Schachner M, Kleene R (2011) Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci 31:7275–7290
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30:1556–1564
Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, Rothstein J, Yang Y (2013) Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem 288:7105–7116
Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K (2006) Alzheimer's disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A 103:11172–11177
Sharples RA, Vella LJ, Nisbet RM, Naylor R, Perez K, Barnham KJ, Masters CL, Hill AF (2008) Inhibition of gamma-secretase causes increased secretion of amyloid precursor protein C-terminal fragments in association with exosomes. FASEB J 22:1469–1478
Bulloj A, Leal MC, Xu H, Castano EM, Morelli L (2010) Insulin-degrading enzyme sorting in exosomes: a secretory pathway for a key brain amyloid-beta degrading protease. J Alzheimers Dis 19:79–95
Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC, Alvarez VE, Lee NC, Hall GF (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287:3842–3849
Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E (2012) The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem 287:43108–43115
Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30:6838–6851
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476
Svensson KJ, Kucharzewska P, Christianson HC, Skold S, Lofstedt T, Johansson MC, Morgelin M, Bengzon J, Ruf W, Belting M (2011) Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci U S A 108:13147–13152
Huwyler J, Wu D, Pardridge WM (1996) Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci U S A 93:14164–14169
Zhang Y, Zhang YF, Bryant J, Charles A, Boado RJ, Pardridge WM (2004) Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin Cancer Res 10:3667–3677
de Boer AG, Gaillard PJ (2007) Strategies to improve drug delivery across the blood–brain barrier. Clin Pharmacokinet 46:553–576
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345
Stewart MJ, Plautz GE, Del Buono L, Yang ZY, Xu L, Gao X, Huang L, Nabel EG, Nabel GJ (1992) Gene transfer in vivo with DNA-liposome complexes: safety and acute toxicity in mice. Hum Gene Ther 3:267–275
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18:1606–1614
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19:1769–1779
Alfarano C, Roubeix C, Chaaya R, Ceccaldi C, Calise D, Mias C, Cussac D, Bascands JL, Parini A (2012) Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transplant 21(9):2009–2019
Yagi H, Soto-Gutierrez A, Kitagawa Y, Tilles AW, Tompkins RG, Yarmush ML (2010) Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant 19:823–830
Yang F, Leung VY, Luk KD, Chan D, Cheung KM (2009) Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol Ther 17:1959–1966
Pittenger MF, Martin BJ (2004) Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res 95:9–20
Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M (2002) Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology 59:514–523
van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ (2010) Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J Neurosci 30:9603–9611
Donega V, van Velthoven CT, Nijboer CH, van Bel F, Kas MJ, Kavelaars A, Heijnen CJ (2013) Intranasal mesenchymal stem cell treatment for neonatal brain damage: long-term cognitive and sensorimotor improvement. PLoS One 8:e51253
Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, Capelli C, Introna M, Remuzzi G, Benigni A (2013) Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev 22:772–780
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK (2010) Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4:214–222
Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S (2012) Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126:2601–2611
Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710
Zhang RL, Zhang ZG, Zhang L, Chopp M (2001) Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience 105:33–41
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963–970
Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo AB, Padmanabhan J, Lee CN, de Kleijn DP, Lim SK (2011) Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med 9:47
Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Mi QS, Xin M, Wang F, Appel B, Lu QR (2010) MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 65:612–626
Acknowledgment
This study was supported in part by EU-7 Neurobid (HEALTH-F2-2009-241778) from the European Union.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Braccioli, L., van Velthoven, C. & Heijnen, C.J. Exosomes: A New Weapon to Treat the Central Nervous System. Mol Neurobiol 49, 113–119 (2014). https://doi.org/10.1007/s12035-013-8504-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8504-9