Abstract
Glia are key players in a number of nervous system disorders. Besides releasing glial and neuronal signaling molecules directed to cellular homeostasis, glia respond also to pro-inflammatory signals released from immune-related cells, with the mast cell being of particular interest. A proposed mast cell–glia communication may open new perspectives for designing therapies to target neuroinflammation by differentially modulating activation of non-neuronal cells normally controlling neuronal sensitization—both peripherally and centrally. Mast cells and glia possess endogenous homeostatic mechanisms/molecules that can be upregulated as a result of tissue damage or stimulation of inflammatory responses. Such molecules include the N-acylethanolamines, whose principal family members are the endocannabinoid N-arachidonoylethanolamine (anandamide), and its congeners N-stearoylethanolamine, N-oleoylethanolamine, and N-palmitoylethanolamine (PEA). A key role of PEA may be to maintain cellular homeostasis when faced with external stressors provoking, for example, inflammation: PEA is produced and hydrolyzed by microglia, it downmodulates mast cell activation, it increases in glutamate-treated neocortical neurons ex vivo and in injured cortex, and PEA levels increase in the spinal cord of mice with chronic relapsing experimental allergic encephalomyelitis. Applied exogenously, PEA has proven efficacious in mast cell-mediated experimental models of acute and neurogenic inflammation. This fatty acid amide possesses also neuroprotective effects, for example, in a model of spinal cord trauma, in a delayed post-glutamate paradigm of excitotoxic death, and against amyloid β-peptide-induced learning and memory impairment in mice. These actions may be mediated by PEA acting through “receptor pleiotropism,” i.e., both direct and indirect interactions of PEA with different receptor targets, e.g., cannabinoid CB2 and peroxisome proliferator-activated receptor-alpha.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is fundamentally a protective cellular response aimed at removing injurious stimuli and initiating the healing process. Yet, prolonged inflammation, known as chronic inflammation, goes beyond physiological control, and eventually destructive effects override the beneficial effects. We have now come to appreciate persistent inflammation as an underlying contributor to virtually every chronic disease, including neuropathic pain. Recent studies highlight the view that chronic inflammation in the central nervous system (CNS) is also a hallmark of various neurodegenerative disorders in which progressive loss of structure and function of neurons and neuronal cell death are observed [1–5]. For example, the concentration of nitrite, a metabolite of nitric oxide, increases in the cerebrospinal fluid of patients with Parkinson's disease (PD) and Alzheimer's disease (AD) in comparison with age-matched controls [6]. Consistently, the ablation of inducible nitric oxide synthase (iNOS) in mutant mice significantly protects dopaminergic neurons from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity, indicating that iNOS is essential in MPTP-induced substantia nigra pars compacta dopaminergic neurodegeneration [7]. A variety of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, eicosanoids, and other immune neurotoxins, are found in either cerebrospinal fluid or affected brain regions of patients with neurodegenerative disorders [8]. Further, nuclear factor-κB (NF-κB), a transcription factor required for the transcription of most pro-inflammatory molecules, is activated in the substantia nigra pars compacta of PD patients and MPTP-intoxicated mice and monkeys, and selective inhibition of NF-κB in mice and monkeys by NF-κB essential modifier-binding domain peptides protects dopaminergic neurons from MPTP toxicity [9, 10]. Therefore, inflammation is an important target for neuronal protection in neurodegenerative disorders and neuropathic pain, the latter which typically develops when peripheral nerves are damaged due to surgery, bone compression in cancer, diabetes, or infection. Intriguingly, neuroinflammation may also raise the brain's sensitivity to stress [11–13].
A fundamental advance in neuroscience research has been the understanding that an extensive communication exists between the immune system and the CNS. Pro-inflammatory cytokines occupy a key role in this communication, as they regulate host responses to infection, inflammation, and reactions to stress or trauma. Activation of glial cells (microglia and astroglia) has been implicated in the pathogenesis of a variety of neurodegenerative diseases, including AD, PD, Creutzfeldt–Jacob disease, HIV-associated dementia, stroke, multiple sclerosis [2, 9, 14–16], and amyotrophic lateral sclerosis [17]—and may even contribute to schizophrenia, depression, and other psychiatric disorders [18, 19]. Microglia-mediated neuroinflammatory processes are thought to be implicated in brain aging as well [20]. Glia provide a link between neuroinflammation and neuropathic pain [21]. Activated microglia and astroglia accumulate at sites of injury or plaques in neurodegenerative CNS diseases [14, 22]. While the former scavenges dead cells from the CNS and secretes neurotrophic factors to promote neuronal cell survival, and the latter may have important beneficial effects in the recovery of injured CNS by actively monitoring and controlling the extracellular water, pH, and ion homeostasis. Inappropriate and prolonged activation of these glia cause various autoimmune responses leading to brain injury and neuronal cell death [2, 14, 22]. During activation, microglia and astroglia express various genes related to inflammation, such as pro-inflammatory cytokines, enzymes, and adhesion molecules. Clearly, characterization of signaling pathways required for the activation of glial cells is an active area of investigation because compounds capable of antagonizing such signaling steps may have therapeutic benefit in neurodegenerative disorders and neuropathic pain.
Glia can respond to pro-inflammatory signals released from cells of immune origin, which includes mast cells. These effector cells of the innate immune system derive from a distinct precursor in the bone marrow [23]. Mast cells are commonly found at sites in close contact with the external environment such as the gastrointestinal tract and airways, and are distributed in virtually all organs and vascularized tissues [24]. Like macrophages, they reside in the brains of many species, where they enter during development via penetrating blood vessels with which they remain associated [25]. Mast cells can move through normal blood–brain barrier [26], but may also cross the blood–spinal cord and blood–brain barriers when the barrier is compromised as a result of CNS pathology. Mast cells participate in innate host defense reactions, and are found in peripheral tissues innervated by small caliber sensory nerve fibers and within the endoneurial compartment, where they orchestrate inflammatory processes. This last point is important, as systemic inflammation can give rise to signals that communicate with the brain and lead to changes in metabolism and behavior, including the expression of a pro-inflammatory phenotype by microglia [27, 28].
Mast cells produce a wide spectrum of mediators, including (but not limited to) biogenic amines such as histamine and serotonin, cytokines (IL-1β and TNF-α in particular), enzymes, lipid metabolites, ATP, neuropeptides, nerve growth factor (NGF), and heparin [29]. In addition to rapid mediator release via degranulation, longer-term activation results in the release of de novo formed mediators [30]. In terms of their immune regulatory role, mast cells release chemoattractants that recruit eosinophils [31] and monocytes [32]. Nervous system mast cells may play a role in the pathogenesis of the experimental autoimmune demyelinating diseases, experimental allergic neuritis, and experimental allergic encephalomyelitis [33]; are degranulated in the brain of rats with experimental allergic encephalomyelitis [34]; and are associated with multiple sclerosis lesions [35]. Mast cell tryptase is elevated in the cerebrospinal fluid of patients with multiple sclerosis [36]. Mast cells can be activated by myelin [37] and myelin basic protein [38], and activated mast cells cause demyelination [39]. Brain mast cells may also furnish a bridge between the immune system and anxiety-like behavior [40].
Glia, Mast Cells, and Neuropathology
Central neuropathic pain is found in spinal cord injury, multiple sclerosis, and some strokes. Aside from diabetes and other metabolic conditions, the common causes of painful peripheral neuropathies are herpes zoster infection, HIV-related neuropathies, nutritional deficiencies, toxins, remote manifestations of malignancies, immune-mediated disorders, and physical trauma to a nerve trunk. Neuropathic pain is common in cancer as a direct result of cancer on peripheral nerves (e.g., compression by a tumor), or as a side effect of chemotherapy, radiation injury, or surgery. Besides neuronal pathways, glia (Schwann cells, spinal microglia, and astrocytes) and elements of the peripheral immune system participate in triggering and maintaining neuropathic pain states [41, 42]. Inflammation or nerve injury can result, e.g., in the synthesis and release of IL-1β that modulates neuronal cell activity [43]. Microglia express purinergic receptors which act in pain signaling in the spinal cord under pathological conditions [44, 45]. In such settings, dorsal horn microglia become activated and show upregulated expression of purinergic receptors [46, 47]. After nerve injury, mitogen-activated protein kinases are differentially activated in spinal microglia and astrocytes, leading to the synthesis of pro-inflammatory/pro-nociceptive mediators to enhance and prolong pain. Their inhibition can attenuate neuropathic and inflammatory pain in different animal models [48].
Resident peripheral nerve mast cells are the first cells activated at the site of nerve damage, release algogenic mediators upon degranulation [49], and contribute to the recruitment of neutrophils and macrophages [50]. Mast cell degranulation activates trigemino-cervical and lumbosacral pain pathways and elicits widespread tactile pain hypersensitivity [51]. The key mast cell mediator histamine has sensitizing effects on nociceptors [52]. Mast cell degranulation is a principal source of rapidly released NGF, and mast cells respond in a paracrine/autocrine fashion to NGF [53, 54]. This NGF can sensitize nociceptors directly via their trkA receptors, and indirectly via other peripheral cell types [52]. These events promote recruitment of T-cells, which reinforce and maintain inflammatory reactions. Such mediators/factors may either induce activity in axons or are transported retrogradely to cell bodies in the dorsal root ganglia neurons to alter gene expression. Mast cells may also contribute indirectly by enhancing the recruitment of other key immune cell types which, in turn, release pro-nociceptive mediators, e.g., IL-1β and IL-6 [55, 56]. Recent data show that systemic glucocorticoid therapy reduces pain and the number of TNF-α-positive mast cells in rats with chronic constrictive injury [57].
Stroke and trauma result in a prolonged inflammatory response involving microglial activation and infiltration of macrophages and neutrophils, which can ultimately lead to secondary injury [58]. Attenuation of microglial activation has protective value, and there are examples making the case for damage-limiting action [59]. While concerted efforts have been directed to inhibiting the inflammatory cascade of blood-borne neutrophil and phagocyte infiltration in ischemia, very few studies have focused on resident brain cell types able to mount an immediate host response in the brain and meninges—the mast cell. The latter are normally resident in the CNS [60], in close association with cerebral blood vessels during development and adulthood [61]. As with peripheral nerve damage, and in contrast to long-standing belief [62], mast cell activation is the “first responder” in this injury—not microglia [63]. Granted that TNF-α is produced by many cells in response to stimuli, mast cells arrive “armed and ready” to initiate acute inflammation with stores of preformed TNF-α [64]. CNS microglia/macrophages [65] and endothelial cells [66] produce TNF-α as well; however, the presence and release of TNF-α from mast cells preceded its detection in other cells. Inhibition of immediate mast cell activation limits hypoxic-ischemic brain damage [63, 67–70]. Mast cells perform as early responders in the regulation of acute blood–brain barrier changes after cerebral ischemia and hemorrhage [71], through their complement of vasoactive and matrix-degrading components like histamine, and proteases capable of activating matrix metalloproteinases. Cerebral mast cells can regulate acute microvascular gelatinase (matrix metalloproteinases-2 and −9) activation and consequent blood–brain barrier disruption following transient cerebral ischemia [72].
In spite of the capability of activated microglia to damage neurons, oligodendrocytes, or extracellular matrix molecules, there is another side to the coin. For example, depletion or blockade of microglia prevents disease progression in demyelinating disorders [73], yet microglial paralysis inhibits the development/maintenance of inflammatory CNS lesions in toxin-induced models of de- and re-myelination [74]. Microglia may support myelin regeneration by phagocytic removal of obstructive myelin debris [75] or through activation and recruitment of endogenous oligodendrocyte precursor cells to the lesion site [76]. Furthermore, genetically modified mice which lack mast cells are resistant to myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (EAE) [77]; reconstitution of these animals with normal bone marrow-derived mast cells restores susceptibility to EAE induction [78]. Using mast cell transplantation and genetic mutations, Bennett et al. [79] showed that while bone marrow-derived mast cells are actively recruited to the CNS during EAE, the disease developed unabated in the complete absence of mast cells or bone marrow-derived mast cell reconstitution.
A microglia role in cerebral amyloidosis/AD pathogenesis remains a matter of discussion [80]. Although microglia can be found alongside amyloid deposits [81] and their suppression is reported to be beneficial in mouse model of AD [82], a marked reduction or a virtually complete ablation of resident microglia (including bone marrow-derived microglia) failed to alter amyloid plaque load in two distinct transgenic AD mouse models [83]. The case for brain ischemia is also complex, as microglia produce cytotoxic molecules, as well as growth and repair factors [59]. In mice in which microglia have been ablated, a transient ischemic insult produces a larger infarct compared to normal mice [84], while injection of microglia into the bloodstream of Mongolian gerbils (which home to an ischemic hippocampal lesion) resulted in greater neuron survival [85]. Microglia may also protect hippocampal neurons from excitotoxicity [86]. Microglia have been suggested to play a critical role in developmental synaptic pruning, either promoting synapse development and plasticity during the early postnatal period [87] or triggering long-lasting impairment of adult neurogenesis [88].
Glia and Mast Cells: A Common Ground?
A growing body of evidence points to the potential for contact between microglia and mast cells. These include the Toll-like receptors (TLRs), especially TLR-2 and TLR-4 (upregulation of cytokine/chemokine release and recruitment of immune cells to site of injury); purinergic (ATP) P2 receptors (e.g., IL-33 from microglia binds to its receptor on mast cells and induces secretion of IL-6, IL-13, and monocyte chemoattractant protein 1 which in turn can modulate microglia activity); proteinase-activated receptor 2 (PAR2; mast cell tryptase cleaves/activates PAR2 on microglia, resulting in P2X4 receptor upregulation and brain-derived neurotrophic factor release, while IL-6 and TNF-α from microglia upregulate mast cell expression of PAR2, resulting in mast cell activation and TNF-α release); C5a receptor (C5aR; microglial C5aR is upregulated upon activation, C5a peptide is released in neuroinflammation, and there is crosstalk between C5a and TLR4; mast cell C5aR is upregulated upon activation, and C5aR is a strong mast cell chemoattractant signal towards C5a peptide; there is also crosstalk between C5a and TLR4). These points are discussed in greater detail elsewhere [89]. Interestingly, mast cells and astrocytes may also interact. For example, astrocytes have receptors for histamine [90], and astrocyte-derived cytokines/chemokines trigger mast cell degranulation [91]. Co-culturing mouse bone marrow mast cells with cortical astrocytes leads to autocrine/paracrine actions, with release of histamine and leukotrienes [92]; mast cells and astrocytes display enhanced surface expression of CD40L and CD40, respectively, whose cross-talk results in production of inflammatory cytokines [93].
N-Palmitoylethanolamine: A Broad-Acting Anti-inflammatory and Neuroprotective Lipid Mediator
Pharmacological attenuation of microglial and mast cell activation is emerging as promising avenue for neuropathic pain [94]. Chemical genetics of neuroinflammation has been used to identify natural and synthetic compounds as microglial inhibitors in vivo [95], while the established mast cell degranulation stabilizer sodium cromoglycate suppresses hyperalgesia induced by nerve injury and postoperative pain [49, 50, 96]. Apart from neuropathic pain, detrimental effects of neuroinflammation have been noted in association with psychiatric and neurodegenerative diseases. Within this context, much attention has been directed to therapeutic strategies aimed at inhibiting neurotoxic glial cell activation [97].
We now know of the existence of molecules involved in endogenous protective mechanisms activated in the body as a result of different types of tissue damage or stimulation of inflammatory responses and nociceptive fibers. In this context, consider the N-acylethanolamines, a class of naturally occurring lipidic mediators composed of a fatty acid and ethanolamine, namely the fatty acid ethanolamines (FAEs). The main FAE family members comprise the endocannabinoid N-arachidonoylethanolamine (anandamide or 5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-tetraenamide), and its congeners N-stearoylethanolamine (N-(2-hydroxyethyl)stearamide), N-oleoylethanolamine (N-2-hydroxyethyl-9(Z)-octadecenamide), and N-palmitoylethanolamine (PEA or palmitoylethanolamide) (N-(2-hydroxyethyl)hexadecanamide) [98]. PEA (Fig. 1) is abundant in the mammalian brain, and PEA, as well as other FAEs, occurs also in marine species such as bivalve molluscs [99] and sea urchin ovaries [100]. Moreover, PEA has been detected in the CNS of the leech Hirudo medicinalis [101]. PEA is produced within the lipid bilayer via on-demand synthesis, where N-phosphatidylethanolamine-specific phospholipase D (NAPE-PLD) releases it from its membrane precursor, N-palmitoylphosphatidylethanolamine [102].
The first hint that FAEs could have beneficial actions came about when Coburn and Moore in 1943 [103] reported dried chicken egg yolk to have antipyretic properties in children with rheumatic fever. Some 10 years passed before this group identified the lipid fraction from egg yolk as the bioactive fraction [104], and PEA as the active molecule [105]. The therapeutic applications of PEA remained largely overlooked, however, until the realization of its anti-inflammatory [106], analgesic [107], and anticonvulsant [108] properties began to take hold. These past 15 years or so have seen a remarkable rise in the number of studies published on PEA anti-inflammatory actions [109, 110].
PEA is produced and hydrolyzed by microglia [111], it inhibits mast cell activation [112, 113], and its content increases in glutamate-treated neocortical neurons ex vivo [114] and in cortex after CNS injury [115–117], as well as in muscle dialysate of women with chronic neck/shoulder pain [118]. PEA levels are elevated in spinal cord of spastic mice with chronic relapsing experimental allergic encephalomyelitis (an animal model of multiple sclerosis, induced by repeated administration to mice of syngeneic spinal cord homogenate emulsified in Freund's complete adjuvant) [119]. Taken together, these findings propose that a PEA key role may be to maintain cellular homeostasis in the face of external stressors provoking, for example, inflammation. At the same time, there could well be pathological scenarios where PEA endogenous production is inadequate to control the ensuing inflammatory cascade.
Taking the above premise to the next level, orally administered PEA was reported to be efficacious in mast cell-mediated experimental models of immunogenic (passive cutaneous anaphylaxis-induced extravasation of leucocytes) and neurogenic (subcutaneous injection of substance P) inflammation, as well as carrageenan or dextran- and formalin-induced hindpaw edema in rats [106, 120]. PEA reduced pain elicited by subcutaneous formalin injection [107, 121, 122], and was effective even when administered after induction of the acute inflammation event [123]. This formalin model is characterized by a marked microglia and astroglia activation, which is normalized by PEA, together with increased glial expression of the anti-inflammatory cytokine IL-10 in the PEA-treated animals [124]. In the carrageenan-induced paw model of hyperalgesia, intracerebroventricular administration of PEA 30 min before carrageenan injection markedly reduced mechanical hyperalgesia up to 24 h following the inflammatory insult [125]. In a rat model of chronic granulomatous inflammation, locally administered PEA reduced mast cell degranulation and the expression/release of NGF, prevented nerve fiber formation and sprouting, reduced mechanical allodynia, and inhibited sensory ganglia activation [126]. Of note, PEA has anti-inflammatory activity and elicits pain relief in rodent neuropathic pain models, as well [127, 128].
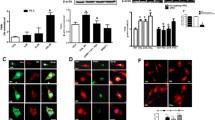
Spinal cord injury in rats is accompanied by alterations in the endocannabinoid system. For example, one finds lesion-induced, early stage increases of anandamide and PEA content together with an upregulation of NAPE-PLD and a downregulation of the degradative enzyme fatty acid amide hydrolase (FAAH), while in delayed stages, 2-arachidonoylglycerol increases [129]. In this injury paradigm, PEA displays also neuroprotective effects. In one study using a mouse compression model of spinal cord trauma (in which an aneurysm clip is applied to the spinal cord to mimic the persistence of cord compression seen in human injury), the systemic administration of PEA 6 and 12 h post-injury induction produced a clear reduction in the severity of spinal cord trauma by limiting mast cell infiltration and activation [130]. Further, PEA reduced the activation of microglia and astrocytes expressing cannabinoid CB2 receptors, and its protective effect appeared to involve changes in neurotrophic factor expression and in spinal cord dopaminergic function. In an earlier study using this experimental model of spinal cord injury, the authors showed that intraperitoneal administration of PEA reduced spinal cord inflammation and tissue injury, neutrophil infiltration, nitrotyrosine formation, pro-inflammatory cytokine expression, NF-κB activation, iNOS expression and apoptosis, and ameliorated recovery of motor limb function [131]. Utilizing a model of mixed neuron-glia cultures from hippocampus, Skaper et al. [38] showed that the introduction of stimulated mast cells precipitated a loss of neurons as a consequence of mast cells releasing TNF-α, thereby triggering astrocyte production of nitric oxide. In this setting, PEA decreased neuron loss resulting from mast cell stimulation in the mixed cultures (Fig. 2), but not that caused by direct cytokine induction of astrocytic nitric oxide synthase [38].
N-Palmitoylethanolamine (PEA) reduces hippocampal neuron death caused by antigen- or myelin basic protein (MBP)-treated mast cells. Mixed neuron–glia cultures were incubated for 12 h with transwell membrane inserts containing 5 × 104 mast cells treated with either anti-dinitrophenol lgE/dinitrophenol-human serum albumin (“antigen”) or 20 μM MBP, alone or together with 30 μM PEA. Hippocampal cell cultures were fixed 60 h after insert removal, and neurofilament-immunopositive (NF+) neurons quantified. Values are means ± SD (four to five experiments). *p < 0.01 or **p < 0.001 compared with the same condition but without PEA. [Modified from Fig. 3 of reference [38], copyright (1996) with permission from Wiley]
In another ex vivo model, these authors showed that PEA was neuroprotective in a delayed post-glutamate paradigm of excitotoxic death [132]. Several very recent reports describe the neuroprotective action of PEA against amyloid β-peptide(25–35)-induced learning and memory impairment in mice [133], or organotypic hippocampal slices challenged with amyloid β-peptide(1–42) [134].
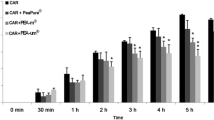
The biochemical and cellular changes that occur following treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine (MPTP) are remarkably similar to those of idiopathic PD. This chronic neurodegenerative disorder is characterized by the loss of dopaminergic nigrostriatal neurons, which leads to disabling motor disturbances. Activation of glial cells and the consequent neuroinflammatory response is increasingly recognized as a prominent neuropathological feature of PD. However, there is currently no evidence for the use of non-steroidal anti-inflammatory drugs in the secondary prevention of PD. Non-aspirin non-steroidal anti-inflammatory drugs, particularly ibuprofen, may reduce the risk (albeit non-significantly) of developing PD. However, little is known of the effects of other individual drugs, and at present, no recommendations can be made regarding their use in primary prevention [135, 136]. Esposito et al. [137] now report that chronic systemic PEA treatment protects against MPTP-induced neurotoxicity, microglial and astrocyte activation, and the ensuing functional deficits even when given after the insult has been initiated. At 8 days after the MPTP injection, wild-type mice exhibited a significant motor dysfunction as indicated by a decrease in the time period on rotarod and by an increased numbers of falls (Fig. 3a, b). Genetic ablation of peroxisome proliferator activated receptor (PPAR)-α alone did not impair performance on the rotarod tests, but its absence did significantly increase the motor dysfunction induced by MPTP. Treatment with PEA in wild-type mice but not in PPAR-α knockout mice reduced the MPTP-induced motor dysfunction (Table 1).
Effect of palmitoylethanolamide (PEA) treatment on motor function assessed by rotarod. At 8 days after the MPTP injection, PPARα wild-type (WT) and PPARα knockout (KO) mice exhibited a significant motor dysfunction as indicated by a decrease in the time period on rotarod and by an increased numbers of falls (a, b). The absence of PPARα gene significantly increased motor dysfunction induced by MPTP. PEA treatment limited motor dysfunction in PPARα WT mice but not in PPARα KO mice. *p < 0.05; **p < 0.01 vs. sham; # p < 0.05; ## p < 0.01 vs. MPTP mice (one-way ANOVA followed by a Bonferroni post-hoc test for multiple comparisons). [Modified from Fig. 2 of reference [137]; this is an open-access article distributed under the terms of the Creative Commons Attribution License]
Stroke is the third leading cause of death and the prime cause of long-term disability in adults. Current therapeutic strategies for stroke, including thrombolytic drugs such as tissue plasminogen activator, are limited in their treatment scope. In a newly published study by Ahmad et al. [138], PEA was administered to mice undergoing middle cerebral artery occlusion. This fatty acid amide reduced edema and lesion size, blocked infiltration of astrocytes, and restored the ischemia-mediated reduced expression of neurotrophic factors such as brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. Moreover, PEA-treated injured animals displayed improved neurobehavioral functions as evaluated by motor deficits.
Traumatic brain injury (TBI) is a major cause of preventable death and morbidity in young adults, and is characterized blood–brain barrier leakage, cerebral ischemia, inflammation, and redox imbalances in the traumatic penumbra of the injured brain. Using a controlled cortical impact in adult mice as a model of TBI (this produces full thickness lesions of the forelimb region of the sensorimotor cortex), PEA treatment was able to reduce edema and brain infarction size [139]. PEA treatment also blocked astrocyte infiltration and inhibited TBI-mediated decreases in expression of activated c-Jun N-terminal kinase and the key pro-inflammatory transcription factor NF-κB. Importantly, PEA-treated injured animals showed improved neurobehavioral functions (Table 1).
Evidence continues to mount pointing to PEA as an endogenous ligand for PPARα, one of a group of nuclear receptor proteins that function as transcription factors regulating the expression of genes. PPARα- and γ-isoforms in particular are associated with pro-inflammatory events. Lo Verme et al. [140] were the first to show, in 2005, that PPARα mediates the anti-inflammatory effects of PEA and suggested that this fatty acid ethanolamine may serve—like its analog oleoylethanolamine—as an endogenous ligand of PPARα. As discussed above, PEA failed to rescue memory deficits induced by amyloid β-peptide(25–35) injection in PPAR-α null mice, while a synthetic PPAR-α agonist mimicked the effect of PEA [133]; the neuroprotective action of PEA in organotypic hippocampal slices challenged with amyloid β-peptide(1–42) was blocked by selective PPARα, but not PPARγ, antagonists [134]; and PEA neuroprotection against the dopaminergic neurotoxin MPTP was in part dependent on PPARα. PEA induced allopregnanolone synthesis in astrocytes in a PPARα-dependent fashion [141], and PPAR-α antagonists reduced the PEA ability to counteract amyloid β-peptide(1–42)-induced reactive gliosis [142]—effects which were absent in PPARα null mice. Other studies have demonstrated that acute intracerebroventricular administration of PEA is able to modulate carrageenan-induced paw edema in mice in a PPARα-dependent fashion [143]. Microinjection of PEA in the ventrolateral periaqueductal grey of male rats reduced the ongoing activity of ON and OFF cells in the rostral ventromedial medulla and produced an increase in the latency of the nociceptive reaction (the periaqueductal grey—rostral ventromedial medulla pathway is a key circuitry in pain processing), effects that were prevented by a selective PPARα antagonist [144].
An “entourage effect hypothesis” has also been put forward to account for the pharmacological actions of PEA. This hypothesis posits that PEA may enhance the anti-inflammatory and anti-nociceptive activity of other endogenous compounds by potentiating their affinity for a receptor or by inhibiting their metabolic degradation [145]. Anandamide is a candidate molecule in this respect, as it possesses anti-inflammatory and anti-nociceptive effects. A possible “contact” point between anandamide and its congeners like PEA is the transient receptor potential vanilloid type 1 (TRPV1) receptor. The TRPV1 receptor, a non-selective cation channel expressed in small diameter sensory neurons, is activated by noxious heat, low pH, and capsaicin. Anandamide is also an agonist for TRPV1 receptors, and PEA enhances anandamide stimulation of the human TRPV1 receptor [146]. The cannabinoid CB2 receptor antagonist SR144528 inhibits some of the analgesic responses to PEA in vivo (although PEA lacks affinity for either CB1 or CB2 receptors) which, conceivably, reflects PEA acting indirectly by potentiating anandamide actions [107]. Mast cells [147] and cortical [148] and spinal cord [149] microglia have all been reported to express TRPV1 receptors. Given the close association of mast cells and microglia in nervous tissue, these findings further strengthen the view that a line of communication exists between these two immune cell types.
The intracellular integral membrane protein FAAH belongs to the amidase family of enzymes, which catalyze the hydrolysis of FAEs into the corresponding fatty acid and ethanolamine [150]. In 2001, Ueda et al. cloned another enzyme that preferentially hydrolyzes PEA [151]. Nominated N-acylethanolamine-hydrolyzing acid amidase (NAAA) is not related to FAAH but bears structural homology to ceramidase and belongs to the family of choloylglycine hydrolases. NAAA is lysosomal in location. Inhibition of PEA degradation thus represents a potential complementary therapeutic approach to treat inflammation, and today is an area of active investigation. A number of selective NAAA inhibitors have been published [152–154], which dampen responses induced by inflammatory stimuli in vivo and in vitro, while at the same time elevating PEA levels in vitro [152]. The most recently identified compound, 1-(2-biphenyl-4-yl)ethyl-carbonyl pyrrolidine, is a reversible and competitive NAAA inhibitor which reduces mRNA expression levels of iNOS and IL-6, while it increases intracellular PEA levels in mouse macrophages with lipopolysaccharide-induced inflammation [155].
Conclusions and Outlook
Inflammatory molecules can profoundly affect a broad range of CNS functions. These effectors derive both from the innate and adaptive immune systems, as well as CNS glia. Microglia, in particular, act as sensors for disturbed brain tissue homeostasis and accumulate locally in response to neuronal injury or entry of foreign material in the brain [156]. In contrast, scarce attention has been directed to resident brain cell types able to mount immediate host responses, namely the mast cell. In addition to their “first responder” action in injury (as opposed to microglia), the longer-lasting activation of mast cells leads to the release of de novo formed mediators. Another key feature of mast cells is their ability to survive and deliver repetitive hits [157].
In human chronic pain, we still lack unequivocal demonstration that glial and mast cell activation occurs in hypersensitized patients. Systematic studies are needed to demonstrate a correlation between the magnitude of glial and/or mast cell markers in the cerebrospinal fluid or in spinal tissue and the intensity of pain in patients.
Therapeutic strategy for neuropathic pain continues to focus on designing drugs to hit neuronal targets and block neurotransmission. Unfortunately, they address pain symptoms but not the underlying pathology of neuropathic pain, and provide no more than transient relief in only a fraction of patients while producing severe CNS side effects. Mast cell stabilizers suppress the development of hyperalgesia but do not touch microglia. On the other side of the coin, glial inhibitors for pain rely in large part on their anti-inflammatory properties, and are burdened with issues such as non-selectivity in targeting one cell population, while risk of either acute or cumulative toxicity could hamper long-term use. Targeting regulators of neuroinflammation may prove to be a more viable therapeutic strategy to affect a diverse array of nervous system disorders. Future studies should investigate the role of mast cells in inflammatory diseases as a network, which requires a critical examination of specific tissue localization, function, and dynamic interaction with endogenous cells.
The capacity of PEA to modulate the protective responses of animals during inflammation and pain led to the hypothesis that endogenous PEA may be a component of the complex homeostatic system controlling the basal threshold of both inflammation and pain. The production of PEA during inflammatory conditions supports this role, and emerging data that selective inhibition of PEA degradation is anti-inflammatory provides more direct evidence for the involvement of PEA in the control of pain and inflammation. As an endogenous compound, PEA has no adverse effects at pharmacological doses, while possessing a double therapeutic effect (i.e., anti-inflammatory and anti-nociceptive).
Although clinical data are only now being to emerge, PEA has been reported to improve myelinated fiber function in patients with chemotherapy-induced painful neuropathy [158], and to reduce neuropathic pain in a patient with multiple sclerosis [159]. Hesselink and Hekker [160] recently presented a case series describing the application and potential efficacy and safety of micronized and ultra-micronized PEA in the treatment of various syndromes associated with chronic pain that is poorly responsive to standard therapies. In addition, nearly 40 clinical trials have been conducted to date, with a total of more than 2,000 patients having been entered in these trials (reviewed in [161]). Lastly, a case study reported on the effects of ultramicronized PEA in sporadic amyotrophic lateral sclerosis, in which treatment led to an improved clinical picture, as evidenced by electromyographic analysis and pulmonary function [162] (Table 1).
It is clear that we still have much to learn about signaling mechanisms that regulate neuroinflammation. PEA, its analogues, and agents that inhibit specifically its degradation are likely to result in the development of new therapeutic strategies for the treatment of pathological conditions where neuroinflammation is a factor.
References
Giovannini MG, Scali C, Prosperi C, Bellucci A, Vannucchi MG, Rosi S, Pepeu G, Casamenti F (2002) β-Amyloid-induced inflammation and cholinergic hypofunction in the rat brain in vivo. Involvement of the p38MAPK pathway. Neurobiol Dis 11:257–274. doi:10.1006/nbdi.2002.0538
Dauer W, Przedborski S (2003) Parkinson disease. Mechanisms and models. Neuron 39:889–909. doi:10.1016/S0896-6273(03)00568-3
Gao HM, Liu B, Zhang W, Hong JS (2003) Novel anti-inflammatory therapy for Parkinson disease. Trends Pharmacol Sci 24:395–401. doi:10.1016/S0165-6147(03)00176-7
Jantaratnotai N, Ryu JK, Kim SU, McLarnon JG (2003) Amyloid β peptide-induced corpus callosum damage and glial activation in vivo. Neuroreport 14:1429–1433. doi:10.1097/01.wnr.0000086097.47480.a0
Barnum CJ, Tansey MG (2010) Modeling neuroinflammatory pathogenesis of Parkinson disease. Prog Brain Res 184:113–132. doi:10.1016/S0079-6123(10)84006-3
Qureshi GA, Baig S, Bednar I, Södersten P, Forsberg G, Siden A (1995) Increased cerebrospinal fluid concentration of nitrite in Parkinson disease. Neuroreport 6:1642–1644
Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB (2000) Deficiency of inducible nitric-oxide synthase protects against MPTP toxicity in vivo. J Neurochem 74:2213–2216. doi:10.1046/j.1471-4159.2000.0742213.x
Nagatsu T, Mogi M, Ichinose H, Togari A (2000) Changes in cytokines and neurotrophins in Parkinson disease. J Neural Transm Suppl 60:277–290
Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K (2007) Selective inhibition of NF-κB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson disease. Proc Natl Acad Sci USA 104:18754–18759. doi:10.1073/pnas.0704908104
Mondal S, Roy A, Jana A, Ghosh S, Kordower JH, Pahan K (2012) Testing NF-κB-based therapy in hemiparkinsonian monkeys. J Neuroimmune Pharmacol 7:544–556. doi:10.1007/s11481-012-9377-9
Skaper SD, Giusti P (2009) P2X7 receptors as a transducer in the co-occurrence of neurological/psychiatric and cardiovascular aisorders: a hypothesis. Cardiovasc Psychiatry Neurol 2009:545263. doi:10.1155/2009/545263
Rivat C, Becker C, Blugeot A, Zeau B, Mauborgne A, Pohl M, Benoliel JJ (2010) Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain 150:358–368. doi:10.1016/j.pain.2010.05.031
Vichaya EG, Young EE, Frazier MA, Cook JL, Welsh CJ, Meagher MW (2011) Social disruption induced priming of CNS inflammatory response to Theiler's virus is dependent upon stress induced IL-6 release. J Neuroimmunol 239:44–52. doi:10.1016/j.jneuroim.2011.08.006
González-Scarano F, Baltuch G (1999) Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci 22:219–240. doi:10.1146/annurev.neuro.22.1.219
Sailasuta N, Harris K, Tran T, Ross B (2011) Minimally invasive biomarker confirms glial activation present in Alzheimer's disease: a preliminary study. Neuropsychiatr Dis Treat 7:495–499. doi:10.2147/NDT.S23721
Barcia C, Ros CM, Annese V, Gómez A, Ros-Bernal F, Aguado-Yera D, Martínez-Pagán ME, de Pablos V, Fernandez-Villalba E, Herrero MT (2011) IFN-γ signaling, with the synergistic contribution of TNF-α, mediates cell specific microglial and astroglial activation in experimental models of Parkinson's disease. Cell Death Dis 2:e142. doi:10.1038/cddis.2011.17
Appel SH, Zhao W, Beers DR, Henkel JS (2011) The microglial-motoneuron dialogue in ALS. Acta Myol 30:4–8
Mitterauer BJ (2011) Possible role of glia in cognitive impairment in schizophrenia. CNS Neurosci Ther 17:333–344. doi:10.1111/j.1755-5949.2009.00113.x
Hinwood M, Morandini J, Day TA, Walker FR (2012) Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex. Cereb Cortex 22:1442–1454
Rosano C, Marsland AL, Gianaros PJ (2012) Maintaining brain health by monitoring inflammatory processes: a mechanism to promote successful aging. Aging Dis 3:16–33
Jha MK, Jeon S, Suk K (2012) Glia as a link between neuroinflammation and neuropathic pain. Immune Netw 12:41–47. doi:10.4110/in.2012.12.2.41
Carson MJ (2002) Microglia as liaisons between the immune and central nervous systems. Functional implications for multiple sclerosis. Glia 40:218–231. doi:10.1002/glia.10145
Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ (2005) Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci USA 102:11408–11413. doi:10.1073/pnas.0504197102
Gilfillan AM, Austin SJ, Metcalfe DD (2011) Mast cell biology: introduction and overview. Adv Exp Med Biol 716:2–12. doi:10.1038/ni1158
Lambracht-Hall M, Dimitriadou V, Theoharides TC (1990) Migration of mast cells in the developing rat brain. Dev Brain Res 56:151–159. doi:10.1016/0165-3806(90)90077-C
Silverman AJ, Sutherland AK, Wilhelm M, Silver R (2000) Mast cells migrate from blood to brain. J Neurosci 20:401–408
Engler H, Doenlen R, Engler A, Riether C, Prager G, Niemi MB, Pacheco-López G, Krügel U, Schedlowski M (2011) Acute amygdaloid response to systemic inflammation. Brain Behav Immun 25:1384–1392. doi:10.1016/j.bbi.2011.04.005
Moreno B, Jukes JP, Vergara-Irigaray N, Errea O, Villoslada P, Perry VH, Newman TA (2011) Systemic inflammation induces axon injury during brain inflammation. Ann Neurol 70:932–942. doi:10.1002/ana.22550
Johnson D, Krenger W (1992) Interactions of mast cells with the nervous system—recent advances. Neurochem Res 17:939–951. doi:10.1007/BF00993271
Galli SJ, Nakae S, Tsai M (2005) Mast cells in the development of adaptive immune responses. Nat Immunol 6:135–142. doi:10.1038/ni1158
Wardlaw AJ, Moqbel R, Cromwell O, Kay AB (1986) Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest 78:1701–1706. doi:10.1172/JCI112765
Perry VH, Andersson P-B, Gordon G (1993) Macrophages and inflammation in the central nervous system. Trends Neurosci 16:268–273. doi:10.1016/0166-2266(93)90180-T
Johnson D, Yasui D, Seeldayers P (1991) An analysis of mast cell frequency in the rodent nervous system: numbers vary between different strains and can be reconstituted in mast cell-deficient mice. J Neuropathol Exp Neurol 50:227–234. doi:10.1097/00005072-19910005-00005
Brenner T, Soffer D, Shalit M, Levi-Schaffer F (1994) Mast cells in experimental allergic encephalomyelitis: characterization, distribution in the CNS and in vitro activation by myelin basic protein and neuropeptides. J Neurol Sci 122:210–213. doi:10.1016/0022-510X(94)90300-X
Theoharides TC (1990) Mast cells: the immune gate to the brain. Life Sci 46:607–617. doi:10.1016/0024-3205(90)90129-F
Rozniecki JJ, Hauser SL, Stein M, Lincoln R, Theoharides TC (1995) Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann Neurol 37:63–66. doi:10.1002/ana.410370112
Medic N, Vita F, Abbate R, Soranzo MR, Pacor S, Fabbretti E, Borelli V, Zabucchi G (2008) Mast cell activation by myelin through scavenger receptor. J Neuroimmunol 200:27–40. doi:10.1016/j.jneuroim.2008.05.019
Skaper SD, Facci L, Romanello S, Leon A (1996) Mast cell activation causes delayed neurodegeneration in mixed hippocampal cultures via the nitric oxide pathway. J Neurochem 66:1157–1166. doi:10.1046/j.1471-4159.1996.66031157.x
Theoharides TC, Baloyannis SJ, Manolidis LS (1991) Activated rat peritoneal mast cells can cause syngeneic brain demyelination in vitro. Int J Immunopathol Pharmacol 4:137–144
Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R (2008) Brain mast cells link the immune system to anxiety-like behavior. Proc Natl Acad Sci USA 105:18053–18057. doi:10.1073/pnas.0809479105
DeLeo JA, Yezierski RP (2001) The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 90:1–6. doi:10.1016/S0304-3959(0)00490-5
Watkins LR, Milligan ED, Maier SF (2001) Spinal cord glia: new players in pain. Pain 93:201–205. doi:10.1016/S0304-3959(01)00359-1
Watkins LR, Milligan ED, Maier SF (2003) Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol 521:1–21
Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K (2008) P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci 28:4949–4956. doi:10.1523/JNEUROSCI.0323-08.2008
Burnstock G, Krügel U, Abbracchio MP, Illes P (2011) Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol 95:229–274. doi:10.1016/j.pneurobio.2011.08.006
Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114:386–396. doi:10.1016/j.pain.2005.01.002
Tsuda M, Kuboyama K, Inoue T, Nagata K, Tozaki-Saitoh H, Inoue K (2009) Behavioral phenotypes of mice lacking purinergic P2X4 receptors in acute and chronic pain assays. Mol Pain 5:28. doi:10.1186/1744-8069-5-28
Ji RR, Gereau RW 4th, Malcangio M, Strichartz GR (2009) MAP kinase and pain. Brain Res Rev 60:135–148. doi:10.1016/j.brainresrev.2008.12.011
Xanthos DN, Gaderer S, Drdla R, Nuro E, Abramova A, Ellmeier W, Sandkühler J (2011) Central nervous system mast cells in peripheral inflammatory nociception. Mol Pain 7:42. doi:10.1186/1744-8069-7-42
Zuo Y, Perkins NM, Tracey DJ, Geczy CL (2003) Inflammation and hyperalgesia induced by nerve injury in the rat: a key role of mast cells. Pain 105:467–479. doi:10.1016/S0304-3959(03)00261-6
Levy D, Kainz V, Burstein R, Strassman AM (2012) Mast cell degranulation distinctly activates trigemino-cervical and lumbosacral pain pathways and elicits widespread tactile pain hypersensitivity. Brain Behav Immun 26:311–317. doi:10.1016/j.bbi.2011.09.016
Koda H, Mizumura K (2002) Sensitization to mechanical stimulation by inflammatory mediators and by mild burn in canine visceral nociceptors in vitro. J Neurophysiol 87:2043–2051. doi:10.1152/jn.00593.2001
Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R (1994) Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci USA 91:3739–3743. doi:10.1073/pnas.91.9.3739
Levi-Montalcini R, Skaper SD, Dal Toso R, Petrelli L, Leon A (1996) Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci 19:514–520. doi:10.1016/S0166-2236(96)10058-8
Vallières L, Rivest S (1997) Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1β. J Neurochem 69:1668–1683. doi:10.1046/j.1471-4159.1997.69041668.x
Leal-Berumen I, Conlon P, Marshall JS (1994) IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J Immunol 152:5468–5476
Hayashi R, Xiao W, Kawamato M, Yuge O, Bennett GJ (2011) Systemic glucocorticoid therapy reduces pain and the number of endoneurial tumor necrosis factor-alpha (TNFα)-positive mast cells in rats with a painful peripheral neuropathy. J Pharmacol Sci 106:559–565. doi:10.1254/jphs.FP0072181
Wang Q, Tang XN, Yenari MA (2007) The inflammatory response in stroke. J Neuroimmunol 184:53–68. doi:10.1016/j.jneuroim.2006.11.014
Hanisch U-K, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394. doi:10.1038/nn1997
Silver R, Silverman A, Vitkovic L, Lederhendler I (1996) Mast cells in the brain: evidence and functional significance. Trends Neurosci 19:25–31. doi:10.1016/0166-2236(96)81863-7
Michaloudi H, Grivas I, Batzios C, Chiotelli M, Papadopoulos G (2003) Parallel development of blood vessels and mast cells in the lateral geniculate nuclei. Brain Res Develop Brain Res 140:269–276. doi:10.1016/S0165-3806(02)00613-2
Chew LJ, Takanohashi A, Bell M (2006) Microglia and inflammation: impact on developmental brain injuries. Ment Retard Dev Disabil Res Rev 12:105–112. doi:10.1002/mrdd.20102
Jin Y, Silverman AJ, Vannucci SJ (2009) Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke 40:3107–3112. doi:10.1161/STROKEAHA.109.549691
Gordon JR, Galli SJ (1991) Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med 174:103–107. doi:10.1084/174.1.103
Gregersen R, Lambertsen K, Finsen B (2000) Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab 20:53–65. doi:10.1097/00004647-200001000-00009
Hallenbeck JM (2002) The many faces of tumor necrosis factor in stroke. Nat Med 8:1363–1368. doi:10.1038/nm1202-1363
Jin Y, Silverman AJ, Vannucci SJ (2007) Mast cell stabilization limits hypoxic-ischemic brain damage in the immature rat. Dev Neurosci 29:373–384. doi:10.1159/000105478
Strbian D, Karjalainen-Lindsberg ML, Tatlisumak T, Lindsberg PJ (2006) Cerebral mast cells regulate early ischemic brain swelling and neutrophil accumulation. J Cereb Blood Flow Metab 26:605–612. doi:10.1038/sj.jcbfm.9600228
Biran V, Cochois V, Karroubi A, Arrang JM, Charriaut-Marlangue C, Heron A (2008) Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol 18:1–9. doi:10.1111/j.1750-3639.2007.00092.x
Lozada A, Maegele M, Stark H, Neugebauer EM, Panula P (2005) Traumatic brain injury results in mast cell increase and changes in regulation of central histamine receptors. Neuropathol Appl Neurobiol 31:150–162. doi:10.1111/j.1365-2990.2004.00622.x
Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML (2010) Mast cells as early responders in the regulation of acute blood–brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab 30:689–702. doi:10.1038/jcbfm.2009.282
Mattila OS, Strbian D, Saksi J, Pikkarainen TO, Rantanen V, Tatlisumak T, Lindsberg PJ (2011) Cerebral mast cells mediate blood–brain barrier disruption in acute experimental ischemic stroke through perivascular gelatinase activation. Stroke 42:3600–3605. doi:10.1161/STROKEAHA.111.632224
Kim SU, de Vellis J (2005) Microglia in health and disease. J Neurosci Res 81:302–313. doi:10.1002/jnr.20562
Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hövelmeyer N, Waisman A, Rülicke T, Prinz M, Priller J, Becher B, Aguzzi A (2005) Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 11:146–152. doi:10.1038/nm1177
Filbin MT (2003) Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci 4:703–713. doi:10.1038/nrn1195
Olah M, Amor S, Brouwer N, Vinet J, Eggen B, Biber K, Boddeke HW (2012) Identification of a microglia phenotype supportive of remyelination. Glia 60:306–321. doi:10.1002/glia.21266
Secor VH, Secor WE, Gutekunst CA, Brown MA (2000) Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med 191:813–822. doi:10.1084/jem.191.5.813
Tanzola MB, Robbie-Ryan M, Gutekunst CA, Brown MA (2003) Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. J Immunol 171:4385–4391
Bennett JL, Blanchet MR, Zhao L, Zbytnuik L, Antignano F, Gold M, Kubes P, McNagny KM (2009) Bone marrow-derived mast cells accumulate in the central nervous system during inflammation but are dispensable for experimental autoimmune encephalomyelitis pathogenesis. J Immunol 182:5507–5514. doi:10.4049/jimmunol.0801485
Wyss-Coray T (2006) Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med 12:1005–1015. doi:10.1038/nm1474
Martín-Moreno AM, Reigada D, Ramírez BG, Mechoulam R, Innamorato N, Cuadrado A, de Ceballos ML (2011) Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer's disease. Mol Pharmacol 79:964–973. doi:10.1124/mol.111.071290
Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, Van Nostrand WE (2007) Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci 27:3057–3063. doi:10.1523/JNEUROSCI.4371-06.2007
Grathwohl SA, Kälin RE, Bolmont T, Prokop S, Winkelmann G, Kaeser SA, Odenthal J, Radde R, Eldh T, Gandy S, Aguzzi A, Staufenbiel M, Mathews PM, Wolburg H, Heppner FL, Jucker M (2009) Formation and maintenance of Alzheimer's disease β-amyloid plaques in the absence of microglia. Nat Neurosci 12:1361–1363. doi:10.1038/nn.2432
Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J (2007) Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci 27:2596–2605. doi:10.1523/JNEUROSCI.5360-06.2007
Imai F, Suzuki H, Oda J, Ninomiya T, Ono K, Sano H, Sawada M (2007) Neuroprotective effect of exogenous microglia in global brain ischemia. J Cereb Blood Flow Metab 27:488–500. doi:10.1038/sj.jcbfm.9600362
Vinet J, van Weering HR, Heinrich A, Kälin RE, Wegner A, Brouwer N, Heppner FL, van Rooijen N, Boddeke HW, Biber K (2012) Neuroprotective function for ramified microglia in hippocampal excitotoxicity. J Neuroinflammation 9:27. doi:10.1186/1742-2094-9-27
Tremblay ME, Lowery RL, Majewska AK (2010) Microglial interactions with synapses are modulated by visual experience. PLoS Biol 8:e1000527. doi:10.1371/journal.pbio.1000527
Lazarini F, Gabellec M-M, Torquet N, Lledo PM (2012) Early activation of microglia triggers long-lasting impairment of adult neurogenesis in the olfactory bulb. J Neurosci 32:3652–3664. doi:10.1523/JNEUROSCI.6394-11.2012
Skaper SD, Giusti P, Facci F (2012) Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J 26:3103–3117. doi:10.1096/fj.11-197194
Hösli L, Hösli E, Schneider U, Wiget W (1984) Evidence for the existence of histamine H1- and H2-receptors on astrocytes of cultured rat central nervous system. Neurosci Lett 48:287–291. doi:10.1016/0304-3940(84)90052-1
Dong Y, Benveniste EN (2001) Immune function of astrocytes. Glia 36:180–190. doi:10.1002/glia.1107
Kim DY, Jeoung D, Ro JY (2010) Signaling pathways in the activation of mast cells cocultured with astrocytes and colocalization of both cells in experimental allergic encephalomyelitis. J Immunol 185:273–283. doi:10.4049/jimmunol.1000991
Kim DY, Hong GU, Ro JY (2011) Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L. J Neuroinflammation 8:25. doi:10.1186/1742-2094-8-25
Gosselin RD, Suter MR, Ji RR, Decosterd I (2010) Glial cells and chronic pain. Neuroscientist 16:519. doi:10.1177/1073858409360822
Suk K, Ock J (2011) Chemical genetics of neuroinflammation: natural and synthetic compounds as microglial inhibitors. Inflammopharmacology 20:151. doi:10.1007/s10787-011-0108-2
Oliveira SM, Drewes CC, Silva CR, Trevisan G, Boschen SL, Moreira CG, de Almeida CD, Da Cunha C, Ferreira J (2011) Involvement of mast cells in a mouse model of postoperative pain. Eur J Pharmacol 672:88–95. doi:10.1016/j.ejphar.2011.10.001
Ralay Ranaivo H, Craft JM, Hu W, Guo L, Wing LK, Van Eldik LJ, Watterson DM (2006) Glia as a therapeutic target: selective suppression of human amyloid-beta-induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration. J Neurosci 26:662–670. doi:10.1523/JNEUROSCI.4652-05.2006
Pacher P, Bátkai S, Kunos G (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58:389–462. doi:10.1124/pr.58.3.2
Sepe N, De Petrocellis L, Montanaro F, Cimino G, Di Marzo V (1998) Bioactive long chain N-acylethanolamines in five species of edible bivalve molluscs. Possible implications for mollusc physiology and sea food industry. Biochim Biophys Acta 1389:101–111. doi:10.1016/S0005-2760(97)00132-X
Bisogno T, Ventriglia M, Milone A, Mosca M, Cimino G, Di Marzo V (1997) Occurrence and metabolism of anandamide and related acyl-ethanolamides in ovaries of the sea urchin Paracentrotus lividus. Biochim Biophys Acta 1345:338–348. doi:10.1016/S0005-2760(97)00009-X
Matias I, Bisogno T, Melck D, Vandenbulcke F, Verger-Bocquet M, De Petrocellis L, Sergheraert C, Breton C, Di Marzo V, Salzet M (2001) Evidence for an endocannabinoid system in the central nervous system of the leech Hirudo medicinalis. Mol Brain Res 87:145–159. doi:10.1016/S0169-328X(00)00290-4
Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N (2004) Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem 279:5298–5305. doi:10.1074/jbc.M306642200
Coburn AF, Moore LV (1943) Nutrition as conditioning factor in the rheumatic state. Am J Dis Child 65:744–756
Coburn AF, Graham CE, Hahinger J (1954) Effect of egg yolk in diets on anaphylactic arthritis (passive Arthus phenomenon) in the guinea pig. J Exp Med 100:425–435. doi:10.1084/jem.100.5.425
Kuehl FA Jr, Jacob TA, Ganley OH, Ormond RE, Meisinger MAP (1957) The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J Am Chem Soc 79:5577–5578. doi:10.1021/ja01577a066
Mazzari S, Canella R, Petrelli L, Marcolongo G, Leon L (1996) N-(2-hydroxyethyl)hexadecanamide is orally active in reducing edema formation and inflammatory hyperalgesia by downmodulating mast cell activation. Eur J Pharmacol 300:227–236. doi:10.1016/0014-2999(96)00015-5
Calignano A, La Rana G, Giuffrida A, Piomelli D (1998) Control of pain initiation by endogenous cannabinoids. Nature 394:277–281. doi:10.1038/28393
Lambert DM, Vandevoorde S, Diependaele G, Govaerts SJ, Robert AR (2001) Anticonvulsant activity of N-palmitoylethanolamide, a putative endocannabinoid, in mice. Epilepsia 42:321–327. doi:10.1046/j.1528-1157.2001.41499.x
Petrosino S, Iuvone T, Di Marzo V (2010) N-palmitoyl-ethanolamine: biochemistry and new therapeutic opportunities. Biochimie 92:724–727. doi:10.1016/j.biochi.2010.01.006
Skaper SD (2012) Conference report: 1st workshop on “Palmitoylethanolamide: biochemistry, pharmacology and therapeutic use of a pleiotropic anti-inflammatory lipid mediator”. CNS Neurol Disord Drug Targets 11:191. doi:10.2174/187152712800672427
Muccioli GG, Stella N (2008) Microglia produce and hydrolyze palmitoylethanolamide. Neuropharmacology 54:16–22. doi:10.1016/j.neuropharm.2007.05.015
Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A (1995) Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci USA 92:3376–3380. doi:10.1073/pnas.92.8.3376
Cerrato S, Brazis P, della Valle MF, Miolo A, Puigdemont A (2010) Effects of palmitoylethanolamide on immunologically induced histamine, PGD2 and TNFα release from canine skin mast cells. Vet Immunol Immunopathol 133:9–15. doi:10.1016/j.vetimm.2009.06.011
Hansen HS, Lauritzen L, Strand AM, Vinggaard AM, Frandsen A, Schousboe A (1997) Characterization of glutamate-induced formation of N-acylphosphatidylethanolamine and N-acylethanolamine in cultured neocortical neurons. J Neurochem 69:753–761. doi:10.1061/j.1471-4159.1997.69020753x
Franklin A, Parmentier-Batteur S, Walter L, Greenberg DA, Stella N (2003) Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J Neurosci 23:7767–7775
Berger C, Schmid PC, Schabitz WR, Wolf M, Schwab S, Schmid HH (2004) Massive accumulation of N-acylethanolamines after stroke. Cell signalling in acute cerebral ischemia? J Neurochem 88:1159–1167. doi:10.1046/j.1471-4159.2003.02244.x
Schäbitz WR, Giuffrida A, Berger C, Aschoff A, Schwaninger M, Schwab S, Piomelli D (2002) Release of fatty acid amides in a patient with hemispheric stroke: a microdialysis study. Stroke 33:2112–2114. doi:10.1161/01.STR.0000023491.63693.18
Ghafouri N, Ghafouri B, Larsson B, Turkina MV, Karlsson L, Fowler CJ, Gerdle B (2011) High levels of N-palmitoylethanolamide and N-stearoylethanolamide in microdialysate samples from myalgic trapezius muscle in women. PLoS One 6:e27257. doi:10.1371/journal.pone.0027257
Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, Khanolkar A, Layward L, Fezza F, Bisogno T, Di Marzo V (2001) Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J 15:300–302. doi:10.1096/fj.00-0399fje
Conti S, Costa B, Colleoni M, Parolaro D, Giagnoni G (2002) Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Brit J Pharmacol 135:181–187. doi:10.1038/sj.bjp.0704466
Jaggar SI, Hasnie FS, Sellaturay S, Rice AS (1998) The antihyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain 76:189–199. doi:10.1016/S0304-3959(98)00041-4
Calignano A, La Rana G, Piomelli D (2001) Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. Eur J Pharmacol 419:191–198. doi:10.1016/S0014-2999(01)00988-8
Costa B, Conti S, Giagnoni G, Colleoni M (2002) Therapeutic effect of the endogenous fatty acid amide, palmitoylethanolamide, in rat acute inflammation: inhibition of nitric oxide and cyclo-oxygenase systems. Br J Pharmacol 137:413–420. doi:10.1038/sj.bjp.0704900
Luongo L, Guida F, Boccella S, Bellini G, Gatta L, Rossi F, de Novellis V, Maione S (2013) Palmitoylethanolamide reduces formalin-induced neuropathic-like behaviour through spinal glial/microglial phenotypical changes in mice. CNS Neurol Disord Drug Targets 12:45–54
D'Agostino G, La Rana G, Russo R, Sasso O, Iacono A, Esposito E, Mattace Raso G, Cuzzocrea S, Loverme J, Piomelli D, Meli R, Calignano A (2009) Central administration of palmitoylethanolamide reduces hyperalgesia in mice via inhibition of NF-κB nuclear signalling in dorsal root ganglia. Eur J Pharmacol 613:54–59. doi:10.1016/j.ejphar.2009.04.022
De Filippis D, Luongo L, Cipriano M, Palazzo E, Cinelli MP, de Novellis V, Maione S, Iuvone T (2011) Palmitoylethanolamide reduces granuloma-induced hyperalgesia by modulation of mast cell activation in rats. Mol Pain 10:3. doi:10.1186/1744-8069-7-3
Helyes Z, Németh J, Thán M, Bölcskei K, Pintér E, Szolcsányi J (2003) Inhibitory effect of anandamide on resiniferatoxin-induced sensory neuropeptide release in vivo and neuropathic hyperalgesia in the rat. Life Sci 73:2345–2353. doi:10.1016/S0024-3205(03)00651-9
Costa B, Comelli F, Bettoni I, Colleoni M, Giagnoni G (2008) The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB1, TRPV1 and PPARγ receptors and neurotrophic factors. Pain 139:541–550. doi:10.1016/j.pain.2008.06.003
Garcia-Ovejero D, Arevalo-Martin A, Petrosino S, Docagne F, Hagen C, Bisogno T, Watanabe M, Guaza C, Di Marzo V, Molina-Holgado E (2009) The endocannabinoid system is modulated in response to spinal cord injury in rats. Neurobiol Dis 33:57–71. doi:10.1016/j.nbd.2008.09.015
Esposito E, Paterniti I, Mazzon E, Genovese T, Di Paola R, Galuppo M, Cuzzocrea S (2011) Effects of palmitoylethanolamide on release of mast cell peptidases and neurotrophic factors after spinal cord injury. Brain Behav Immun 25:1099–1112. doi:10.1016/j.bbi.2011.02.006
Genovese T, Esposito E, Mazzon E, Di Paola R, Meli R, Bramanti P, Piomelli D, Calignano A, Cuzzocrea S (2008) Effects of palmitoylethanolamide on signaling pathways implicated in the development of spinal cord injury. J Pharmacol Exp Ther 326:12–23. doi:10.1124/jpet.108.136903
Skaper SD, Buriani A, Dal Toso R, Petrelli L, Romanello S, Facci L, Leon A (1996) The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons. Proc Natl Acad Sci USA 93:3984–3989. doi:10.1073/pnas,93.9.3984
D'Agostino G, Russo R, Avagliano C, Cristiano C, Meli R, Calignano A (2012) Palmitoylethanolamide protects against the amyloid-β25-35-induced learning and memory impairment in mice, an experimental model of Alzheimer disease. Neuropsychopharmacology 37:1784–1792. doi:10.1038/npp.2012.25
Scuderi C, Valenza M, Stecca C, Esposito G, Carratù MR, Steardo L (2012) Palmitoylethanolamide exerts neuroprotective effects in mixed neuroglial cultures and organotypic hippocampal slices via peroxisome proliferator-activated receptor-α. J Neuroinflammation 9:49. doi:10.1186/1742-2094-9-21
Rees K, Stowe R, Patel S, Ives N, Breen K, Clarke CE, Ben-Shlomo Y (2011) Non-steroidal anti-inflammatory drugs as disease-modifying agents for Parkinson's disease: evidence from observational studies. Cochrane Database Syst Rev 11, CD008454. doi:10.1002/14651858.CD008454.pub2
Driver JA, Logroscino G, Lu L, Gaziano JM, Kurth T (2011) Use of non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease: nested case–control study. Brit Med J 342:d198. doi:10.1136/bmj.d198
Esposito E, Impellizzeri D, Mazzon E, Paterniti I, Cuzzocrea S (2012) Neuroprotective activities of palmitoylethanolamide in an animal model of Parkinson's disease. PLoS One 7(8):e41880. doi:10.1371/journal.pone.0041880
Ahmad A, Genovese T, Impellizzeri D, Crupi R, Velardi E, Marino A, Esposito E, Cuzzocrea S (2012) Reduction of ischemic brain injury by administration of palmitoylethanolamide after transient middle cerebral artery occlusion in rats. Brain Res 1477:45–58. doi:10.1016/j.brainres.2012.08.006
Ahmad A, Crupi R, Impellizzeri D, Campolo M, Marino A, Esposito E, Cuzzocrea S (2012) Administration of palmitoylethanolamide (PEA) protects the neurovascular unit and reduces secondary injury after traumatic brain injury in mice. Brain Behav Immun 26:1310–1321. doi:10.1016/j.bbi.2012.07.021
Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D (2005) The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol 67:15–19. doi:10.1124/mol.104.006353
Raso GM, Esposito E, Vitiello S, Iacono A, Santoro A, D'Agostino G, Sasso O, Russo R, Piazza PV, Calignano A, Meli R (2011) Palmitoylethanolamide stimulation induces allopregnanolone synthesis in C6 cells and primary astrocytes: involvement of peroxisome-proliferator activated receptor-α. J Neuroendocrinol 23:591–600. doi:10.1111/j.1365-2826.2011.02152.x
Scuderi C, Esposito G, Blasio A, Valenza M, Arietti P, Steardo L Jr, Carnuccio R, De Filippis D, Petrosino S, Iuvone T, Di Marzo V, Steardo L (2011) Palmitoylethanolamide counteracts reactive astrogliosis induced by β-amyloid peptide. J Cell Mol Med 15:2664–2674. doi:10.1111/j.1582-4934.2011.01267.x
D'Agostino G, La Rana G, Russo R, Sasso O, Iacono A, Esposito E, Raso GM, Cuzzocrea S, Lo Verme J, Piomelli D, Meli R, Calignano A (2007) Acute intracerebroventricular administration of palmitoylethanolamide, an endogenous peroxisome proliferator-activated receptor-alpha agonist, modulates carrageenan-induced paw edema in mice. J Pharmacol Exp Ther 322:1137–1143. doi:10.1124/jpet.107.123265
de Novellis V, Luongo L, Guida F, Cristino L, Palazzo E, Russo R, Marabese I, D'Agostino G, Calignano A, Rossi F, Di Marzo V, Maione S (2012) Effects of intra-ventrolateral periaqueductal grey palmitoylethanolamide on thermoceptive threshold and rostral ventromedial medulla cell activity. Eur J Pharmacol 676:41–50. doi:10.1016/j.ejphar.2011.11.034
Smart D, Jonsson KO, Vandevoorde S, Lambert DM, Fowler CJ (2002) ‘Entourage’ effects of N-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Brit J Pharmacol 136:452–458. doi:10.1038/sj.bjp.0704732
Petrocellis D, Davis JB, Di Marzo V (2001) Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett 506:253–256. doi:10.1016/S0014-5793(01)02934-9)
Bíró T, Maurer M, Modarres S, Lewin E, Brodie C, Acs G, Acs P, Paus R, Blumberg PM (1998) Characterization of functional vanilloid receptors expressed by mast cells. Blood 91:1332–1340
Kim SR, Kim SU, Oh U, Jin BK (2006) Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. J Immunol 177:4322–4329
Katsura H, Obata K, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Sakagami M, Noguchi K (2006) Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. J Neurosci 26:8680–8690. doi:10.1523/JNEUROSCI.1771-06.2006
Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87. doi:10.1038/384083a0
Ueda N, Yamanaka K, Yamamoto S (2001) Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J Biol Chem 276:35552–35557. doi:10.1074/jbc.M106261200
Solorzano C, Zhu C, Battista N, Astarita G, Lodola A, Rivara S, Mor M, Russo R, Maccarrone M, Antonietti F, Duranti A, Tontini A, Cuzzocrea S, Tarzia G, Piomelli D (2009) Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc Natl Acad Sci USA 106:20966–20971. doi:10.1073/pnas.0907417106
Saturnino C, Petrosino S, Ligresti A, Palladino C, De Martino G, Bisogno T, Di Marzo V (2010) Synthesis and biological evaluation of new potential inhibitors of N-acylethanolamine hydrolyzing acid amidase. Bioorg Med Chem Lett 20:1210–1213. doi:10.1016/j.bmcl.2009.11.134
Yamano Y, Tsuboi K, Hozaki Y, Takahashi K, Jin XH, Ueda N, Wada A (2012) Lipophilic amines as potent inhibitors of N-acylethanolamine-hydrolyzing acid amidase. Bioorg Med Chem 20:3658–3665. doi:10.1016/j.bmc.2012.03.065
Li Y, Yang L, Chen L, Zhu C, Huang R, Zheng X, Qiu Y, Fu J (2012) Design and synthesis of potent N-acylethanolamine-hydrolyzing acid amidase (NAAA) inhibitor as anti-inflammatory compounds. PLoS One 7:8. doi:10.1371/journal.pone.0043023
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388–399. doi:10.1038/nrn3053
Schäfer T, Starkl P, Allard C, Wolf RM, Schweighoffer T (2010) A granular variant of CD63 is a regulator of repeated human mast cell degranulation. Allergy 65:1242–1255. doi:10.1111/j.1398-9995.2010.02350.x
Truini A, Biasiotta A, Di Stefano G, La Cesa S, Leone C, Cartoni C, Federico V, Petrucci M, Cruccu G (2011) Palmitoylethanolamide restores myelinated-fibre function in patients with chemotherapy-induced painful neuropathy. CNS Neurol Disorders Drug Targets 10:916–920. doi:10.2174/187152711799219307
Kopsky DJ, Hesselink JM (2012) Multimodal stepped care approach with acupuncture and PPAR-α agonist palmitoylethanolamide in the treatment of a patient with multiple sclerosis and central neuropathic pain. Acupunct Med 30:53–55. doi:10.1136/acupmed-2011-010119
Hesselink JM, Hekker TA (2012) Therapeutic utility of palmitoylethanolamide in the treatment of neuropathic pain associated with various pathological conditions: a case series. J Pain Res 5:437–442. doi:10.2147/JPR.S32143
Hesselink JMK (2012) New targets in pain, non-neuronal cells, and the role of palmitoylethanolamide. The Open Pain Journal 5:12–23
Clemente S (2012) Amytrophic lateral sclerosis treatment with ultramicronized palmitoylethanolamide: a case report. CNS Neurol Disord Drug Targets 11:933–936. doi:10.2174/1871527311201070933
Acknowledgements
The authors wish to thank Stefano Lovison for excellent graphic design assistance. L. Facci was supported by Fondazione CARIPARO “Progetto Dottorati di Ricerca” Anno 2009.
Conflicts of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skaper, S.D., Facci, L. & Giusti, P. Glia and Mast Cells as Targets for Palmitoylethanolamide, an Anti-inflammatory and Neuroprotective Lipid Mediator. Mol Neurobiol 48, 340–352 (2013). https://doi.org/10.1007/s12035-013-8487-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8487-6