Abstract
In this study, all-solid-state contact PVC membrane silver(I)-selective sensor has been prepared and 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine used as ionophore. Optimum membrane composition; 3.5% ionophore, 59.6% Bis(2-ethylhexyl) sebacate (BEHS), 1.0% potassium tetrakis (p-chlorophenyl) borate (KTpClPB) and 35.9% polyvinyl chloride (PVC). The developed silver(I)-selective sensor exhibited a linear response (E = −51,57 (−log[Ag+]) + 1690,6 and R2 = 0,9986) in the varying concentration range of 1.0 × 10−1 to 1.0 × 10−5 mol L−1 silver ions. The limit of detection of the sensor was calculated as 1.9 × 10−6 mol L−1. The silver(I)-selective sensor exhibited good selectivity towards some alkali, alkali earth and heavy metal ions. The sensor can be used in the pH range from 4.0 to 10.0. The sensor has been shown to have good reusability and response time less than 8 s. The sensor was used as an indicator electrode in titrations of silver nitrate with sodium chloride solution and was successfully applied for the determination of the different water samples.
Graphic abstract
In this study, all-solid-state contact PVC membrane silver(I)-selective sensor has been developed. The developed silver(I)-selective sensor exhibited a linear response in the varying concentration range of 1.0 × 10−5 to 1.0 × 10−1 mol L−1 silver ions. The sensor was successfully applied for the determination of the different real samples.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Potentiometric ion sensors or ion-selective electrodes (ISEs) are an important subgroup of electrochemical sensors. The ion-selective electrodes (ISEs) in the class of potentiometric sensors were first described in 1906,1 after the 1960s, great advances have been made in this field and to date, ion-selective sensors have been identified for a large number of species. The studies in the field of ion-selective electrodes are progressing very rapidly in accordance with the requirements by using the knowledge in many fields of science together with the developments in technology. Nowadays ion-selective sensors are used to determine agricultural, environmental and medical fields,2,3 some poly ions such as heparin and protamine,4,5 some neutral species such as CO2, O2, SO2,6,7 ammonia, organic amines, alcohols and nonionic surfactants.8 Potentiometric methods offer great advantages such as easy preparation and procedures, short response time, high selectivity, low-energy consumption, wide linear working range at low cost compared to the other analytical methods such as spectrophotometry, high-performance liquid chromatography (HPLC), inductively coupled plasma mass spectrometry (ICP-MS), inductively coupled plasma optical emission spectrometry (ICP-OES), atomic absorption spectrometry (AAS), atomic fluorescence spectrometry (AFS) and neutron activation analysis.9,10,11,12,13,14,15,16,–17
Silver is an important element that is usually loaded with +1 in its compounds. Silver is used in many areas including photographic industry, electronic parts, ornaments, electrical and electrical equipment, jewellery, alloys, dental and medical products as well as silver-zinc and silver-cadmium batteries.18,19,20,21,22,–23 Therefore, the determination of silver in different biological and environmental samples is highly important.
Porphyrin compounds have been the subject of intensive research due to their importance in biological systems and material chemistry. Initial studies on porphyrins started with the isolation of these compounds from natural compounds and then synthetically synthesized in laboratory conditions. The synthesis of porphyrin was first made by Rothemund in 1935.24 Porphyrin is a molecule system consisting of four pyrrole rings connected by four methines (-CH=) bridges. Porphyrins and their structural derivatives are intensely reported as an ionophore for ion-sensitive electrodes. Porphyrins are one of the nitrogen-containing macrocyclic aromatic structures. Due to their rich redox chemistry, delocalized aromatic π-system and synthetic flexibility, they are frequently used in ionophore sensors.25,26,–27 Because of all these properties, porphyrin derivatives are preferred as ionophores and different ions sensitive sensors have been reported in the literature.28,29,30,–31
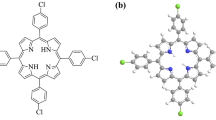
In this study, 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine (Figure 1) was used as ionophore and silver(I)-selective potentiometric PVC membrane sensor was developed. Potentiometric properties of the developed sensor were investigated.
2 Experimental
2.1 Apparatus
The potentiometric measurements were conducted by using a laboratory-made computer-controlled multi-channel potentiometric measurement system. The system has a home-made software program. The potential values as steady-state responses of the PVC membrane silver(I)-selective sensor were performed for different concentrations of standard solutions of silver(I) respectively. A micro-sized solid silver/silver chloride electrode (obtained from Isedo medical instruments, Turkey) was used as reference electrode with the silver(I)-selective PVC membrane sensor throughout the measurements.
2.2 Materials
All the chemicals were of analytical-reagent grade unless otherwise stated, and deionized water was used throughout. The 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine (ionophore) were obtained from Sigma Aldrich. High molecular mass polyvinylchloride (PVC, high molecular weight), Bis(2-ethylhexyl)sebacate (BEHS, density 0.914 g/mL at 25 °C), o-nitrophenyl octyl ether (o-NPOE, density 1.04 g/mL at 25 °C), Dibutyl phthalate (DBF, density 1.043 g/mL at 25 °C) and Bis(2-ethylhexyl) adipate (DEHA, density 0.925 g/mL at 20 °C), Potassium tetrakis (4-chlorophenyl)borate (KTpClPB), tetrahydrofuran (THF, anhydrous, density, 0.889 g/mL at 25 °C) and graphite obtained Sigma Aldrich. Epoxy (Macroplast Su 2227) from Henkel (Istanbul, Turkey) and hardener (Desmodur RFE) from Bayer AG (Darmstadt, Germany), were obtained and used in the preparation of all state solid contact. Deionized water was obtained by using a DI 800 Model deionize water system. All reagent and nitrate salts of the cations used in selectivity studies were in analytical grade and obtained from Sigma Aldrich. Stock solutions were prepared by using distilled deionized water. The stock solution was diluted gradually from 1.0 × 10−1 to 1.0 × 10−6 mol L−1.
2.3 Method
2.3.1 Preparation of silver(I)-selective sensors
Sensors were prepared at two steps. At the first step, solid-state contact was prepared. A mixture of conductive material consisting of 50.0% (w/w) graphite, 35.0% (w/w) epoxy and 15.0% (w/w) hardener was prepared by solving in sufficient THF (5 mL).32 At the second step, PVC membrane sensor was prepared. The optimum PVC membrane was prepared by thorough mixing of 59.60% plasticizer (BEHS), 35.90% PVC, 3.5% ionophore and 1.0% additive KTpClPB in 5 mL of THF. This mixture was placed on the sensors surface and allowed to dry for 24 h. Prior to first use, the prepared sensors were conditioned in a 1.0 × 10−2 mol L−1 AgNO3 solution for 12 h. Preparation of other membranes and optimized of membrane ingredients are summarized Table 1.
The preparation of the sensors and the potentiometric measurement system are summarized in Figure 2.
2.3.2 Potentiometric measurements
Potentials were measured using Ag/AgCl reference electrodes. Potential measurements were carried out at 25 ± 0.1 °C by setting up the following cell assembly:
3 Results and Discussion
3.1 Potentiometric performance of the PVC membrane silver(I)-selective membrane sensor
The PVC membrane silver(I)-selective membrane sensor was evaluated by potentiometric performance for optimizing membrane composition. PVC membrane silver(I)-selective membrane sensor was determined by the calibration curve of the over silver(I) concentration range from 1.0 × 10−1 to 1.0 × 10−6 mol L−1. Figure 3 represents that PVC membrane silver(I)-selective membrane sensor have highly reproducible. PVC membrane silver(I)-selective sensor exhibited a linear response, the graph of the linear response was defined by the equation of E = −51.57 (−log[Ag+]) + 1690,6 with a correlation coefficient R2 = 0.9986.
The potentiometric response to exhibits of silver(I)-selective membrane sensor (a: 10−1 mol L−1, b: 10−2 mol L−1, c: 10−3 mol L−1, d: 10−4 mol L−1, e: 10−5 mol L−1 and f: 10−6 mol L−1) and the variation of average potential values (mV) with log[Ag+] for silver(I) solutions in the range 10−1–10−5 mol L−1.
3.2 Calibration curve
Figure 4 depicts the potentiometric response of silver(I)-selective sensor. The potential response of sensor exhibited a linear concentration range 1.0 × 10−5–1.0 × 10−1 mol L−1 of silver ion in the calibration solution. The linear range of the graph and the tangent points from the deviation were plotted. The detection limit was calculated by substituting the potential value that is the projection of the cut-off point (1396.0 mV) in the correct equation. The developed sensor displays a low detection limit of 1.9 × 10−6 mol L−1.
3.3 The selectivity of silver(I)-selective PVC membrane sensor
The potentiometric selectivity coefficients are one of the most important characteristic parameters of an ion-selective electrode and measure its relative response for the primary ion in the presence of other ions present in the solution. The nitrate salts of different cations were measured at varying concentrations by the developed sensor. In this study, the selectivity coefficients of silver ion and other cations were calculated according to the following equation at a concentration of 1.0 × 10−2 mol L−1 by using the fixed interference method by IUPAC.33
The obtained logarithmic values are reported for NH4+, K+, Na+, Li+, Cd2+, Zn2+, Mg2+, Cu2+, Sr2+, Co2+, Ca2+, Pb2+, Ni2+, Al3+, Cr3+. The calculated selectivity coefficients are summarized in Table 2. In Table 2, we observed that the prepared silver(I)-selective sensor is highly selective with respect to a variety of other cations.
The developed sensor as a result of selectivity studies showed the highest potential value and linear operating range compared to other metals. The ionophore has a great effect at this point.
Silver sensitivity of the macrocyclic ring could be driven by both pyridine group attached the meso carbon and pyrrolic nitrogen in the structure. When the molecular structure of 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphine is considered, nitrogen groups attract to silver ions which increases the sensitivity of electrodes toward to silver ions.
3.4 The reusability of silver(I)-selective membrane sensor
The all-solid-state PVC membrane silver(I)-selective sensor investigated reusability. In this study, silver(I)-selective sensor was determined by taking repeated measurements at 10−2, 10−3, and 10−4 mol L−1 silver(I) concentrations. Figure 5 shows that exhibit to results can be good repeatedly.
3.5 The pH dependence of silver(I)-selective membrane sensor
The prepared sensor dependence of pH-potential profiles, and adjusted to various pH values in the range of 2 to11 by using NaOH and HNO3. The silver(I)-selective membrane sensor showed a potential change against the silver concentration of 10−3 mol L−1 in acidic and basic mediums. In Figure 6, it is clear that there is no potential remained unaffected over a pH range of 4–10.
3.6 The response time of silver(I)-selective membrane sensor
The response time is known as the time period for the presented potential of the sensor to reach equilibrium with the sensible part of the membrane.34 In this work, the response time was measured from 1.0 × 10−1 mol L−1 to 1.0 × 10−6 mol L−1 for sensor. The sensor response time obtained was always less than 8 s.
3.7 Analytical applications
3.7.1 The potentiometric titration of silver(I)-selective membrane sensor
The analytical application of the sensor was investigated by using it as an indicator sensor in the potentiometric titration of 1.0 × 10−2 mol L−1 silver nitrate solution with 1.0 × 10−2 mol L−1 sodium chloride solution. In this work, 1.0 × 10−2 mol L−1 silver nitrate solution was taken and 1.0 × 10−2 mol L−1 sodium chloride solution were added continuously and the results are shown in Figure 7. As seen, prepared silver(I)-selective PVC membrane sensor was used successfully as an indicator electrode in potentiometric titration of AgNO3 with NaCl.
3.7.2 Analysis of real samples
To prove that the silver(I)-selective sensor can be applied to real samples was carried out by adding a standard silver(I) ion to the water samples including tap water precipitated rainwater and some commercially available bottled waters. By using the silver(I)-selective sensor, the concentration was adjusted with standard silver(I) ion solutions ranging from 1.0 × 10−5 to 1.0 × 10−1 mol L−1 and the correct equation was obtained. Then, the potential values of each sample prepared by standard addition method were measured. Measured values were written in the correct equation created by using the calibration graph and the silver(I) quantities of each sample were calculated. Table 3 shows potentiometric determination of silver(I) ions in water samples which have been successfully performed with developed silver(I)-selective PVC membrane sensor.
3.8 Comparison with other reported sensors
A comparative study of the response characteristics of the developed sensor with some of the reported sensors for silver(I) is shown in Table 4. The achieved results provide enough proof that the developed silver(I) ion-selective sensor is superior to the previously reported silver(I) sensors in terms of concentration range, the limit of detection, pH working range, response time and real samples applications.
In this study, the potentiometric properties of silver(I)-selective PVC membrane sensor are generally better than other membrane sensors in the literature (Table 4). The pH of the silver(I)-selective PVC membrane sensor we developed is wider than the working range of pH of others. In this study, the response time of the sensor was obtained for 8 s, while in other studies the response times ranged from 10 to 30 s. The concentration range of the silver(I)-selective PVC membrane sensor we developed was between 1.0 × 10−1–1.0 × 10−6 mol L−1 and the limit of detection was determined as 1.9 × 10−6 mol L−1. The potentiometric results of the silver(I)-selective PVC membrane sensor obtained are consistent with the literature results given in Table 4.
4 Conclusions
In this stıdy, a novel potentiometric PVC membrane sensor that is sensitive and selective for the determination of silver ion in aqueous solutions was developed. The silver(I)-selective sensor showed a short response time and high sensitivity. The detection limit of the silver(I)-selective PVC membrane sensor was measured as 1.9 × 10−6 mol L−1 and the response time was shorter than 8 seconds. The linear operating range of the sensor was found to be between 1.0 × 10−1 and 1.0 × 10−6 mol L−1. The sensor exhibited good potentiometric behavior and its stability remained unchanged at 4–6 °C and dry atmosphere for one month. The potential change of the silver(I)-selective sensor in the concentration range from 1.0 × 10−1 to 1.0 × 10−6 mol L−1 was found to be compatible with the Nernst equation. In addition, it was determined that silver(I)-selective membrane sensor could be used as an indicator electrode as a result of potentiometric titration studies. The sensor developed will make a significant contribution to the work to be done in this area.
References
Cremer M Z 1906 Origin of electromotor properties of tissues, and instructional contribution for polyphasic electrolyte chain Z. Biol. 47 562
Mahajan R K and Parkash O 2000 Silver(I) ion selective PVC membrane based on bis-pyridine tetramide macrocycle Talanta 52 691
Mashhadizadeh M H, Mostafavi A, Allah-Abadi H and Sheikhshoai I 2006 New Schiff base modified carbon paste and coated wire PVC membrane electrode for silver ion Sens. Actuat. B-Chem. 113 930
Qin Y, Mi Y and Bakker E 2000 Determination of complex formation constants of 18 neutral alkali and alkaline earth metal ionophores in poly(vinyl chloride) sensing membranes plasticized with bis(2-ethylhexyl)sebacate and o-nitrophenyloctylether Anal. Chem. Acta 421 207
Shvarev A and Bakker E 2003 Reversible Electrochemical Detection of Nonelectroactive Polyions J. Am. Chem. Soc. 125 11192
Collison M E, Aebli G V, Petty J and Meyerhoff M E 1989 Potentiometric combination ion-carbon dioxide sensors for in vitro and in vivo blood measurements Anal. Chem. 61 2365
Severinghaus J W and Bradley A F 1958 Electrodes for blood pO2 and pCO2 determination J. Appl. Physiol. 13 515
Pressman C, Harris E J, Jagger W S and Johnson J H 1967 Antibiotic-mediated transport of alkali ions across lipid barriers Proc. Natl. Acad. Sci. USA 58 1949
Shahrokhian S, Taghani A and Moattar F 2002 Iodide-selective electrode based on copper phthalocyanine Electroanalysis 14 1621
Amini M K, Ghaedi M, Rafi A, Mohamadpoor-Baltork I and Niknamb K 2003 Silver selective electrodes based on methyl-2-pyridyl ketone oxime, phenyl-2-pyridyl ketone oxime and bis[2-(o-carboxythiophenoxy)methyl]-4-bromo-1-methoxybenzene carriers Sens. Actuat. B-Chem. 96 669
Lu J Q, Pang D W, Zeng X S and He X W 2004 A new solid-state silver ion-selective electrode based on a novel tweezer-type calixarene derivative J. Electroanal. Chem. 568 37
Reinoso-García M M, Dijkman A, Verboom W, Reinhoudt D N, Malinowska E, Wojciechowska D, Pietrzak M and Selucky P 2005 Metal complexation by tripodal N-Acyl(thio)urea and picolin(thio)amide compounds: synthesis/extraction and potentiometric studies Eur. J. Org. Chem. 10 2131
Gupta V K, Ganjali M R, Norouzi P, Khani H, Nayak A and Agarwal S 2011 Electrochemical analysis of some toxic metals by ion–selective electrodes Int. J. Eng. Appl. Sci. 41 282
Ding J, Qin W Y, Zhang Y and Wang X 2013 Potentiometric aptasensing based on target-induced conformational switch of a DNA probe using a polymeric membrane silver ion-selective electrode Biosens. Bioelectron. 45 148
Tadayon F, Katebi A, Afkhami A, Panahi Y and Bagheri H 2014 A new potentiometric sensor based on a high-performance composite for nanomolar determination of mercury (II) in environmental samples Int. J. Environ. Anal. Chem. 94 901
Zhang W, Feng J, Chen T, Wang G, Yang T, Huang R, Liang L and Wu X 2018 Novel potentiometric sensor based on molecular imprinted polyacrylamide for the determination of shikimic acid in herbal medicine Anal. Lett. 51 2920
Topcu C, Lacin G, Yilmaz V, Coldur F, Çaglar B, Cubuk O and Isildak I 2018 Electrochemical determination of copper(II) in water samples using a novel ion-selective electrode based on a graphite oxide–imprinted polymer composite Anal. Lett. 51 1890
Chen L, Ju H, Zeng X, He X and Zhang Z 2001 Silver ion-selective electrodes based on novel containing benzothiazolyl calix[4]arene Anal. Chim. Acta 437 191
Malinowska E, Brzozka Z, Kasiura K, Egberink R J M and Reinhoudt D N 2004 Lead selective electrodes based on thioamide functionalized calix[4]arenes as ionophores Anal. Chim. Acta 298 253
Purcell T W and Peters J J 1998 Sources of silver in the environment Environ. Toxicol. Chem. 17 539
Furno F, Morley K S, Wong B, Sharp B L, Arnold P L, Howdle S M, Bayson R, Brown P D, Winship P D and Reid H J 2004 Silver nanoparticles and polymeric medical devices: a new approach to prevention of infection? J. Antimicrob. Chemother. 54 1019
Farghaly O A, Abdel Hameed R S and Abu-Nawwas A H 2014 Analytical application using modern electrochemical techniques Int. J. Electrochem. Sci. 9 3287
Isildak O, Deligönül N and Özbek O 2019 A novel silver(I)-selective PVC membrane sensor and its potentiometric applications Turk. J. Chem. 43 1149
Rothemund P 1936 A new porphyrin synthesis. The synthesis of porphin J. Am. Chem. Soc. 58 625
Lvova L, Galloni P, Floris B, Lundström I, Paolesse R and Natale C 2013 A ferrocene-porphyrin ligand for multi-transduction chemical sensor development Sensors 13 5841
Li Z, Zhang X B, Guo C C, Shen G L and Yu R Q 2001 Porphyrin dimer as neutral ionophore for a berberine-sensitive potentiometric sensor Anal. Lett. 34 2035
Paolesse R, Nardis S, Monti D, Stefanelli M and Natele C 2017 Porphyrinoids for chemical sensor applications Chem. Rev. 117 2517
Gupta V K, Chauhan D K, Saini V K, Agarwal S, Antonijevic M M and Lang H 2003 A porphyrin based potentiometric sensor for Zn2+ determination Sensors 3 223
Fakhari A R, Alaghemand M and Shamsipur M 2001 Iron(III)-selective membrane potentiometric sensor based on 5,10,15,20-tetrakis-(Pentafluorophenyl)-21H,23H-porphyrin Anal. Lett. 34 1097
Jaworska E, Pomarico G, Berna B B, Maksymiuka K, Paolesse R and Michalska A 2018 All-solid-state paper based potentiometric potassium sensors containing cobalt(II) porphyrin/cobalt(III) corrole in the transducer layer Sens. Actuat. B-Chem. 277 306
Vlascici D, Pruneanu S, Olenic L, Pogacean F, Ostafe V, Chiriac V, Pica E M, Bolundut L C, Nica L and Fagadar-Cosma E 2010 Manganese(III) Porphyrin-based potentiometric sensors for diclofenac assay in pharmaceutical preparations Sensors 10 8850
Isildak O 2009 Determination of inorganic anions in mushrooms by ion chromatography with potentiometric detection J. Anal. Chem. 64 1242
IUPAC 1976 Recommendations for nomenclature of ion-selective electrodes Pure Appl. Chem. 48 127
Isildak O and Durgun O 2015 All solid state PVC membrane Fe III selective electrode based on 2 hydroxymethyl 15 crown 5 Int. J. Eng. Appl. Sci. 2 1
Topcu C, Coldur F, Andaç M, Isildak I, Senyuz N and Bati H 2011 Ag+-selective poly(vinyl chloride) membrane electrode based on [N,N′-ethylenebis-(3-methoxy salicylaldimine)] Curr. Anal. Chem. 7 136
Mahajan R K, Inderpreet Kaur, Sharma V and Kumar M 2002 Sensor for silver(I) ion based on Schiff-base-p-tertbutylcalix[4]arene Sensors 2 417
Mashhadizadeh M H and Shamsipur M 1999 Silver(I)-selective membrane electrode based on hexathia-18-crown-6 Anal. Chim. Acta 381 111
Mazloum M, Niassary M S, Chahooki M and Amini M K 2001 Silver-selective coated-wire electrode based on Resorc[4]arene neutral carrier Electroanalysis 14 376
Omran O A, Elgendy F A and Nafady A 2016 Fabrication and applications of potentiometric sensors based on p-tert-butylthiacalix[4]arene comprising two triazole rings ionophore for silver ion detection Int. J. Electrochem. Sci. 11 4729
Zeng X, Weng L, Chen L, Leng X, Zhang Z and He X 2000 Improved silver ion-selective electrodes using novel 1,3-bis(2-benzothiazolyl)thioalkoxy-p-tert-butylcalix[4]arenes Tetrahedron Lett. 41 4917
Ardakani M M, Dehghani H, Jalayer M and Zare H R 2004 Potentiometric determination of silver(I) by selective membrane electrode based on derivative porphyrin Anal. Sci. 20 1667
Isildak I, Yolcu M, Isildak O, Demirel N, Topal G and Hosgoren H 2004 All-solid-state PVC membrane Ag1-selective electrodes based on diaza-18-crown-6 compounds Microchim. Acta 144 177
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Isildak, Ö., Özbek, O. Silver(I)-selective PVC membrane potentiometric sensor based on 5,10,15,20-tetra(4-pyridyl)-21H, 23H-porphine and potentiometric applications. J Chem Sci 132, 29 (2020). https://doi.org/10.1007/s12039-019-1734-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-019-1734-2