Abstract
In this study, 5,10,15,20-tetrakis(p-chlorophenyl)porphyrin has been synthesized and used as an ionophore for the preparation of a poly(vinyl chloride) (PVC) membrane sensor for aluminium(III) ions. The optimum composition of the best performing membrane contained ionophore, bis(2-ethylhexyl) adipate, PVC and potassium tetrakis(p-chlorophenyl) borate in the ratio of 4.0:63.0:32.0:1.0 (mg). The prepared PVC membrane morphology has been analysed by scanning electron microscopy. The developed aluminium(III)-selective sensor works in a wide linear concentration range of 1.0 × 10–5 to 1.0 × 10–1 mol l–1 and, the detection limit of this sensor is 2.81 × 10−6 mol l–1. The sensor displays near-Nernstian slope of 25.0 ± 2.7 mV per decade for Al3+ ions. The aluminium(III)-selective sensor has a wide working pH range of 5.0–10.0. The sensor shows good reusability, long-term stability and a fast response time of less than 5 s. In addition, the sensor shows good selectivity for Al3+ ions over different cations. This aluminium(III)-selective sensor was successfully used as an indicator electrode in the potentiometric titration of Al3+ ions with EDTA. The developed sensor was successfully applied to the direct determination of Al3+ in different water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aluminium exists in nature extensively and it is the third most abundant element in the earth’s crust following oxygen and silicon [1]. Also, it is an essential element in diverse biological systems. Aluminium is an important metal, which has tremendous and multifunctional usage in many industries and other fields, such as telecommunication, the development of electrical equipment, machinery, household appliances, transport, storage, cosmetics, pharmaceutical production, catalysis, culinary utensils, packaging materials, architecture and potable water treatment units [2,3,4,5,6,7]. Aluminium is a powerful neurotoxicant, and considerable evidence suggests that it plays a role in the development of Parkinson’s disease (PD), Alzheimer’s disease (AD) and some kidney diseases [8,9]. Therefore, the determination of aluminium in different areas is very crucial. Many different analytical techniques of aluminium(III) determination have been applied such as inductively coupled plasma mass spectrometry [10,11], atomic absorption spectrometry (AAS) [12,13], UV–Vis spectroscopy [14] and voltammetry [15]. However, these methods require high-energy consumption, sample pre-treatment, long application time and they are mostly too expensive. Therefore, potentiometric-based ion sensors or ion-selective electrodes offer great advantages, including wide linear working range, low-energy consumption, low cost, good sensitivity and short response time [16,17,18,19,20,21]. As a result, these methods have been extensively studied by researchers.
Porphyrins are an important class of macrocyclic compounds, and they play very important roles in the metabolism of living organisms [22]. Porphyrins have gained interest by researchers working in the sensor field; because the porphyrin nucleus is a tetradentate ligand providing a space for a coordinated metal [23]. In addition, porphyrin derivatives used as electron mediators exhibit great electrocatalytic activity due to their conjugated two-dimensional π-system and special electrochemical properties [24]. Until today, a variety of porphyrin-based potentiometric sensors have been developed for the detection of different metal ions [25,26].
In the present work, 5,10,15,20-tetrakis(p-chlorophenyl)porphyrin (figure 1) was synthesized according to a procedure similar to that reported in the literature [27]. Then, this synthesized molecule was used as an ionophore and a novel aluminium(III)-selective PVC membrane sensor was developed. The potentiometric behaviour of the developed sensor was tested under laboratory conditions.
2 Materials and methods
2.1 Chemicals and reagents
Aluminium(III) stock solution (1.0 × 10–1 mol l–1) was prepared by dissolving Al(NO3)3·9H2O (Sigma Aldrich) in deionized water and making up the final volume to 50 ml. Other metal solutions were prepared in the same way as nitrate salts. Other reagents such as ortho-nitrophenyloctyl ether (o-NPOE), dibutyl phthalate (DBP), bis(2-ethylhexyl) adipate (DEHA), bis(2-ethylhexyl)sebacate (BEHS), high relative molecular weight PVC, ethylenediaminetetraacetic acid (EDTA), potassium tetrakis (p-chlorophenyl)borate (KTpClPB), sodium tetraphenyl borate (NaTPB), tetrahydrofuran (THF), hydrochloric acid (HCl), sodium hydroxide (NaOH) and graphite were purchased from the Fluka, Merck and Sigma Aldrich Chemical companies. Epoxy (Macroplast Su 2227) from Henkel (Istanbul, Turkey) and hardener (Desmodur RFE) from Bayer AG (Darmstadt, Germany) were obtained. Deionized water was produced by using a DI 800 Model deionized water system.
2.2 Apparatus
The potential measurements were performed by using a computer-controlled multichannel potentiometric system (Medisen Medical Ltd. Sti., Turkey). This system has a lab-made software program. A micro-sized solid Ag/AgCl (Thermo-Orion) was used as a reference electrode in the potentiometric cell. 1H spectra were measured on a Bruker Avance DPX-400 instrument. Fourier transform infrared (FT–IR) spectra were saved by Jasco FT/IR-4700 spectrometer. The melting point was measured on an Electrothermal 9100 apparatus. pH measurements were performed with a digital pH meter (Mettler Toledo Model S220-K). Surface images and energy-dispersive X-ray (EDX) analysis of prepared aluminium(III)-selective electrode were carried out with a scanning electron microscope (SEM; Quanta FEG 450-FEI). Atomic absorption spectrometry (Perkin Elmer Analysis 700 model) was used in comparison studies.
2.3 Method
2.3.1 Synthesis of ionophore
5,10,15,20-Tetrakis(p-chlorophenyl)porphyrin was synthesized by following the procedure in the literature [27]. A quantity of 1.406 g (0.01 mol) of 4-chlorobenzaldehyde and 0.69 ml (0.01 mol) of pyrrole were refluxed for 1 h in 70 ml propionic acid. The reaction mixture was then cooled down to room temperature, and the solution was washed several times with hot water and subsequently with methanol. The obtained purple crystals were allowed to dry at room temperature for 48 h. The synthesis scheme of the porphyrin derivative molecule is shown in figure 2. The spectroscopic data are identical to the data reported in the literature [28]. Purple crystals, Mp: > 300°C; 1H NMR (400 MHz, CDCl3): δ –2.82 (br. s, 2-NH), 7.77 (d, J = 8.4 Hz, 8Ar–H), 8.16 (d, J = 8.4 Hz, 8Ar–H), 8.87 (s, 8-pyrrole–H). IR (cm−1): 1709, 1484, 1463, 1387, 1346, 1220, 1176, 1085, 1009, 965, 843, 790, 718, 700.
2.3.2 Preparation of PVC membrane sensors
All-solid-state contact material was prepared according to the literature [29,30]. For the preparation of all-solid-state contact material, the powdered 50 mg of graphite, 35 mg of epoxy and 15 mg of hardener were mixed in approximately 5 ml of THF and, after obtaining an appropriate viscosity, a copper wire (approximately 1 mm of thickness and 5–15 cm length) was immersed in the solution. The copper wires coated with the conductive solid contact mixture were left to dry in the dark for 24 h. Then, PVC membrane sensors were prepared. For this purpose, ionophore (5,10,15,20-tetrakis(p-chlorophenyl)porphyrin), plasticizers (o-NPOE, DBP, BEHS and DEHA), KTpClPB and PVC in different proportions were dissolved in 5 ml of THF (table 1). The pre-prepared all-solid-state contact electrodes were immersed in the PVC membrane mixture. Finally, the prepared aluminium(III)-selective sensors were allowed to dry for approximately 12 h. Aluminium(III)-selective sensors were prepared by coating the membrane cocktail on the surface of the all-solid-state contact (figure 3). Optimum PVC membrane cocktail was determined as 4.0% (w/w) ionophore, 63.0% (w/w) plasticizer (DEHA), 32.0% (w/w) PVC and 1.0% (w/w) KTpClPB (table 1).
2.4 Potential measurements
Potentials were measured using Ag/AgCl reference electrode. All potential studies were carried out at 25 ± 1.0°C temperature by using the following cell assembly: Ag/AgCl; KCl (saturated) ||Al3+ sample solution|Al3+ selective sensor|conductive solid contact|Cu wire.
3 Results and discussion
In this study, an aluminium(III)-selective PVC membrane sensor was developed. The potentiometric characteristics of the sensor such as working concentration range, selectivity, reusability, response time, lifetime and pH working range were further investigated under laboratory conditions.
3.1 Characterization of 5,10,15,20-tetrakis(p-chlorophenyl)porphyrin
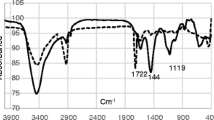
The 5,10,15,20-tetrakis(p-chlorophenyl)porphyrin was characterized by 1H-NMR and FT–IR techniques. Figure 4 shows the FT–IR and 1H-NMR spectra for the 5,10,15,20-tetrakis(p-chlorophenyl)porphyrin. Figure 4 indicates a peak at 1709 cm–1, which belongs to the C=N of the porphyrin ring. The peak at 1463 cm–1 belongs to the chemical bond stretching of the C=C of aromatic groups, while the peak at 1176 cm–1 belongs to the chemical bond stretching of the C–H of structure molecule. In 1H-NMR, two protons in –2.82 ppm belong to –NH of the porphyrin ring. Doublet splittings found in 7.77 and 8.16 ppm belong to the protons in the aromatic ring. Finally, the peak at 8.87 ppm belongs to the protons in the porphyrin ring. The FT–IR and 1H-NMR spectra of the structure are shown in figure 4a and b. As a result, we can state that the spectroscopic data obtained in this study are in harmony with the structure.
3.2 Electrode surface characterization
Surface characterization of the developed aluminium(III)-selective electrode was investigated by SEM technique. SEM images of the PVC membrane electrode are given in figure 5. As seen, prepared optimum membrane mixture exhibited porous polymer matrix, physically tight, homogeneous and crack-free structure as expected. EDX analysis was performed with prepared all-solid-state PVC membrane (figure 6a) and all-solid-state PVC membrane electrode conditioned in aluminium(III) solution for 3 h (figure 6b). EDX analysis shows that there is only carbon, oxygen and chloride in the all-solid-state PVC membrane structure (figure 6a). On the other hand, the PVC membrane electrode conditioned in 1.0 × 10–2 mol l–1 aluminium solution for 3 h has aluminium peaks (figure 6b). These results reveal that the porphyrin derivative molecule (ionophore) is efficient to form the complex with aluminium(III) ions.
3.3 Working concentration range, calibration curve and response time
The potential behaviour of electrodes prepared with all-solid-state, all-solid-state + DEHA and all-solid-state + DEHA + KTpClPB membrane components against aluminium(III) ion was tested. Thus, the effect of the ionophore was determined. Obtained potentiometric measurement results are given in figure 7. As seen in figure 7, all electrodes without ionophore showed a non-linear potentiometric response to Al3+ ions, while PVC membrane sensor with ionophore in their composition showed a linear potentiometric response to Al3+ ions.
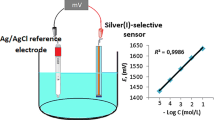
The potential response of the aluminium(III)-selective sensor as a function of Al3+ activity was studied in the concentration range of 1.0 × 10–5 to 1.0 × 10–1 mol l–1 of Al3+. Membrane 1 exhibited a narrow working concentration range of 1.0 × 10–4 to 1.0 × 10–1 mol l–1. Membranes 2–6 exhibit behaviour at concentrations from 1.0 × 10–5 to 1.0 × 10–1 mol l–1, but their correlations are low. The optimum membrane (membrane no. 7) showed a linear behaviour with the highest correlation value (R2 = 0.9978) in the 1.0 × 10–5 to 1.0 × 10–1 mol l-1 concentration range (figure 8). This sensor displayed a near-Nernstian slope of 25.0 ± 2.7 mV per decade and a detection limit of 2.81 × 10–6 mol l–1 (figure 8). In addition, the developed sensor reached equilibrium very quickly at every 10-fold concentration change. The response time of the sensor was determined according to the IUPAC recommendations and was found to be 5 s [31].
3.4 Potentiometric selectivity
Selectivity is one of the most important characteristics of any sensor and it is defined as the ability to determine a particular species without being affected by other species present in the sample mixture [32]. The nitrate salts of different ions such as K+, Na+, Li+, NH4+, Pb2+, Mg2+, Cu2+, Cd2+, Ni2+, Sr2+, Ca2+, Co2+, Zn2+ and Cr3+ were measured at concentrations ranging from 1.0 × 10–5 to 1.0 × 10–1 mol l–1 by the developed sensor. Figure 9 shows that the sensor is highly selective against Al3+ ions even in the presence of other metal ions. The selectivity coefficients of aluminium(III) ion and other cations were calculated according to the following equation at a concentration of 1.0 × 10–2 mol l–1 by using the separate solution method (SSM) by IUPAC [33].
where \({K}_{\mathrm{A},\mathrm{B}}^{\mathrm{pot}}\) = selectivity coefficient, aA = activity of aluminium(III) ion, aB = activity of interfering ion, \(Z\)A = charge of aluminium(III) ion, \(Z\)B = charge of interfering ion; R is the gas constant equal to 8.314510 J K−1 mol−1; T the absolute temperature, K; F is Faraday constant, 9.6485309 × 104 C mol−1.
The selectivity coefficient was calculated by two solution methods (TSM) based on measuring the potentials of a pure solution of Al3+ ion (EA), and a mixed solution containing Al3+ and interference ions (EA+B). The potential difference (ΔE = EA+B – EA) was added to the following equation to calculate the selectivity coefficient [33]:
The selectivity coefficients calculated according to both methods are given in table 2. Figure 9 and table 2 indicate that the aluminium(III)-selective sensor performed a highly selective response to Al3+ ion over the other cations.
3.5 pH effect
The pH dependence of the potential of the aluminium(III)-selective sensor at 1.0 × 10–2 mol l–1 Al3+ was tested over the pH range of 2.0–12.0. pH 2.0–7.0 was prepared with hydrochloric acid and pH 8.0–12.0 was prepared with sodium hydroxide. The pH dependence of the sensor is shown in figure 10. Considering this data, it is clear that the potentials are independent of pH in the range 5.0–10.0. The potential decrease in the electrode response at high pH values (>10.0) may be due to the formation of hydroxyl complexes of Al3+ cation in the solution. On the other hand, at lower pH values (<5.0) the potential increases, indicating that the electrode responds to hydrogen ions.
3.6 Reusability and lifetime
The aluminium(III)-selective sensor was repetitively exposed to Al3+ ion solutions at three different concentrations (1.0 × 10–2 to 1.0 × 10–4 mol l–1) and its reusability was determined. Figure 11 demonstrates that the aluminium(III)-selective sensor is highly reusable.
The lifetime of the developed sensor was determined by monitoring the potential behaviour in potentiometric measurements taken against Al3+ ions at different times. At the end of the study, we determined that the sensor had a lifetime of 1 month.
3.7 Analytical applications
3.7.1 Potentiometric titration
The analytical application of the aluminium(III)-selective electrode was tested by using it as an indicator electrode. For this purpose, potentiometric titration of Al3+ with EDTA was performed and, the endpoint was determined. An aliquot of 10 ml of 1.0 × 10–3 mol l–1 Al(NO3)3 solution was titrated against 1.0 × 10–2 mol l–1 EDTA solution. The potential data were plotted against the volume of EDTA and the titration graph is shown in figure 12. As seen in this figure, the turning point of the titration was determined to be 1.0 ml. In the study, a sigmoid shape could not be obtained due to the interference effect of sodium ions in the structure of EDTA. Similar results were also reported in the literature [34,35]. Consequently, the proposed aluminium(III)-selective electrode was successfully used in the potentiometric titration of Al3+ ions with EDTA, and the data showed that it can be used as an indicator electrode.
3.7.2 Real sample applications
The developed aluminium(III)-selective sensor was determined to work well under laboratory conditions. Then, to assess the applicability of the developed sensor to real samples, using the developed sensor, we determined Al3+ in local tap water (Tokat, Turkey), purification water and two different commercially available water products. The study was carried out according to the standard addition method. Validation of the developed sensor was tested by comparing its AAS. The results are summarized in table 3. As seen from table 3, high recovery values ranging from 96.00 to 97.91% was achieved with the proposed sensor, and the results were in good agreement with AAS. As a result, the proposed sensor can be used to detect Al3+ ions in different samples.
3.8 Comparison study
The comparison of the developed aluminium(III)-selective sensor with the previously reported aluminium(III)-selective sensors is given in table 4. The comparison was performed in terms of concentration range, detection limit, pH working range and response time properties. The developed sensor was found to be superior to other sensors in terms of its wide pH working range and fast response time. Also, considering detection limit property, it has a value close to other sensors. This comparative study indicates that the proposed aluminium(III)-selective sensor is better than existing electrodes or sensors when some of the characteristics analysed in this study are taken into account.
4 Conclusions
There are various analytical methods in the literature for macro and micro analysis of Al3+ ions. Although the reported analytical methods are highly sensitive for the determination of Al3+ ions, some complications might be encountered in their application, especially the pre-treatment and the requirement for experienced personnel. Potentiometric ion-selective sensors provide the rapid determination of various ions with their easy preparation and simple use. On the other hand, these sensors offer low-energy consumption and low-cost analysis [40]. In this study, a novel all-solid-state contact PVC membrane sensor, which is highly selective to aluminium(III) ions, has been developed using a porphyrin-derivative molecule as an ionophore. The developed aluminium(III)-selective sensor has high stability, good reusability, good selectivity, fast response time, a wide working range and a wide pH working range. The developed sensor was successfully used as an indicator electrode in the potentiometric titration of Al3+ against EDTA. In addition, it was determined that the developed sensor is applicable for the determination of aluminium(III) ions in water samples and, the results are compatible with AAS.
References
Ma Y-H, Yuan R, Chai Y-Q and Liu X-L 2010 Mater. Sci. Eng. C 30 209
Ahmed M J, Hoque M R, Khan A S M and Bhattacharjee S C 2010 Eurasian. J. Anal. Chem. 5 1
Silvestre A L P, Milani M I, Rossini E L, Pezza L and Pezza R P 2018 Spectrochim. Acta A Mol. Biomol. Spectrosc. 204 432
Arvand M and Asadollahzadeh S A 2008 Talanta 75 1046
Gürdere M B, Özbek O and Ceylan M 2016 Synt. Comm. 46 322
Bera R T, Sahoo S K, Mittal S K and Ashok Kumar S K 2010 Int. J. Electrochem. Sci. 5 29
Kumar R S and Ashok Kumar S K 2019 Inorg. Chem. Commun. 106 165
Flaten T P 2001 Brain Res. 55 187
Yari A, Darvishi L and Shamsipur M 2006 Anal. Chim. Acta 555 329
Harigaya K, Kuwahar Y and Nishi H 2008 Chem. Pharm. Bull. 56 475
Frankowski M, Zioła-Frankowska A, Kurzyca I, Novotný K, Vaculoviˇc T, Kanický, et al 2011 Environ. Monit. Assess. 182 71
Krishnan S S, Gillespie K A and Crapper D R 1972 Anal. Chem. 44 1469
Bradley C and Leung F Y 1994 Clin. Chem. 40 431
Zanjanchi M A, Noei H and Moghimi M 2006 Talanta 70 933
Zuziak J, Reczyński W, Baś B and Jakubowska M 2018 Anal. Biochem. 558 69
Işıldak Ö, Deligönül N and Özbek O 2019 Turk. J. Chem. 43 1149
Isildak Ö, Özbek O and Gürdere M B 2020 J. Anal. Test. 4 273
Isildak Ö, Özbek O and Yigit K M 2020 Bulg. Chem. Commun. 52 448
Özbek O, Isildak Ö and Isildak I 2021 Biochem. Eng. J. 176 108181
Isildak O and Özbek O 2021 Crit. Rev. Anal. Chem. 51 218
Özbek O, Isildak Ö, Gürdere M B and Cetin A 2021 Org. Commun. 14 228
Temelli B and Unaleroglu C 2009 Tetrahedron 65 2043
Zhao L, Zhao Y, Li R, Wu D, Xu R, Li S et al 2020 Chemosphere 238 24552
Huang D, Li X, Chen M, Chen F, Wan Z, Rui R et al 2019 J. Electroanal. Chem. 841 101
Özbek O, Isildak Ö and Berkel C 2020 J. Incl. Phenom. Macrocycl. Chem. 98 1
Isildak O and Özbek O 2020 J. Chem. Sci. 132 29
Adler A D, Longo F R, Finarelli J D, Goldmacher J, Assour J and Korsakoff L 1967 J. Org. Chem. 32 476
Liu F, Duan L, Wang Y-L, Zhang Q and Wang J-Y 2009 Synt. Comm. 39 3990
Isildak O, Özbek O and Yigit K M 2021 Int. J. Environ. Anal. Chem. 101 2035
Topcu C 2016 Talanta 161 623
Buck R P and Lindner E 1994 Pure Appl. Chem. 66 2527
Özbek O and Isildak Ö 2021 Int. J. Environ. Anal. Chem. https://doi.org/10.1080/03067319.2021.1877283
Umezawa Y, Bühlmann P, Umezawa K, Tohda K and Amemiya A S 2000 Pure Appl. Chem. 72 1851
Gupta V K, Jain A K, Singh L P and Khurana U 1997 Anal. Chim. Acta 355 33
Gupta V K, Jain A K and Kumar P 2006 Electrochim. Acta 52 736
Mousavi M F, Arvand-Barmchi M and Zanjanchi M A 2001 Electroanal. 13 1125
Abbaspour A, Esmaeilbeig A R, Jarrahpour A A, Khajeh B and Kia R 2002 Talanta 58 397
Saleh M B, Hassan S S M, Abdel Gaber A A and Abdel Kream N A 2001 Anal. Chim. Acta 434 247
Soleimani M and Afshar M G 2014 Russ. J. Electrochem. 50 554
Özbek O and Isildak Ö 2022 ChemistrySelect 7 e202103988
Acknowledgements
We thank the Tokat Gaziosmanpasa University Scientific Research Projects Commission (Project Number 2019/43) for the financial support. In addition, we would like to thank Research Assistant Caglar Berkel and M.Sc. student Alper Cetin for their contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özbek, O., Isildak, Ö. Use of 5,10,15,20-tetrakis(p-chlorophenyl)porphyrin as sensor material: potentiometric determination of aluminium(III) ions. Bull Mater Sci 45, 114 (2022). https://doi.org/10.1007/s12034-022-02696-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-022-02696-3