Abstract
Here, we investigated the possible involvement of gamma-aminobutyric acid B1 receptor (GABAB1R) in mediating the protective effects of black soybean anthocyanins against ethanol-induced apoptosis in prenatal hippocampal neurons because GABARs are known to play an important role in the development of central nervous system. Treatments were performed on primary cultures of prenatal rat hippocampal neurons transfected with or without GABAB1R small interfering RNA (siRNA). The results showed that, when ethanol treatment was followed by anthocyanins treatment, cellular levels of proapoptotic proteins such as Bax, activated caspase-3, and cleaved poly (ADP-ribose) polymerase 1 (PARP-1) were decreased, and the cellular level of the antiapoptotic protein Bcl-2 was increased compared to treatment with ethanol alone. Furthermore, the effects of ethanol on cellular levels of GABAB1R and its downstream signaling molecules such as protein kinase A, calcium/calmodulin-dependent protein kinase II (CaMKII), and phosphorylated cAMP response element binding protein were diminished or reversed by anthocyanins treatment. The ability of anthocyanins to reverse the effects of ethanol on cellular levels of Bax, Bcl-2, active caspase-3, cleaved PARP-1, GABAB1R, and CaMKII were abrogated in cells transfected with GABAB1R siRNA. In a GABAB1R-dependent manner, anthocyanins also inhibited the ability of ethanol to elevate intracellular free Ca2+ level and increase the proportion of cells with low mitochondrial membrane potential in the population. Cell apoptosis assay and morphological studies also confirmed the neuroprotective effect of anthocyanins against ethanol via GABAB1R. Our data suggest that GABAB1R plays an important role in the neuroprotective effects of anthocyanins against ethanol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to high levels of ethanol in utero results in lifelong mental and physical abnormalities that are collectively referred to as fetal alcohol syndrome (FAS) [1]. Damage to the central nervous system of fetuses due to ethanol exposure is the main cause of FAS [2, 3]. The cortex, hippocampus, and thalamus are brain structures that are particularly sensitive to alcohol exposure [4].
Ethanol affects the central nervous system in many ways. Many of the effects of ethanol such as dependence and behavior impairment are mediated by receptors for gamma-aminobutyric acid (GABA), the principal inhibitory neurotransmitter in the central nervous system [5]; GABAB receptors (GABABRs) belong to the G-protein coupled receptor family and are involved in various physiological and pathological processes [6–8]. They play an important role in the development of the central nervous system because they are involved in memory and learning. They are important for synaptic plasticity and mediate behavior modifying actions of ethanol. Gamma aminobutyric acid B receptor 1 has been reported to improve behavior impaired by ethanol in Drosophila [9]. GABABR signaling activates adenylyl cyclase and protein kinase A (PKA). cAMP is generated by the action of adenylyl cyclise, and it is an activator of PKA. Phosphorylation and activation of the transcription factor cAMP response element binding protein (CREB) takes place after the activation of PKA. Transcription of cAMP inducible genes by phosphorylated CREB (p-CREB) is important for maintaining synaptic plasticity. Besides activation by GABABR, adenylyl cyclase activity is also modulated upon phosphorylation by Ca+2/calmodulin kinase (CaMKII) [10]. Ethanol crosses the blood–brain barrier and induces neurodegenaration through the intrinsic apoptotic pathway by activating Bax and promoting cleavage of caspase-3 [11]. Reactive oxygen species (ROS) are generated during ethanol metabolism by the brain [12, 13]. The fetal brain develops in a low oxygen environment and has low antioxidant capacity [14, 15].
Natural antioxidants extracted from fruits and vegetables are ideal candidates to ameliorate oxidative stress and scavenge ROS induced by ethanol. Anthocyanins and flavonoids are plant-derived polyphenols that differ in their oxidation states. Flavonoids have been shown to prevent ethanol-induced apoptosis in cultured fetal rhombencephalic neurons [16]. Flavonoids originating from grape seed have a neuroprotective role against ethanol in the cerebellum and hippocampus of mice [17]. In fact, it has been demonstrated that dietary flavonoids can protect brain and liver against ethanol-induced damage [18]. Electron delocalization properties and the ability to form resonance structures make anthocyanins the most potent antioxidants among flavonoids [19, 20]. In fact, anthocyanins are one of the most effective natural antioxidants. They have cardioprotective, anti-inflammatory, antidiabetic, and anticarcinogenic properties [19, 21]. Anthocyanins are the main flavonoids components of red wine and have been shown to reduce ethanol-induced lipid peroxidation, modulate redox status, and improve spatial memory in the hippocampus and cerebellum of adult rats [22–24]. Anthocyanins extracted from berries can improve cognitive brain function and reduce age-associated oxidative stress [19, 21, 25, 26]. They have been shown to prevent learning and memory loss in estrogen-deficient rats [27].
Black soybean is rich in anthocyanins and has been used for centuries as a medicinal food in China, Korea, and Japan [28]. Therefore, it is probable that black soybean anthocyanins have healthful effects. The neuroprotective effects of anthocyanins against ethanol-induced damage to prenatal brain tissues and the involvement of GABABRs in this process have not been examined in detail. Here, we compared GABAB1R siRNA transfected and nontransfected prenatal hippocampal neuronal cells and showed that anthocyanins from black soybeans acted via GABAB1R to protect cultured prenatal hippocampal neurons against ethanol-induced apoptosis.
Materials and Methods
Treatment of Animals
Female Spargue–Dawly rats (n = 10, 250 g body weight) were housed in a temperature-controlled environment (Geyongsang National University Neurobiology Laboratory, Jinju, Korea) with lights on from 0800–2000 hours (12 h light/dark) cycle and allowed ad libitum access to food. At gestational day (GD) 17.5, pregnant rats were killed by decapitation after an intravenous injection (i.v.) of pentobarbital sodium (3 mg/100 g b.w). All the experimental procedures were approved by the animal ethics committee of the Division of Applied Life Sciences, Department of Biology, Gyeongsang National University, South Korea.
Primary Cultures of Hippocampal Neurons
The primary cultures of hippocampal neurons were prepared from brains of GD 17.5 fetuses. Hippocampal tissues were treated with 0.25 % trypsin-EDTA for 20 min, dissociated by mechanical trituration in ice-cold calcium- and magnesium-free Hank's balanced salt solution and collected by centrifugation. Cells (1 × 106 cells/ml) were resuspended in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % heat-inactivated fetal bovine serum, 1 mM pyruvate, 4.2 mM sodium bicarbonate, 20 mM HEPES, 0.3 g/l bovine serum albumin, 50 U/ml penicillin, and 50 mg/l streptomycin and plated in poly-lysine (0.02 g/l) coated cell culture plates or chamber slides. Cultures were maintained at 37 °C in a humidified atmosphere with 5 % CO2. Proliferation of neuroglia cells was inhibited by adding 10 μM cytosine β-d-arabinofuranoside (Sigma, St. Louis, MO, USA) to the growth medium followed by incubation for 12 h.
siRNA Transfection and Drug Treatment
GABAB1R complementary DNA (cDNA) plasmid (Novartis Pharma, Basel, Switzerland) was digested with XbaI and EcoRI to release the insert from pCI vector. The PCR primers used for amplification of GABAB1R cDNA were 5′CGGTAATACGACTCACTATAGGGAGACGCTACCATCCAACAGACCA-3′ and 5′ GCGTAATACGACTCACTATAGGGAGATCCTGTGAGCTCATGTTGGAA-3′ and contained T7 promoter sequences (underlined) at their 5′ ends. The PCR reaction amplified a 420-bp fragment of GABAB1R cDNA 1,096–1,516 bp from that is known to have the highest silencing activity. The PCR product was used as template for double-stranded RNA (dsRNAs) synthesis using the MEGA script® RNAi kit (Ambion, Austin, TX, USA). Subsequent processing into short fragments for transfection was performed using a ShortCut RNAi Kit (New England Biolabs, Buckinghamshire, UK). Liposome solution (DMEM containing Lipofectamine2000TM, Invitrogen, Carlsbad, CA, USA) and an equal volume of dsRNAs (21 bp) solution were incubated separately for 5 min at room temperature and then combined and incubated for 20 min. The mixture was added to cells (1 × 106 cells/ml) that had been starved by 24 h incubation in DMEM without antibiotics and serum at a final concentration of 40 nM GABAB1R siRNA, and negative control siRNA was purchased from (Qiagen, Inc). After 48 h, cells were transferred to the normal growth medium with or without drug supplements. The seven treatment groups included in this study were the following: (1) control; (2) ethanol, 100 mM; (3) baclofen, 50 μM; (4) anthocyanins, 0.1 mg/ml; (5) ethanol, 100 mM plus anthocyanins, 0.1 mg/mL; (6) ethanol, 100 mM plus GABAB1R siRNA, 40 nM; (7) and ethanol, 100 mM plus GABAB1R siRNA 40 nM, plus anthocyanins, 0.1 mg/mL. After 20 min of drug treatment, the cells were harvested for analyses. In all experiments, ethanol treatment was followed by anthocyanins treatment for additional 20 min.
Extraction of Anthocyanins
Anthocyanins were extracted from Korean black soybeans provided by the agriculture research facility of GNU. Anthocyanins were extracted from whole beans a total weight (1,500 g) three times by stirring with 1,500 mL of 95 % methanol/1 % HCl for 72 h at room temperature in the dark. The methanol extract was concentrated using a rotary evaporator, made up to 150 ml and loaded on a XAD-7 column. The column was washed with distilled water until the elute was a soft red color. The column was then washed with ethyl acetate until the color on the entire column changed to purple except for a 1-cm layer at the very bottom of the column. Anthocyanins were then eluted by passing 95 % methanol/1 % HCl through the column until the color on the entire column changed to red. The elute containing anthocyanins was concentrated to 100 ml using a rotary evaporator, and fines were removed by passing through a 0.45-μm filter. The anthocyanins concentrate was loaded onto a Sephadex column and eluted using a 50 % methanol/50 % distilled water/1 % HCl solution until between 800 and 1,000 mL of a red colored elute was collected. The red colored anthocyanin elute was evaporated to dryness using a rotary evaporator. The resulting anthocyanins powder was stored at −20 °C.
Western Blotting
Cultured hippocampal cells were homogenized in cell lysis buffer (Cell Signaling no. 9803, Danvers, MA, USA). Cell lysates were cleared by two rounds of centrifugation at 12,000 rpm for 10 min at 4 °C, and the supernatant saved for analyses. Protein concentration was determined using the Bio-Rad protein assay kit. Thirty micrograms of total proteins were resolved on duplicate 12 % sodium dodecyl sulfate polyacrylamide gels and transferred to a polyvinylidene fluoride membrane for immunoblot analysis as we described previously [11]. The following primary antibodies were used for the immunoreactions: guinea pig antirat GABAB1R (1:500 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit antirat PKA-α (1:500 dilution, Santa Cruz Biotechnology), rabbit antirat CaMKII (1:500 dilution, Cell Signaling), rabbit antirat p-CREB (1:500 dilution, Cell Signaling), Bax and Bcl-2 rabbit antirat (1:500 dilution, Santa Cruz Biotechnology), PARP-1 (1:500 dilution, Santa Cruz Biotechnology), rabbit antirat caspase-3 (1:500 dilution, Cell Signaling), or antiactin (1:1,000 dilution, Sigma-Aldrich, Jerusalem, Israel; used as loading control). Incubation with primary antibody was performed for 24 h at 4 °C. After rinsing the blots, horseradish peroxidase-conjugated goat antimouse, mouse antigoat, or goat antirabbit IgG-HRPs (Santa Cruz Biotech 1:1,000) were incubated with the blots for 2 h at room temperature, and the antigens were detected by enhanced chemiluminescence (Western blotting detection reagents, Amersham Pharmacia Biotech, Piscataway, NJ, USA) according to manufacturer’s instructions. In some cases, blots were stripped and reprobed as follows. After washing in Tris-buffered saline containing 0.1 % (v/v) Tween-20 (TBST) the membrane was stripped using ReBlot Plus Strong Antibody stripping solution (Millipore, Temecula, CA, USA) according to manufacturer’s instructions. The stripped membrane was washed with TBST (4 washes of 5 min) and then used for reprobing. Western blots were analyzed by densitometry using the computer-based Sigma Gel system (SPSS Inc., Chicago, IL, USA). Density values were expressed as mean ± SEM.
Intracellular Free Ca2+ Measurement
The intracellular Ca2+ concentration was measured with the fluorescent Ca2+ indicator fura-2 acetoxymethyl ester (Fura-2AM). Drug treatments were performed as described under “siRNA transfection and drug treatment” in triplicate plates; each containing 1 × 106 cells. After treatment, cells were washed twice with Krebs buffer and then incubated for 60 min in DMEM media containing 5 μM Fura-2AM at 37 °C in a humidified incubator with 5 % CO2. Cells were washed twice with Locke’s solution (pH 7.8), and Fura-2AM fluorescence signals of Ca2+ were measured using a luminescence spectrophotometer (LS50B, Perkin Elmer, Boston, MA, USA) with excitation wavelengths of 340 and 380 nm and emission measurements at 510 nm. The 340 nm/380 nm fluorescence ratio, averaged over a period of 2 s, was monitored. Fluorescence signals were acquired stored and analyzed using a computer with universal imaging software or a MicroVAX II computer with origin 7 software. Intracellular calcium was determined from the Grynkiewicz equation [29]:
where K d is the dissociation constant of the Fura-2AM Ca2+ interaction was taken to be 225 nM in the cytosolic environment; R is the 340 nm/380 nm fluorescence ratio; R min is ratio with zero Ca2+; R max is the ratio with saturating Ca2+ (using calcium chloride); Sf2 is the fluorescence at 380 nm with zero Ca2+; and Sb2 is the fluorescence at 380 nm with saturating Ca2+.
Flouro-Jade B and Propidium Iodide Staining
Flouro-Jade B (FJB) staining was performed as previously described [30]. Primary cultures of hippocampal neurons from the GABAB1R siRNA transfected and nontransfected groups were plated 48 h after transfection in poly-d-lysine coated chambers. After 4 days growth, cultures were treated as indicated in the figure legends for 12 h at 37 °C. Then, cells were fixed in 4 % paraformaldehyde in phosphate-buffered saline (PBS) for 5 min and stored at −70 °C. Slides were air-dried for 3 h and then subjected, in order, to the following treatments: 10 min in 0.06 % potassium permanganate solution, distilled water rinse, 20 min in 0.1 % acetic acid containing 0.0004 % FJB (Calbiochem, San Diego, CA, USA) and three washes in distilled water. The slides were allowed to dry at 55 °C for 10 min and viewed under a confocal microscope using an FITC filter with (Olympus Fluoview FV1000, Japan). For propidium iodide (PI) staining, slides were immersed with gentle mixing in PI solution (1 μg/ml in PBS) for 20 min at room temperature and then washed twice with PBS for 10 min. Glass cover slips were mounted on the slides with mounting medium prior to viewing.
Flow Cytometric Analysis of Mitochondrial Membrane Potential (ΔΨm)
Mitochondrial membrane potential (ΔΨm) was monitored using the JC-1 mitochondrial membrane potential detection kit (Biotium Inc., Hayward, CA, USA) according to the manufacturer’s protocol. Primary cultures of hippocampal neurons from GD 17.5 fetal brain were transfected or not with siGABAB1R RNA and treated with test compounds as described under “siRNA transfection and drug treatment.” After drug treatment, cells in were harvested, stained with JC-1 reagents at 37 °C for 15 min, washed twice in 1× assay buffer, and resuspended in 0.5 ml PBS for FACS analysis (FACSCalibur Flow Cytometer; Becton Dickinson, San Jose, CA, USA). Analyses were performed on triplicate samples. JC-1 aggregates in healthy polarized mitochondria and emits a red fluorescence at 590 nm. JC-1 monomers that leak from stressed depolarized mitochondria emit green fluorescence at 530 nm. The red and green fluorescence were measured in the green (FL-1) and red (FL-2) channels of the flow cytometer, respectively. The cells were then immediately observed under a fluorescence microscope using a “dual-band pass” filter designed to simultaneously detect fluorescein and rhodamine dyes.
Flow Cytometric Analysis for Apoptosis Using Fluorescence-Activated Cell Sorting Assay
Fragmentation of genomic DNA to sub-G1 DNA was assessed with the aid of fluorescence-activated cell sorting (Becton Dickinson, Franklin Lakes, NJ, USA) analysis as described [31]. GD 17.5 hippocampal cells were cultured in DMEM medium same to that used for primary culture until 80 % confluence. Total cells were collected and resuspended in 100 μl PBS, fixed with 1 ml 70 % ethanol (−20 °C), and washed with PBS. Each pellet was then resuspended in 250 μl PBS containing 1 mg/ml RNase and incubated on ice for 30 min. After the incubation with 250 μl propidium iodide solution (0.5 mg/ml in PBS; Sigma) for 30 min at room temperature then cells were subjected to fluorescence-activated cell sorting assay.
Statistical Analysis
All the bands from Western blot were scanned and analyzed by densitometry using the Sigma Gel System (SPSS Inc., Chicago, IL, USA). All the density values were expressed as the mean ± SE. Statistical analysis of the results were carried out by one-way ANOVA, followed by the Duncan’s least significant difference post hoc test to determine group differences (F values). Differences with a p < 0.05 were considered statistically significant.
Results
Anthocyanins Abrogate the Negative Effects of Ethanol on Cellular Levels of Some Apoptosis Marker Proteins Through GABAB1R
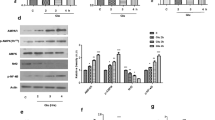
We measured cellular levels of a few apoptosis marker proteins to ascertain whether anthocyanins can protect against ethanol-induced apoptosis in primary cultures of prenatal rat hippocampal neurons. The cellular level of the proapoptotic protein Bax increased upon exposure to ethanol for 20 min, whereas the cellular level of the antiapoptotic protein Bcl-2 was reduced significantly increasing Bax/Bcl-2 ratio [F(1, 8) = 8.7, p < 0.05; Fig. 1] as expected. Furthermore, we measured the expressions levels of both activated caspase-3 and cleaved PARP-1 as they are important executioners of apoptosis. Activated caspase-3 cleaves PARP-1 and ultimately results in apoptotic and necrotic cell death [32]. Significant increases in active caspase-3 [F(1, 10) = 16.8, p < 0.05] level and cleaved PARP-1 [F(1, 8) = 11.6, p < 0.05] level were observed in ethanol-treated cells (Fig. 1). Therefore, ethanol treatment increases levels of some but not all proapoptosis markers.
Treatment of anthocyanins following ethanol treatment abrogates the negative effects of ethanol on cellular levels of some apoptosis marker proteins through GABAB1R. Shown are the results of Western blot analysis of apoptosis marker proteins in protein extracts of cultured prenatal rat hippocampal cells. The various groups that were analyzed are as follows: control (C); 100 mM ethanol (E), 0.1 mg/ml anthocyanins (An), 100 mM ethanol + 0.1 mg/ml anthocyanins (E + An), 100 mM ethanol added to cells transfected with 40 nM GABAB1R siRNA (E + siRNA), and 100 mM ethanol + 0.1 mg/ml anthocyanins added to cells transfected with 40 nM GABAB1R siRNA (E + siRNA + An). The groups that did not receive siRNA (C, E, An, E + An) received the vehicle that was used to deliver siRNA at the same time as the other two groups received siRNA (E + siRNA, E + siRNA + An). All treatments were performed 48 h after transfection. The duration of treatment was for 20 min of ethanol followed by additional 20 min of anthocyanins at 37 °C. a Immunoblot of Bax, Bcl-2, Caspase-3, PARP-1, and β-actin. β-actin was used as loading control. b Histogram showing the ratio of proapoptotic protein Bax and antiapoptotic protein Bcl-2 quantification of signals on the Western blots in protein extracts of cultured prenatal rat hippocampal neuronal cells. c Quantification of signals on the Western blots. Bars represent mean ± SEM (n = 3). *p < 0.05, significantly different from control; # p < 0.05 significantly different from ethanol
Treatment of anthocyanins for 20 min following ethanol treatment protected against the ethanol-induced increase in cellular Bax level and decrease in Bcl-2 level. It also significantly [F(1, 4) = 12.6, p < 0.05] reduced their ratio while simultaneously decreased cellular levels of active caspase-3 [F(1, 10) = 21.4, p < 0.05) and cleaved PARP-1 [F(1, 8) = 17.7, p < 0.05] (Fig. 1). These results indicated that anthocyanins protect against ethanol-induced apoptosis. In cells transfected with GABAB1R siRNA, the ability of anthocyanins to reverse the effect of ethanol on Bax/Bcl-2 ratio, active caspase-3 and cleaved PARP-1 levels was abrogated or diminished, suggesting that GABAB1R was required for the protective action of anthocyanins.
Anthocyanins Modulate the Effect of Ethanol on Cellular Levels of GABAB1R and its Downstream Signaling Molecules
To extend our study to sort out the mechanism involved in ethanol induced apoptosis in both hippocampal neuronal cells, we measured the expressions levels of GABAB1R and its downstream molecules. GABAB1R was transfected with targeted siRNA in prenatal hippocampal neuronal cells. This was confirmed through Western blot analysis using agonist and antagonist of GABAB1R (Fig. 2a). Ethanol treatment significantly increased the levels of GABAB1R and its downstream signaling molecules, CaMKII and p-CREB. It decreased the expressions level of PKA-α in no siRNA transfection groups but not in the transfection group (Fig. 2b). Compared to the ethanol-treated group, GABAB1R, CaMKII, and p-CREB levels were lower, but PKA-α level is high in the ethanol plus anthocyanin-treated group, suggesting that anthocyanins interfere with ethanol signaling. Baclofen, a specific agonist of GABAB1R, was used for comparison.
a The expression levels of control and targeted siRNA GABAB1R protein along with or without drug treatment in prenatal hippocampal neuronal cells. a Shown are the results and quantification signals of Western blot analysis of nontargeted and targeted siRNA GABAB1R protein levels in cultured prenatal rat hippocampal neuronal cells. Prenatal hippocampal neuronal cells were transfected with targeted GABAB1R siRNA and then incubated in DMEM media for 48 h. b Shown are the results and quantification signals of Western blot analysis of non-targeted and targeted siRNA with or without drug treated GABAB1R protein levels in cultured prenatal rat hippocampal neuronal cells. The various groups that were analyzed are as follows: control (C); 100 mM ethanol (E); 50 μM Baclofen (Ba); and 100 μM Phaclofen. All treatments were performed 48 h after transfection. The duration of treatment was for 20 min at 37 °C. Bars represent mean ± SEM (n = 3). *p < 0.05. b Treatment of anthocyanins following ethanol treatment abrogates the negative effects of ethanol on cellular levels of GABAB1R and some GABAB1R downstream signaling molecules. Shown are the results of Western blot analysis of GABAB1R and its downstream signaling molecules in protein extracts of cultured fetal rat hippocampal cells. The various groups that were analyzed are as follows: control (C); 100 mM ethanol (E); 50 μM Baclofen (Ba); 0.1 mg/mL anthocyanins (An); 100 mM ethanol + 0.1 mg/mL anthocyanins (E + An); 100 mM ethanol added to cells transfected with 40 nM GABAB1R siRNA (E + siRNA); and 100 mM ethanol + 0.1 mg/ml anthocyanins added to cells transfected with 40 nM GABAB1R siRNA (E + siRNA + An). The groups that did not receive siRNA (C, E, Ba, An, and E + An) received the vehicle that was used to deliver siRNA at the same time as the other two groups received siRNA (E + siRNA, E + siRNA + An). All treatments were performed 48 h after transfection. The duration of treatment was 20 min of ethanol followed by additional 20 min of anthocyanins at 37 °C. a Immunoblot of GABAB1R, PKA-α, CaMKII, p-CREB, and β-actin. β-Actin was used as loading control (b). Quantification of signals on the Western blots. Bars represent mean ± SEM (n = 3). *p < 0.05, significantly different from control; # p < 0.05, significantly different from ethanol
Ethanol-Induced Disturbance of Ca2+ Homeostasis is Inhibited by Anthocyanins Through GABAB1R
Ca2+ homeostasis has an important role in the release of neurotransmitters and in the development of neurons and its disturbance causes neuronal cell death. Ethanol-induced neurotoxicity is mainly linked to the dysregulation of intracellular Ca2+ concentrations [33]. Therefore, cytosolic free Ca2+ concentrations without and with drug treatments were compared using the fluorescent Ca2+ indicator, fura-2AM (Fig. 3). Compared with the control group, ethanol treatment for 20 min significantly increased peak Ca2+ concentration in the nontransfected group than transfected group. In the nontransfected group, the ethanol-induced increase in peak Ca2+ concentration was inhibited almost completely by anthocyanins treatment for 20 min following ethanol treatment. This protective effect of anthocyanins against the ethanol-induced increase in peak Ca2+ concentration was not evident in the GABAB1R siRNA transfected group, suggesting that the protective effect of anthocyanins requires the GABAB1R. The GABAB1R agonist baclofen also decreased the concentration of intracellular Ca2+. These results showed that ethanol elevates intracellular Ca2+ concentrations, while anthocyanins played a role in reversing this increase via GABAB1R, suggesting that anthocyanins may protect against ethanol-induced cell death.
Treatment of anthocyanins following ethanol treatment inhibits ethanol-induced disturbance of Ca+2 homeostasis. Shown are the Fura-2AM fluorescence spectra indicating peaks of intracellular [Ca+2] in cultured hippocampal cells subjected to the following treatments. The various groups that were analyzed are as follows: control (C); 100 mM ethanol (E); 50 μM Baclofen (Ba); 0.1 mg/ml anthocyanins (An); 100 mM ethanol + 0.1 mg/ml anthocyanins added to cells transfected with 40 nM GABAB1R siRNA (E + siRNA + An); 100 mM ethanol added to cells transfected with 40 nM GABAB1R siRNA (E + siRNA); and 100 mM ethanol + 0.1 mg/mL anthocyanins (E + An). The groups that did not receive siRNA (C, E, Ba, An, and E + An) received the vehicle that was used to deliver siRNA at the same time as the other two groups received siRNA (E + siRNA, E + siRNA + An). All treatments were performed 48 h after transfection. The duration of treatment was 20 min of ethanol followed by additional 20 min of anthocyanins at 37 °C. The results are representative of three time experiments with similar results
Ethanol-Induced Collapse of Mitochondrial Membrane Potential (∆ΨM) is Inhibited by Anthocyanins Through GABAB1R
The abnormal increase in intracellular Ca2+ level induced by ethanol leads to collapse of the mitochondrial membrane potential (∆ΨM), and this collapse along with release of cytochrome-c can activate the apoptotic pathway [34]. FACS analysis showed that the ethanol-treated nontransfected population had a significantly higher percentage of cells with lower mitochondrial potential (24.04 %) compared with control (11.56 %). The group treated with anthocyanins for 20 min following ethanol exposure had a lower percentage of cell population with lower mitochondrial potential (15.86 %) compared to the ethanol-treated group, indicating that anthocyanins inhibit ethanol toxicity. However, in cells transfected with GABAB1R siRNA, anthocyanin was unable to reverse the adverse effect of ethanol on mitochondrial membrane potential (Fig. 4).
Treatment of anthocyanins following ethanol treatment inhibits ethanol-induced collapse of mitochondrial membrane potential (∆ΨM). Shown are the results of FACS analysis of JC-1 fluorescence. The various groups that were analyzed are as follows: control (C); 100 mM ethanol (E); 0.1 mg/ml anthocyanins (An); 100 mM ethanol added to cells transfected with 40 nM GABAB1R siRNA (E + siRNA); 100 mM ethanol + 0.1 mg/ml anthocyanins (E + An); and 100 mM ethanol + 0.1 mg/mL anthocyanins added to cells transfected with 40 nM GABAB1R siRNA (E + siRNA + An); the groups that did not receive siRNA (C, E, An, and E + An) received the vehicle that was used to deliver siRNA at the same time as the other two groups received siRNA (E + siRNA, E + siRNA + An). All treatments were performed 48 h after transfection. The duration of treatment was 20 min of ethanol followed by additional 20 min of anthocyanins at 37 °C. Data are the mean of three independent experiments (n = 3) with three plates in each experiment. *p < 0.05, significantly different from control; # p < 0.05, significantly different from ethanol
Anthocyanins Protect Prenatal Rat Hippocampal Neuronal Cells Against Ethanol-Induced Apoptosis
Next, we performed an apoptosis assay to determine whether anthocyanins protected against ethanol-induced apoptosis. Two groups of hippocampal neuronal cells were analyzed; one transfected with GABAB1R siRNA and the other treated exactly the same way except for the addition of siRNA. As expected [35], the percentage of apoptotic cells in the ethanol treated samples was significantly higher in nontransfected population than in control and transfected group (Fig. 5). Treatment with anthocyanins for 20 min following ethanol exposure significantly reduced the percentage of apoptotic cells in the nontransfected population but not in the GABAB1R siRNA transfected population, again indicating that the protective effect of anthocyanins required GABAB1R.
Treatment of anthocyanins following ethanol treatment inhibits ethanol-induced apoptosis. Shown are the results of an apoptosis assay performed on cultured prenatal rat hippocampal neurons. The various groups that were analyzed by FACS are as follows: control (C); 100 mM ethanol (E); 50 μM baclofen (Ba); 0.1 mg/ml anthocyanins (An); 100 mM ethanol + 0.1 mg/ml anthocyanins (E + An); 100 mM ethanol added to cells transfected with 40 nM GABAB1R siRNA (E + siRNA) and 100 mM ethanol + 0.1 mg/ml anthocyanins added to cells transfected with 40 nM GABAB1R siRNA (E + siRNA + An). The groups that did not receive siRNA (C, E, Ba, An, and E + An) received the vehicle that was used to deliver siRNA at the same time as the other two groups received siRNA (E + siRNA, E + siRNA + An). All treatments were performed 48 h after transfection. The duration of treatment was 20 min of ethanol followed by additional 20 min of anthocyanins at 37 °C. The bar graph shows the percentage of apoptotic cells in each population. Data are the mean of three independent experiments (n = 3) with three plates in each experiment. M1 indicates cell cycle arrest phase in mitosis. *p < 0.05, significantly different from control; # p < 0.05, significantly different from ethanol
Morphological Analysis of the Protective Effect of Anthocyanins Against Ethanol Induced Neurodegenaration
For the morphological assessment of cell death, we performed FJB and PI staining (Fig. 6). In cultured nontransfected hippocampal neurons, ethanol-treated cells showed a higher number of dead cells than control. Cells that received anthocyanins for 20 min after ethanol treatment displayed a significant reduction of cell death compared to the ethanol treatment alone indicating that anthocyanins inhibit ethanol-induced apoptosis. In contrast, no significant reduction of cell death was observed in the GABAB1R siRNA transfected group treated with ethanol plus anthocyanins compared to ethanol-treated nontransfected cells.
Treatment of anthocyanins following ethanol treatment inhibits ethanol-induced neurodegenaration. Analysis of neurodegenaration in primary cultures of prenatal rat hippocampal neurons was examined by Flouro Jade B (FJB) and propidium iodide (PI) staining. The various groups that were analyzed are as follows: control (C); 100 mM ethanol (E); 100 mM ethanol + 0.1 mg/ml anthocyanins (E + An); and 100 mM ethanol + 0.1 mg/ml anthocyanins added to cells transfected with 40 nM GABAB1R siRNA (E + siRNA + An). The groups that did not receive siRNA (C, E, and E + An) instead received the vehicle that was used to deliver siRNA. Cultures were exposed to test compounds for 12 h before staining with FJB (green) and PI (red). Shown images were obtained under a confocal microscope. White arrows indicate dead neuronal nuclei. Magnification with ×40 objective field, scale bar = 20 μm
Discussion
The major finding of our in vitro study is that anthocyanins extracted from black soybeans protected against ethanol-induced degeneration of prenatal rat hippocampal neurons. The protective effect of anthocyanins was attenuated upon transfection of cells with GABAB1R siRNA, indicating that the neuroprotective effect of anthocyanins involves GABAB1R. In this study, we compared the effects of ethanol and/or anthocyanins treatment in two groups of prenatal rat hippocampal neuronal cells. One group (transfected) received GABAB1R siRNA, while the other group (nontransfected) did not receive any RNA during the transfection process. Baclofen, an agonist of GABAB1 receptor, was used as a control to monitor events arising from stimulation of GABAB1R signaling.
Ethanol-induced apoptosis and neurodegenaration in the prenatal rat hippocampal neuronal cells (Figs. 1, 5, and 6). We have demonstrated that ethanol not only increased cellular levels of the proapoptotic protein Bax and reduced the cellular level of the antiapoptotic protein Bcl-2 but also increased their ratio in nontransfected cells. In addition to measuring levels of these apoptosis markers, we demonstrated that ethanol induces apoptosis in nontransfected cells by apoptosis assay and histochemical analyses. When ethanol treatment of nontransfected cells was followed by black soybean anthocyanins treatment, levels of apoptosis markers (such as Bax/Bcl-2 ratio, active caspase-3, and cleaved PARP-1) as well as the percentage of apoptotic cells in the population were reduced compared to ethanol-treated cells. All of these protective effects of anthocyanins against ethanol-induced neuronal apoptosis were more intense in the nontransfected group compared to GABAB1R siRNA transfected group, indicating that GABAB1R had a role in mediating the protective effects of anthocyanins.
Ethanol is known to induce neuronal apoptosis in many ways. Among them, oxidative stress is widely recognized as a major pathway by which ethanol induces neuronal apoptosis [12, 13, 36–44]. Since ethanol is known to increase oxidative stress and the antioxidative properties of anthocyanins are well recognized, it is possible to speculate that the protective effect of anthocyanins against ethanol-induced apoptosis could be attributed at least in part to their ability to scavenge the ROS induced by ethanol treatment. It is widely accepted that neuronal degeneration after ethanol administration is associated with increase in intracellular Ca2+ concentrations. Dysregulation of calcium homeostasis and collapse of mitochondrial membrane potential activate apoptosis. Ethanol significantly increased intracellular Ca2+ level and increased the proportion of cells with low mitochondrial membrane potential in nontransfected prenatal rat hippocampal neuronal cells. These effects of ethanol were less marked if the neurons had been transfected with GABAB1R siRNA prior to ethanol treatment [33] (Figs. 3 and 4). Similarly, anthocyanins inhibited the ethanol-induced increase in intracellular Ca2+ level and loss of mitochondrial membrane potential to a greater extent in nontransfected prenatal rat hippocampal neurons than in GABAB1R siRNA transfected neurons (Figs. 3 and 4). Thus, the neuroprotective effects of anthocyanins mediated by GABAB1R include reversal of the detrimental effects of ethanol on Ca2+ homeostasis and mitochondrial membrane potential. Stimulation of GABABRs has been shown to inhibit calcium influx in neurons [45] and to protect against neuronal apoptosis in a cytosolic Ca2+-dependent manner [46].
These observations agree with our results and suggest that anthocyanins directly or indirectly activate GABAB1R. The possibility that they stimulate downstream components of the GABAB1R signaling pathway cannot be excluded. Ethanol and GABABR activation have a major effect on the cyclic AMP signaling pathway in the brain [47]. Ethanol and GABABR activation also affect other signaling molecules related to cAMP, such as G proteins and PKA, and may cause changes in the functions of CREB. Previous study conducted in vitro has reported that ethanol increased the cellular levels of cAMP through activated adenosine A 2 receptors, which results in the phosphorylation of CREB [48]. Several lines of evidences show that CREB phosphorylation (p-CREB) is a crucial event in the early gene expression [49–51]. Our data (Fig. 1) show that ethanol toxicity was accompanied by increase in the levels of GABAB1R and its downstream signaling molecules such as PKA-α CaMKII and p-CREB (Fig. 2b). Black bean anthocyanins reversed the effect of ethanol on cellular levels of GABAB1R and its downstream signaling molecules to a greater extent in nontransfected cells than in GABAB1R siRNA transfected cells. Thus, black soybean anthocyanins modulate the effects of ethanol on GABAB1R levels and also modulate the effects of ethanol on the levels of downstream components of the GABAB1R signaling pathway through GABAB1R.
Conclusion
In conclusion, anthocyanins reverse the adverse effects of ethanol in cultured prenatal rat hippocampal neurons. They inhibit ethanol-induced processes such as apoptosis, increase in cytosolic Ca2+ level, mitochondrial membrane dysfunction, and neurodegenaration via GABAB1R signaling. Thus, anthocyanins have considerable potential as therapeutic interventions for mitigating the deleterious effects of ethanol on prenatal brain. Realization of this potential requires further research to examine their effects in vivo.
References
Clarren SK, Smith DW (1978) The fetal alcohol syndrome. Eng J Med 298:1063–1067
Streissguth AP, Aase JM, Clarren SK, Randels SP, LaDue RA, Smith DF (1991) Fetal alcohol syndrome in adolescents and adults. JAMA: J Am Med Assoc 265:1961–1967
Guerri C (1998) Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunction induced by prenatal alcohol exposure. Alcohol Clin Exp Res 22:304–312
Guerri C (2002) Mechanisms involved in central nervous system dysfunctions induced by prenatal ethanol exposure. Neurotox Res 4:327–335
Mondadori C, Jaekel J, Preiswerk G (1993) The first orally active GABA blocker improves the cognitive performance in mice, rats and rhesus monkeys. Behav Neurol Biol 60:62–68
Bormann J (2000) The ‘ABC’ of GABA receptors. Trends Pharmacol Sci 21:16–19
Bowery NG (1993) GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol 33:109–147
Misgeld U, Bijak M, Jarolinek W (1995) A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol 46:423–462
Dzitoyeva S, Dimitrijevic N, Manev H (2003) Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence". Proc Natl Acad Sci USA 100(9):5485–5490
Cooper DMF, Mons N, Karpen JW (1995) Adenylyl cyclases and the interaction between calcium and cAMP signaling. Nature 374:421–424
Ullah N, Naseer MI, Ullah I, Lee HY, Koh PO, Kim MO (2011) Protective effect of pyruvate against ethanol-induced apoptotic neurodegenaration in the developing rat brain. Neuropharmacology 61:1248–1255
Haorah J, Ramirez SH, Floreani N, Gorantla S, Morsey B, Persidsky Y (2008) Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic Biol Med 45:1542–1550
Zakhari S (2006) Overview: how is alcohol metabolized by the body? Alcohol Res Heal 29:245–254
Buonocore G, Perrone S, Bracci R (2001) Free radicals and brain damage in the newborn. Biol Neonate 79(3–4):180–186
Thompson LP, Al-Hasan Y (2012) Impact of oxidative stress in fetal programming. J Pregnancy 2012:1–8
Antonio AM, Druse MJ (2008) Antioxidants prevent ethanol-associated apoptosis in fetal rhombencephalic neurons. Brain Res 1204:16–23
Guo L, Wang LH, Sun B, Yang JY, Zhao YQ, Dong YX, Spranger MI, Wu CF (2007) Direct in vivo evidence of protective effects of grape seed procyanidin fractions and other antioxidants against ethanol-induced oxidative DNA damage in mouse brain cells. J Agric Food Chem 55:5881–5891
La GL, Wang M, Watkins R, Ortiz D, Sanchez ME, Konst J, Lee C, Reyes E (1999) Protective effects of the flavonoid mixture, silymarin, on fetal rat brain and liver. J Ethnopharmacol 65:53–61
Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D (2007) Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res 51:675–683
Ghosh D, Konishi T (2007) Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J Clin Nutr 16:200–208
Bagchi D, Sen CK, Bagchi M, Atalay M (2004) Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin- rich berry extract formula. Biochemistry (Mosc) 69:75–80
Rivero-Perez MD, Muniz P, Gonzalez-Sanjose ML (2008) Contribution of anthocyanin fraction to the antioxidant properties of wine. Food Chem Toxicol 46:2815–2822
Assuncao M, Santos-Marques MJ, De FM, Carvalho F, Andrade JP, Lukoyanov NV, Paula-Barbosa MM (2007) Red wine antioxidants protect hippocampal neurons against ethanol-induced damage: a biochemical, morphological and behavioral study. Neuroscience 146:1581–1592
Assuncao M, Santos-Marques MJ, De FV, Paula-Barbosa MM, Carvalho F (2008) Modulation of rat cerebellum oxidative status by prolonged red wine consumption. Addict Biol 13:337–344
Barros D, Amaral OB, Izquierdo I, Geracitano L, do Carmo Bassols RM, Henriques AT, Ramirez MR (2006) Behavioral and genoprotective effects of Vaccinium berries intake in mice. Pharmacol Biochem Behav 84:229–234
Hou DX (2003) Potential mechanisms of cancer chemoprevention by anthocyanins. Curr Mol Med 3:149–159
Varadinova MG, Docheva-Drenska DI, Boyadjieva NI (2009) Effects of anthocyanins on learning and memory of ovariectomized rats. Menopause 16:345–349
Xu B, Chang SK (2008) Characterization of phenolic substances and antioxidant properties of food soybeans grown in the North Dakota–Minnesota region. J Agric Food Chem 56:9102–9113
Grynkyewicz G, Poenie M, Tsien RY (1985) A new generation of Ca+2 indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO (2012) Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci 13:11
Yu J, Zhang L, Hwang PM et al (1999) Identification and classification of p53-regulated genes. Proc Natl Acad Sci 96:14517–14522
Sairanen T, Szepesi R, Karjalainen-Lindsberg ML, Saksi J, Paetau A, Lindsberg PJ (2009) Neuronal caspase-3 and PARP-1 correlate differentially with apoptosis and necrosis in ischemic human stroke. Acta Neuropathol 118:541–552
Naseer MI, Lee HY, Ullah N, Ullah I, Park MS, Kim SH, Kim MO (2010) Ethanol and PTZ effects on siRNA mediated GABAB1 receptor: down regulation of intracellular signaling pathway in prenatal rat cortical and hippocampal neurons. Synapse 64:181–190
Simasko MS, Boyadjieva N, De A, Sarkar DK (1999) Effect of ethanol on calcium regulation in rat fetal hypothalamic cells in culture. Brain Res 824:89–96
Barclay DC, Hallbergson AF, Montague JR, Mudd LM (2005) Reversal of ethanol toxicity in embryonic neurons with growth factors and estrogen. Brain Res Bull 67(6):459–465
Bleich S, Spilker K, Kurth C, Degner D, Quintela-Schneider M, Javaheripour K, Ruther E, Kornhuber J, Wiltfang J (2000) Oxidative stress and an altered methionine metabolism in alcoholism. Neurosci Lett 293:171–174
Bondy SC (1992) Ethanol toxicity and oxidative stress. Toxicol Lett 63:231–241
Bondy SC, Guo SX (1994) Effect of ethanol treatment on indices of cumulative oxidative stress. Eur J Pharmacol 270:349–355
Chen G, Ma C, Bower KA, Shi X, Ke Z, Luo J (2008) Ethanol promotes endoplasmic reticulum stress-induced neuronal death: involvement of oxidative stress. J Neurosci Res 86:937–946
Crews FT, Nixon K (2009) Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol 44:115–127
Ramachandran V, Watts LT, Maffi SK, Chen J, Schenker S, Henderson G (2003) Ethanol-induced oxidative stress precedes mitochondrially mediated apoptotic death of cultured fetal cortical neurons. J Neurosci Res 74:577–588
Sandhir R, Kaur K (2006) Influence of ethanol on methanol-induced oxidative stress and neurobehavioral deficits. J Biochem Mol Toxicol 20:247–254
Sun AY, Sun GY (2001) Ethanol and oxidative mechanisms in the brain. J Biomed Sci 8:37–43
Hampton MB, Orrenius S (1998) Redox regulation of apoptotic cell death. Biofactors 8:1–5
Benjamin R, Patrick N, Gudrun AH, Andreas R, Christian R, Joerg B, Dietmar S (2011) Activation of metabotropic GABA receptors increases the energy barrier for vesicle fusion. J Cell Sci 124:1–8
Tu H, Xu C, Zhang W, Liu Q, Rondard P, Pin JP, Liu J (2010) GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation. J Neurosci 30(2):749–759
Diamond I, Gordon AS (1997) Cellular and molecular neuroscience of alcoholism. Physiol Rev 77:1–20
Orna A, Thomas D, Cunningham LY, Adrienne S, Gordon ID (2002) Ethanol stimulates cAMP-responsive element (CRE)-mediated transcription via CRE-binding protein and cAMP-dependent protein kinase. JPET 301:66–70
Bading H, Ginty DD, Greenberg ME (1993) Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 260:181–186
Konradi C, Cole RL, Heckers S, Hyman SE (1994) Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci 14:5623–5634
Yamamoto JK, Sparger E, Ho EW, Andersen PR, OConnor TP, Mandell CP, Lowenstine L, Munn R, Pedersen NC (1998) The pathogenesis of experimentally induced feline immunodeficiency virus (FIV) infection in cats. cAm J Vet Res 49:1246–1258
Acknowledgments
This research was supported by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2012-0009521).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ali Shah, S., Ullah, I., Lee, H.Y. et al. Anthocyanins Protect Against Ethanol-Induced Neuronal Apoptosis via GABAB1 Receptors Intracellular Signaling in Prenatal Rat Hippocampal Neurons. Mol Neurobiol 48, 257–269 (2013). https://doi.org/10.1007/s12035-013-8458-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8458-y