Abstract
To explore a potential methodology for treating aganglionic megacolon, neural stem cells (NSCs) expressing engineered endothelin receptor type B (EDNRB) and glial cell-derived neurotrophic factor (GDNF) genes were transplanted into the aganglionic megacolon mice. After transplantation, the regeneration of neurons in the colon tissue was observed, and expression levels of differentiation-related genes were determined. Primary culture of NSCs was obtained from the cortex of postnatal mouse brain and infected with recombinant adenovirus expressing EDNRB and GDNF genes. The mouse model of aganglionic megacolon was developed by treating the colon tissue with 0.5 % benzalkonium chloride (BAC) to selectively remove the myenteric nerve plexus that resembles the pathological changes in the human congenital megacolon. The NSCs stably expressing the EDNRB and GDNF genes were transplanted into the benzalkonium chloride-induced mouse aganglionic colon. Survival and differentiation of the implanted stem cells were assessed after transplantation. Results showed that the EDNRB and GDNF genes were able to be expressed in primary culture of NSCs by adenovirus infection. One week after implantation, grafted NSCs survived and differentiated into neurons. Compared to the controls, elevated expression of EDNRB and GDNF was determined in BAC-induced aganglionic megacolon mice with partially improved intestinal function. Those founding indicated that the genes transfected into NSCs were expressed in vivo after transplantation. Also, this study provided favorable support for the therapeutic potential of multiple gene-modified NSC transplantation to treat Hirschsprung’s disease, a congenital disorder of the colon in which ganglion cells are absent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Materials and Methods
Materials
Experimental Animals
Newborn Kunming mice (male or female, average body weight 1.5 ± 0.2 g) and adult 8-week-old male Kunming mice (average body weight 29 ± 1.8 g) were purchased from animal core of Tongji Medical College (Wuhan, China; batch no. SYXK (E) 2004–0028). Major reagents are the following: diethyl pyrocarbonate, agarose, and low-melting-point agarose DMEM/F12 (1:1) (Sigma-Aldrich, St. Louis, MO, USA); B27 supplement (Invitrogen, Grand Island, NY, USA); EGF and βFGF (PeproTech, UK); 50 % benzalkonium chloride (BAC) (Sigma, Fluka, Switzerland); nestin polyclonal antibody (Boster, Wuhan, China); neuron-specific enolase (NSE) monoclonal antibody (Abcam, Cambridge, MA, USA); glial fibrillary acidic protein (GFAP) monoclonal antibody (Sigma-Aldrich, St. Louis, MO, USA); SABC-FITC and SABC-CY3 kit (Boster, Wuhan, China); SABC and DAB kit (Boster, Wuhan, China); internal ribosome entry site (IRES) expression vector pIRES (Clontech, Mountain View, CA, USA); HEK293 cell, Escherichia coli DH5-α that were stored by our lab, M-MLV reverse transcriptase, and Taq DNA polymerase (Roach, Indianapolis, IN, USA); RNase inhibitor and dNTPs (Promega, Madison, WI, USA); T4 DNA ligase, endonucleases XbaI and KpnI, DNA marker DL 2000, and Purigene DNA extraction kit (Takara, Dalian, China); diethyl pyrocarbonate, agarose, and low-melting-point agarose (Sigma-Aldrich, St. Louis, MO, USA); and Trizol (Gibco, Grand Island, NY, USA). Primer design upstream and downstream primers were designed based on sequence from Gene Bank and literature report and synthesized by Shanghai Bioengineering Company.
Methods
Construction and Identification of Recombinant Adenovirus Vector with Mouse Glial Cell-Derived Neurotrophic Factor and Endothelin Receptor Type B Gene
Construction of Multiple Gene Expression Adenovirus Vector
The mouse glial cell-derived neurotrophic factor (GDNF) and endothelin receptor type B (EDNRB) cDNAs were derived from the mouse brain and heart, respectively, by reverse transcription polymerase chain reaction (RT-PCR). The primers for amplifying GDNF were as follows: primer F 5′ GCTCGGTACCGACTCTAAGATGAAGTTA 3′ and primer R 5′ GGAGTCTAGAGGGTCAGATACATCCACAC 3′. Sequences of restriction endonuclease sites of KpnI and XbaI were added to the 5′ end of forward and reverse primers, respectively. The primers for amplifying EDNRB were primer F 5′ CCGCTGTCTGGCATTCTC 3′ and primer R 5′ GCCGTGCTGAAGTGCTGA 3′. EcolI and BamHI restriction sites were added to the 5′ end of forward and reverse primers, respectively. The PCR products of GDNF was subcloned into KpnI and XbaI multiple clone sites, followed by subcloning the PCR products EDNRB gene into EcolI and BamHI multiple clone sites of pAdTrack-CMV vector. An IRES sequence from pIRES vector was subcloned between GDNF and EDNRB genes by inserting into XbaI and EcolI sites of pAdTrack-CMV. The new constructed plasmid was named as pAdTrack-CMV-GE and was sequenced by Shanghai Bioengineering Company.
Preparation of Ad-GE Recombinant Adenovirus and Measurement of Virus Titer
AdEasier1 cells and E. coli DH5-α electroporated competent cells were prepared with 10 % pre-cold sterile glycerol. The pAdTrack-CMV-GE plasmid was linearized by digestion with PmeI restriction enzyme. One microgram linear pAdTrack-CMV-GE was mixed with 20 μl AdEasier1 and co-transformed into competent cells by electroporation at setting of 2,500 V, 200 Ω, and 25 μF. Transformed cells were selected by kanamycin (50 mg/L) resistance on LB solid agar plates. Fifteen colonies were randomly selected after incubation for 16 h, and plasmids were extracted from these clones. Positive plasmids with similar size of pAdEasy1 were further transformed into E. coli DH5-α competent cells by electroporation at the same setting that has used above. Recombinant adenoviral plasmids were extracted from clones grown on the plates and identified by Pad endonuclease digestion. The confirmed plasmid was named as pAD-GE and purified for transfection. Twenty-four hours prior to transfection, the 293 cells were seeded onto a 60-mm cell culture plate at 2 × 106 cells per well until grown 60–80 % of confluence. Four micrograms of linearized pAD-GE plasmid digested by PacI endonuclease was transfected into 293 cells by Lipofectamine 2000 reagent according to the manufacture’s instruction. Fresh fetal bovine serum (FBS) medium (100 mL/L) was replaced at 24 h after transfection. Green fluorescent protein (GFP) was observed at 3 days post-transfection with fluorescence microscope. Seven days after transfection, 293 cells were collected, followed by repeated freeze-thaw lysis for four times. After centrifugation, supernatant was collected. Virus expansion was achieved by infecting more 293 cells with the supernatant. After 5 days, infected 293 cells were collected and centrifuged at 1,500 rpm for 7 min. The supernatant was discarded, and the pellets were washed with 2 mL phosphate-buffered saline (PBS) per dish, followed by four repeated freeze-thaw cycles. The infection and collection steps were repeated one more time as described above. The final PBS supernatant was collected, and the adenovirus was purified from the virus resuspension by CsCL density-gradient ultracentrifugation. To calculate the virus titers, 293 cells were seeded at density of 5 × 105 per well onto a six-well plate and infected with a series of virus dilutions. Seventy-two hours later, the Ad-GE virus titers (expression forming unit per liter) were calculated based on the numbers of GFP-positive cells observed.
NSCs Primary Culture and Gene Transfection
Separation and cultivation of neural stem cells are done as follows: select a newly born mouse (born within 24 h) and then cut off successively the head, skin, and skull under aseptic condition after soaking the mouse in 75 % ethanol for 3–5 min and then take out completely the brain tissue; next, remove the meninges and blood tissues in the process of careful striping tissue on glacial table after rinsing the mouse in D-Hanks twice; take the cerebral cortex in a sterile culture dish with D-Hanks’ balanced salt solution and then transfer to balanced salt solution with the cerebral cortex into a sterile centrifuge tube. Next, add the solution after removing supernatant in centrifuging to 3 mL of complete medium and then blow and beat softly the processed solution by thin dripper so as to disperse brain tissue into monoplasts. As a result, obtain single-cell suspension after filtering by 300 cells; count living cells by Trypan Blue; then transfer single-cell suspension into a 50-mL sterile cell culture bottle, followed by adding it to DMEM/F12 complete medium with B27 (20 mL/L) + EGF (20 μg/L) + bFGF (20 μg/L); and inoculate the new medium with adjusted 5 × 105/mL of cell density into a 25-mL disposable cell culture bottle, which was placed in the cell incubator with 5 % CO2. Change the culture solution in half quantity once with the method of adding fresh complete medium every 3 days [1]. SABC immunohistochemistry staining against nestin was performed to assess pre-differentiation status of neurospheres. Glial fibrillary acidic protein and neuron-specific enolase are markers for astrocytes and neuron, respectively, and were stained by immunohistochemistry (IHC) to examine the multiple potent of NSCs. NSCs were infected by adenovirus as described in jetPEITM in vitro transfection protocols. GFP fluorescence was dynamically observed at 24 to 72 h after infection. At 48 h after transfection, the average number of positive cells was calculated from ten random fields under × 100 objects. The average number multiplied by 50 is the positive cell number per well in a 24-well plate. Transfection efficiency was examined by FACStar flow cytometry at 24, 48, and 72 h after infection. Three samples were tested from each group, and 10,000 cells per sample were enumerated. The calculation formula of positive transfection rate was as follows: efficiency (GFP-positive cell ratio) = percentage of GFP-positive cells (M1 region) − percentage of GFP-negative cells (M1 region), and GFP-positive cell region is designated M1 region based on the negative control of non-transfected cells. The gene expression levels were examined at 24, 48, and 72 h after transfection.
-
Primer design, internal reference β-actin 443 bp Tm 52.6 °C
-
Forward 5′-GGGAAATCGTGCGTGA-3′
-
Reverse 5′-CTGGAAGGTGGACAGTGAG-3′
-
-
EDNRB 208 bp Tm 53.1 °C
-
Forward 5′-ACAAGCACGATCCGTAGT-3′
-
Reverse 5′-ACGCCACCCACTAAGACC-3′
-
-
GDNF 240 bp Tm 54.6 °C
-
Forward 5′-TGGTCTATTTGTTCGCCG-3′
-
Reverse 5′-GGATGTCGTGGCTGTCTG-3′
-
Construction and Identification of Mouse Model of Aganglionic Megacolon
Ninety male Kunming mice of 8 weeks old were randomly divided into three groups: (1) BAC group (n = 30), of which colonic serosa was treated with 0.5 % BAC as denervation reagent; (2) normal saline (NS) group (n = 30), which received saline instead of a denervation reagent; and (3) normal control group (n = 30), which has no treatment. For BAC-treated group, mice were operated under anesthesia, and 0.5 % BAC was applied onto the serous layer of colon descendens for 15 min. In NS group, normal saline was applied instead of BAC. The behavior, diet, and bowel movements were observed daily after surgery. Four mice of each group were sacrificed at 2, 7, 14, and 28 days after surgery. The treated section of descending colon was obtained. After flushing the intestinal lumen with saline, the tissues were examined for pathological changes, including the numbers of myenteric neurons, mucous membrane, and submucous and muscle layers. To enumerate neurons, each treated colon segment was continuously cut into 200 slices of 5 μm thickness. Five consecutive slices were combined as one group, and total 40 groups were measured. The number of neurons was counted only from the first slice of each group, and the sum of 40 groups was represented as the total neurons in 1 mm of enteric plexuses of the colon. Immunohistochemistry staining for neurofilaments-200 (NF-200) was performed to examine the removal of enteric plexuses. RT-PCR assay was performed to examine the mRNA expression level of NF-200, GFAP, anti-choline acetyltransferase (ChAT), and neuronal nitric oxide synthase (nNOS). Primers for RT-PCR were as follows:
-
Internal reference β-actin 443 bp
-
Forward 5′-GGGAAATCGTGCGTGA-3′
-
Reverse 5′-CTGGAAGGTGGACAGTGAG-3′
-
-
NF-200 215 bp
-
Forward 5′-TTCAGCCAGACTACCTCCC-3′
-
Reverse 5′-CGCTGCGGATTAACAACC-3′
-
-
GFAP 188 bp
-
Forward 5′-TTGTCCTTCTCGCGGTAC-3′
-
Reverse 5′-GTGGTATCGGTCTAAGTTTGC-3′
-
-
ChAT 159 bp
-
Forward 5′-AAAATGGCGTCCAACGAG-3′
-
Reverse 5′-CAGGCATACCAGGCAGAT-3′
-
-
nNOS 102 bp
-
Forward 5′-ACAACCTCGCTACTATTCCA-3′
-
Reverse 5′-CCTTCTCCATCTCGGGTA-3′
-
Survival, Differentiation, and Gene Expression of NSCs Transplanted in the Colonic Wall of Megacolon Mice
NSCs Transplantation
Two weeks after denervation surgery, the mice survived from BAC group were randomly divided into two groups: (1) NSCs group (n = 30) which received NSCs treatment and (2) NS group (n = 30) which received saline treatment. NSCs introduced with EDNRB and GDNF genes were centrifuged and resuspended in PBS at a density of 1 × 106/100 μL. A total of 200 μL NSC suspensions were injected into the denervated colon at four different sites using a 50-μL microinjector. After injection, the needle was hold in the same position for 5 min to distribute the cells suspension and prevent leakage. The sites of injection were labeled with 8–0 suture. In NS group, saline was injected instead of NSCs.
Survival and Differentiation of NSCs In Vivo
The biological features, behavior, diet, and bowel movements were examined after transplantation surgery. At 7, 14, 21, and 28 days after surgery, respectively, two mice were sacrificed from each group. Colons were harvested, and frozen sections were prepared. GFP fluorescence was observed using fluorescence microscope to examine the survival of NSCs. NSE and GFAP expression in the frozen tissue sections were examined by immunofluorescence staining to determine the differentiation of transplanted NSCs. At the same time, number of neurons was counted using the same method as described above.
Examine the mRNA Levels of ChAT, nNOS, GDNF, and EDNRB Genes by RT-PCR
Two mice of each group were randomly selected and sacrificed at 28 days after transplantation. Segment of colon treated with NSCs or saline was harvested and cleaned. Mucous membrane and submucous layer were carefully removed, and the muscle layer was kept at −70 freezer and subjected to RT-PCR assay to detect the mRNA expression level of target genes. The method used and the primer design were described as above.
Statistical Analysis
Data were analyzed using SPSS 13.0 (IBM SPSS Data Collection, Armonk, NY, USA) and presented as mean ± SEM. A t test was used to compare the two different groups, and the difference between three or more groups was analyzed using a one-way ANOVA followed by the Newman–Keuls or Dunnett’s post hoc tests. P values less than the 0.01 were considered statistically significant.
Results
Identification of pAdTrack-CMV-GE by Restriction Endonuclease Digestion
The recombinant plasmid pAd-GE was confirmed by restriction endonuclease digestion and agarose gel electrophoresis. The size of DNA fragments after digestion was the same as the size of GDNF and EDNRB genes, which matched with the DNA marker (Fig. 1).
Primary Culture of NSCs and Transfection with Recombinant Adenovirus
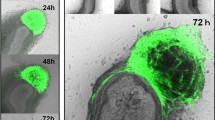
GFP fluorescence was observed in a small amount of NSCs at 24 h after transfection with Ad-GE adenovirus. The intensity of fluorescence was strong, and fluorescence was distributed in the neurospheres in a circular or oval shape. The intensity of fluorescence reached the peak level at 48 h after infection, and the number of positive cells markedly increased (Fig. 2). Cellular toxicity resulted from transfection was observed, including the appearance of less prominent projection on GFP-positive cells, irregular shapes accompanied by distributed green granules, and moderate number of cell deaths. However, the number of GFP-positive cells can reach to 2.42 × 105 per well. Expression of reporter gene GFP suggested successful package of adenovirus particles.
Flow cytometry analysis showed that the efficiency of viral transfection was 15.36, 24.67, and 25.73 % at 24, 48, and 72 h, respectively, and 0.45 % for the control group. Total RNA was extracted from NSCs at 24, 48, 72, and 96 h after Ad-GE infection, respectively. RT-PCR was performed to examine the expression levels of GDNF and EDNRB gene. Result showed that mRNA expression of these two genes can be detected as early as 24 h after infection and reached to the maximum expression at 48 to 72 h and declined gradually after 72 h (Figs. 3 and 4).
Construction of Mouse Model of Aganglionic Megacolon
Histological Measurement
Histological measurement was performed at 2, 7, 14, and 28 days after surgery. Results showed that no morphological change was observed. The colons of sham and NS group of mice showed normal structures of the mucous membrane and submucous and muscle layers with normal distribution of myenteric neurons. In BAC group, mononuclear cell aggregates can be observed in serosa and muscle layers at 2 and 7 days after surgery. The structure of smooth muscle was close to normal at day 2 after surgery. Disordered and hypertrophy of smooth muscle structure appeared at 7 days after surgery (Fig. 5). The number of myenteric neurons in BAC group mice has a significant decrease compared with sham and NS groups (P < 0.01) at all of the time points measured after surgery. As early as 2 days after surgery, the number of myenteric neurons declined to one fifth of the number in NS group, which is statistically significant compared to the other three time points. At 7, 14, and 28 days after surgery, the reduced levels of neuron left were less than 10 % of the number in normal group and remained consistent with no marked difference between each time point (Table 1).
HE staining was performed at 7 days after surgery. The tissues were examined for pathological changes, including the numbers of myenteric neurons, mucous membrane, and submucous and muscle layers. BAC group showed absence of myenteric nerve plexus and a smooth muscle layer with normal structure. Mononuclear cell aggregates can be observed in serosa layer (a). NS group showed normal myenteric nerve plexus shown by the arrowhead (b)
mRNA Expression Levels of NF-200, GFAP, ChAT, and nNOS by RT-PCR
RT-PCR assay was used to examine the mRNA expression levels of NF-200, GFAP, ChAT, and nNOS of each group at 14 days after surgery. Compared to sham and NS control groups, BAC treatment led to a significant decrease of mRNA expression of NF-200, GFAP, ChAT, and nNOS genes (P < 0.01). The decreased mRNA expression in NF-200 and GFAP suggested a smaller number of neurons present in the myenteric nerve plexus. The reduced mRNA expression of ChAT and nNOS suggested an impaired function of cholinergic neurons and nitrergic neurons which regulate the constriction and relaxation of smooth muscle and, consequently, the dysfunctional mobility of a local intestinal segment (Table 2).
Survival, Differentiation, and Gene Expression of Transplanted NSCs in Aganglionic Megacolon Mice
Gross Observation
After transplantation surgery, mice from both NS and NSCs groups have shown constipation, abdominal distension, and even no excretion. The overall symptoms from NSCs transplantation mice were relatively milder than those from NS group, although gross anatomy observation showed stenosis and feces retention at the proximal part of a treated colon segment of both NS and NSCs groups. The symptoms of NSCs group were alleviated at 28 days after transplantation surgery, with moister feces inside the intestinal lumen than that of the NS group and no obvious formation of stercoral (Fig. 6).
Survival and Differentiation of Transplanted NSCs
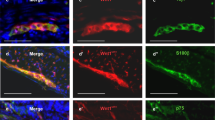
At 7 days after transplantation, green fluorescence can be observed under UV light at a muscle layer of the colon wall in NSCs group. Immunofluorescence assay showed positive nestin signal at a NSC-transplanted colon segment, but little NSE- and GFAP-positive cells. Results indicated that the NSCs can survive in myenteric microenvironment at 7 days after transplantation, but no differentiation occurred yet. No positive signal was observed in NS group. At 21 days after NSCs transplantation, cells with green fluorescence were still present. Immunofluorescence assay showed no nestin-positive cells. Most are NSE-positive cells, and a small portion of cells was expressing GFAP, which suggested that by now, NSCs have differentiated mostly into neurons, and some differentiated into neurogliocytes (Fig. 7). Compared to NSCs treatment group, only a small portion of NSE-positive cells can be detected in NS group.
After NSCs transplantation, fluorescence microscopy observed the muscle layer of colon wall in NSCs group. At 7 days, Hoechst3343-labeled NSCs can be observed under UV light at the muscle layer of the colon wall (a). Immunofluorescence assay showed positive nestin signal at a NSC-transplanted colon segment, but little NSE- and GFAP-positive cells (b). However, at 21 days, Hoechst3343-labeled NSCs were still present (c), with NSE expression determined by immunohistofluorescence staining, FITC labeled (d), and GFAP expression, CY3 labeled (e)
mRNA Expression Levels of ChAT, nNOS, GDNF, and EDNRB by RT-PCR
RT-PCR assay was used to examine the mRNA expression levels of genes in NS and NSCs groups. At 28 days after transplantation surgery, relative expression level of ChAT in NS group was 0.27 ± 0.03 compared to 0.53 ± 0.04 at NSCs group (P < 0.01). The relative expression level of nNOS in NS group was 0.17 ± 0.03 compared to 0.4 ± 0.04 at NSCs group (P < 0.01) (Table 3). The increased expression of ChAT and nNOS mRNAs suggested that the transplanted NSCs are differentiated into functional cholinergic neuron and nNOS-positive neurons which regulate the bowel movement function. RT-PCR results also show an elevated expression of GDNF and EDNRB mRNAs which suggested that the genes introduced into NSCs can be effectively expressed in vivo after transplantation.
Discussion
The enteric nervous system (ENS) is embedded in the lining of the gastrointestinal system (including the pancreas and gallbladder) that consisted of neuron, neural transmitter, proteins, and their supportive cells. The major neurons of the ENS come from myenteric and submucosal nerve plexuses. The major function of ENS includes the control of motility and regulation of fluid exchange and local blood flow. The ENS originates from neural crest cells that colonize the gut during intrauterine life [2]. After colonizing, neural crest-derived cells within the gut wall then migrate, propagate, and differentiate into glial cells plus many different types of neurons and generate the most complex part of the peripheral nervous system under the control of microenvironmental changes from the gut. Hirschsprung’s disease is a disorder of the gut which is caused by the failure of the neural crest cells to migrate properly during fetal development of the intestine and the lack of normal functional neurons in myenteric nerve plexus as a consequence. Currently, surgery is the major therapeutic option; however, there are several concerns such as various complications and large wound resulted from surgical section of an affected intestinal segment. More importantly, the long-term outcome after surgery is still uncertain.
Neural stem cells (NSCs) are the self-renewing, multipotent cells that generate the main phenotypes of the nervous system. The major function of NSCs serves as a reservoir of the cellular backup to repair the nerve system damage and replenish neuron cells lost due to normal death. The characteristic of NSCs includes the following: (1) the ability of self-renewing and proliferation through symmetric and asymmetric divisions; (2) the ability of multidirectional differentiation; NSCs can be induced under varied induction conditions to differentiate into specialized cells such as neurons, astrocytes, or oligodendrocytes [3]; (3) the ability of migration and well tissue compatibility [4]; and (4) low immunogenicity [5]. Because these are key features, NSCs transplantation to treat CNS neurodegeneration disease and spinal cord injury has become a hot spot in neuroscience research field and achieved some affirmative experimental results, such as glioblastoma multiforme [6].
There is relatively little research on treating the enteric nervous system disease with NSCs. Rauch et al. [7] has reported that NSCs derived from the intestine of a fetus or neonate have the ability to differentiate into neural precursor cell for neurons and neuroglia cells. Lindley et al. [8] showed that after mouse neural precursor cells were transplanted into aganglionic mouse fetus, the precursor cells could migrate and differentiate into functional neurons and restore the ability of regulating the construction rate of the colon. Furthermore, Micci et al. [9] transplanted NSCs into the pylorus of gastroparesis mice (nNOS_/_ mice) and examined the gastric empting and pylorus function. They found that after transplantation, NSCs could differentiate into nNOS-positive neurons, and the gastric emptying function was remarkably restored.
Based on the previous research, we designed experiments to study the therapeutic potential of treating mice with aganglionic megacolon by transplantation of NSCs that have been introduced with both GDNF and EDNRB genes by adenovirus infection. Findings of our study would establish a fundamental principle for the treatment of the human Hirschsprung’s disease with stem cell therapy. In this study, we have shown a successful isolation and culture of the NSCs by neurosphere methods, and NSCs were identified by positive nestin IHC staining. Those NSCs can be induced by FBS to differentiate into neurons, astrocytes, or oligodendrocytes. Also, we also successfully constructed an aganglionic megacolon mouse model by selectively denervating the myenteric nerve plexuses with 0.5 % BAC. This model is valuable in the study of NSCs treatment of aganglionic megacolon.
In our study, we used microinjection technique to deliver the NSCs at multiple sites into the muscle layer of a denervated segment of the colon wall. Histology examination showed no obvious inflammatory response in the area around injection site, which suggested the low immunogenicity of NSCs. After 7 days of NSCs transplantation surgery, nestin-positive cells can be detected that indicate the survival of transplanted NSCs within the colon wall. Meanwhile, the negative staining of NSE and GFAP at 7 days suggested that those NSCs have not been differentiated yet. At 21 days after transplantation, no nestin-positive cells were present, and the majority of cells were NSE positive with a small portion of GFAP-positive cells. Those findings indicated that most NSCs have been differentiated into neurons and, to a less extent, glial cells. The expression pattern of NSCs in vivo was different from our in vitro study. We found that in vitro NSCs would differentiate more to astrocytes than to neurons with a ratio of less than 20 %. The reason of this difference is unknown, but it may be closely related to the local cytokines secretion and the different microenvironments for the cells. Therefore, we assume that colon walls of this mouse model may provide the favorable microenvironment for the differentiation of NSCs mostly into neuron rather than glial cells. To validate this, definitely, further investigation of the in vivo differentiation mechanism of NSCs is required. Neural glial cells differentiated from some NSCs could secrete nutrition factors, which provide nutritional supplements to support the growth of neurons and integration with host cells. Thus, neural glial cells might also be important for restoring bowel function.
The fates of transplanted NSCs could be the following: (1) cell death at the transplantation sites because of the different environments between in vitro and in vivo; (2) transplanted cells maintain in G0 phase with no proliferation or differentiation; and (3) cells successfully survive and differentiate into functional neurons and glial cells to repair the injured myenteric nerve plexus. In this study, we injected the NSCs with microinjector at a tilted angle with carefully controlled depth to minimize the traumatic damage to the colon wall. Also, we held the needle in situ for five more minutes after a slow injection to prevent the leakage of the NSCs. The NSCs were transfected with vector-carrying GFP gene, and the survival of these NSCs within the colon wall was observed with GFP fluorescence. However, no nestin-positive cell was found at day 21 post-transplantation, which indicated no continuous propagation of these NSCs within the colon wall. Immunofluorescence assay showed the NSE- and GFAP-positive cells, which reveal that NSCs differentiated into neurons and neural glial cells in vivo. RT-PCR results showed the mRNA expression levels of ChAT and nNOS after NSCs transplantation were significantly higher than those in NS group, which suggests the regeneration of cholinergic neuron and nNOS neuron that may contribute to the repairing of the myenteric nerve plexus and restoration of the intestinal motility. The elevated expression of GDNF and EDNRB mRNA from NSCs transplantation group suggested that the genes introduced into NSCs can be expressed efficiently in vivo. This study establishes a fundamental basis for future study of multiple gene-introduced stem cell therapy for congenital megacolon.
Nonetheless, the research of NSCs therapy for intestinal neuromuscular disorders is still at initial phase, and there are many questions to be investigated such as the following: (1) is the best resource of NSCs and of embryonic and mesenchymal stem cells originated from CNS or neural crest stem cells, which is the best cellular donor? (2) Could the NSCs after transplantation in vivo maintain its biological features as in vitro culture? (3) Are there detailed cellular mechanisms of the proliferation, differentiation, and migration of the NSCs after transplantation and long-term outcome of NSCs transplantation?
In conclusion, we successfully constructed the recombinant adenovirus containing GDNF and EDNRB genes and transfected NSCs in primary culture. By using 0.5 % BAC treatment, we developed the aganglionic megacolon mouse model which resembles the pathohistological changes seen in congenital megacolon. After transplantation of NSCs into the colon wall of mice with aganglionic megacolon, we found out that the NSCs could survive and differentiate into neurons and neural glial cells within the colonic microenvironment. Most importantly, differentiated NSCs were able to repair the damaged myenteric nerve plexuses and partially restore the intestinal function. Meanwhile, expression of the target genes was detected by RT-PCR. Experimental data of this study support the therapeutic potential of multiple gene-transfected neural stem cell therapy as a novel remedy for human Hirschsprung’s disease.
References
Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710
Grundy D, Schemann M (2007) Enteric nervous system. Curr Opin Gastroenterol 23:121–126
Temple S (2001) The development of neural stem cells. Nature 1414:112–117
Amar AP, Zlokovic BV, Apuzzo ML (2003) Endovascular restorative neurosurgery: A novel concept for molecular and cellular therapy of the nervous system. Neurosurgery 52:402–412
Modo M, Rezaie P, Heuschling P et al (2002) Transplantation of neural stem cells in a rat model of stroke: Assessment of short-term graft survival and acute host immunological response. Brain Res 958:70–82
Gonzalez-Gomez P, Sanchez P, Mira H (2011) MicroRNAs regulators of neural stem cell-related pathways in glioblastoma multiforme. Mol Neurobiol 44:235–249
Rauch U, Hänsgen A, Hagl C et al (2006) Isolation and cultivation of neuronal precursor cells from the developing human enteric nervous system as a tool for cell therapy in dysganglionosis. Int J Colorectal Dis 21:554–559
Lindley RM, Hawcutt DB, Connell MG et al (2008) Human and mouse enteric nervous system neurosphere transplants regulate the function of aganglionic embryonic distal colon. Gastroenterology 135:205–216
Micci MA, Kahrig KM, Simmons RS et al (2005) Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology 129:1817–1824
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shu, X., Meng, Q., Jin, H. et al. Treatment of Aganglionic Megacolon Mice via Neural Stem Cell Transplantation. Mol Neurobiol 48, 429–437 (2013). https://doi.org/10.1007/s12035-013-8430-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8430-x