Abstract
In this study, zeolite-based sorbents were prepared and examined for \(\hbox {CO}_{2}\) adsorption from a simulated flue gas mixture using a fixed-bed flow reactor. Various amines such as monoethanolamine, ethylenediamine, diethylenetriamine and triethylenetetramine (TETA) were impregnated on support materials to prepare the adsorbents. Also, the effects of various parameters on \(\hbox {CO}_{2}\) adsorption capacity have been examined in this work. Further, an effort has been made to characterize various physico-chemical properties like surface area, pore volume, chemical composition, etc. of the in-house developed sorbents. Observation showed that the \(\hbox {CO}_{2}\) adsorption capacity enhanced with amine loading up to a certain concentration. The maximum carbon capture capacity of the 30-TETA-ZSM-5 sorbent is around 53 g of \(\hbox {CO}_{2}/\hbox {kg}\) of adsorbent. The thermo-chemical stability of the adsorbents has been tested by reusing the same material for multiple adsorption–desorption cycles, and no significant change in \(\hbox {CO}_{2}\) adsorption capacities was observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, carbon dioxide emission into the biosphere has increased since the industrial revolution. According to the International Energy Agency (IEA) report, the carbon dioxide concentration in air has increased during the last 50 years by more than 100 ppm [1]. Technologies involved in carbon capture and storage (CCS) are thought to be efficient methods for reducing \(\hbox {CO}_{2}\) emission. It is expected that by year 2050 the amount of \(\hbox {CO}_{2}\) captured will be around 240 billion tons using CCS technologies [2]. Conventionally, an aqueous amine or blends of amines are used to separate \(\hbox {CO}_{2}\) from other gases. However, the technology needs significant research and modification to address high-energy consumption and corrosion related problems associated with flue gas application. Degradation of absorbents and emission of aerosols to the environment are also issues related to amine-based absorption technology. Solid adsorbents can provide an alternative to the traditionally used aqueous absorbents for \(\hbox {CO}_{2}\) capture due to relatively lower energy consumption, negligible corrosion problem and easy regeneration of adsorbents. \(\hbox {CO}_{2}\) capture using solid adsorbents such as zeolites, AC, mesoporous silica, \(\hbox {Al}_{2}\hbox {O}_{3}\) and metal–organic frameworks are still under research [3,4,5,6,7,8]. The suitability of zeolite materials for the capture of \(\hbox {CO}_{2}\) has been reported by many research groups. Silica and alumina are the major composition of a zeolite material. The porous structure of a zeolite is advantageous for its \(\hbox {CO}_{2}\) adsorption. Generally, zeolites are acidic in nature due to the presence of Lewis and Brønsted acid sites. Various amines can be added to zeolite to improve the basic strength and the \(\hbox {CO}_{2}\) adsorption. Castellazzi et al [9] have evaluated the \(\hbox {CO}_{2}\) sorption capacity of diethanolamine-impregnated alumina in a fixed-bed reactor. The calculated capture capacity with 36% diethanolamine on alumina was 32 g \(\hbox {kg}^{-1}\) at \(85^{\circ }\hbox {C}\). Chen et al [10] have synthesized Zeolite-13X from bentonite to investigate the capture capacity of \(\hbox {CO}_{2}\). A BELSORP-mini-II instrument was used to perform the adsorption study, and the reported capacity was about 270 g kg\(^{-1}\) at \(25^{\circ }\hbox {C}\). Dantas et al [11] have examined the adsorption of single gas \(\hbox {CO}_{2}\) on zeolite-13X utilizing a fixed-bed reactor. The informed \(\hbox {CO}_{2}\) sorption capacity was around 90 g \(\hbox {kg}^{-1}\) at \(28^{\circ }\hbox {C}\). Madden and Curtin [12] have examined the \(\hbox {CO}_{2}\) capture capacity of an aminopropyltriethoxysilane-modified zeolite material. It is reported that the material shows stability for nine cycles. The average adsorption capacity was around 207 g kg\(^{-1}\) at \(35^{\circ }\hbox {C}\). Girimonte et al [13] have explored the \(\hbox {CO}_{2}\) adsorption capacity of the zeolite-13X material by employing a fluidized-bed reactor. The adsorption capacity of the materials was in the range of 84–107 g kg\(^{-1}\) based on a sorbent particle size and fluidization velocity. Bezerra et al [14] have examined the carbon capture capacity of zeolite-13X impregnated with monoethanolamine (MEA). The study was conducted in the temperature range of 25–\(75^{\circ }\hbox {C}\) and at elevated pressure using a Rubotherm instrument. It is mentioned that amine-impregnated zeolites adsorb less amounts of \(\hbox {CO}_{2}\) compared to non-impregnated materials under similar conditions. The study also demonstrated that \(\hbox {CO}_{2}\) adsorption increased with an increase in temperature. Chatti et al [15] have carried out a \(\hbox {CO}_{2}\) adsorption study using a packed-bed reactor in the presence of amine-modified zeolite-13X sorbents. The \(\hbox {CO}_{2}\) sorption capacity of the MEA and isopropanol amine-loaded zeolite-13X was around 20 and 23 g kg\(^{-1}\), respectively, at \(75^{\circ }\hbox {C}\). Frantz et al [16] have studied the effect of the Si/Al molar ratio in ZSM-5 on \(\hbox {CO}_{2}\) capture capacity. ZSM-5 with Si/Al ratios of 25, 50 and 75 was synthesized and an adsorption study was conducted using a pressure swing adsorption setup. It is mentioned that the Si/Al ratio of 25 zeolite shows relatively better adsorption capacity compared to other two materials. Lee et al [17] have inspected the \(\hbox {CO}_{2}\) capture capacity of a polyethylenimine (PEI)-loaded zeolite material using a thermogravimetric analyser (TGA) instrument. The maximum capture capacity obtained in the study was found to be around 116 g \(\hbox {kg}^{-1}\) of adsorbent with 33 wt% PEI loading in the presence of both N,N,N-triethyl ethanaminium bromide and tetraethylammonium as structure directing agents. The performance of alkali-loaded meso-porous solid sorbents was investigated for \(\hbox {CO}_{2}\) capture in a fixed bed using simulated flue gas [18, 19].
Qi et al [20] have explored \(\hbox {CO}_{2}\) adsorption on amine-based (PEI and tetraethylenepentamine) silica materials using a TGA instrument. Sanz et al [21] have probed the \(\hbox {CO}_{2}\) adsorption on SBA-15 impregnated with PEI in the temperature range of 25–\(75^{\circ }\hbox {C}\) using a high pressure volumetric analyser. The capture capacity was about 90 g \(\hbox {kg}^{-1}\) at \(75^{\circ }\hbox {C}\) for \(\hbox {CO}_{2}\). Xu et al [22] have studied the moisture effect on \(\hbox {CO}_{2}\) adsorption when the MCM-41 material was impregnated with PEI. A packed-bed setup was used for the experiment and the reported capacity of the sorbent was 280 g \(\hbox {kg}^{-1}\). Aruldoss et al [23] have performed experiment with SBA-15-impregnated triethylenetetramine (TETA) using a packed bed reactor for \(\hbox {CO}_{2}\) adsorption. The capacity was around 210 g \(\hbox {kg}^{-1}\) for the 50 wt% TETA-impregnated SBA-15 adsorbent for \(\hbox {CO}_{2}\).
Based on the reported studies, it was observed that the studies concerning to amine-based zeolite adsorbents for \(\hbox {CO}_{2}\) separation from a flue gas stream are in very few and scattered numbers. Again, in most of the articles related to \(\hbox {CO}_{2}\) adsorption, either a thermogravimetric or an adsorption instrument was employed to investigate the \(\hbox {CO}_{2}\) capture capacity of a material. In very few cases, the adsorption experiments are performed using reactors like a packed bed or fluidized bed reactor. The point to highlight here is that the estimated value of capture capacity of a material can differ significantly depending on the experimental setup used for the study. In the case of thermogravimetric or adsorption instruments, only a few milligrams of adsorbent materials are generally used to find its capture capacity. However, in the case of lab-scaled fixed or fluidized bed reactors, a large amount (generally gram or kilogram scale) of the adsorbent material is used for a study. The scale-dependent properties like heat effects, non-ideal behaviour will be more realistic and the percentage error in measurements will be relatively small for a bigger scale study. It has also been observed that the effects of various parameters such as nature of amines, concentration of amines, temperature on \(\hbox {CO}_{2}\) adsorption and optimization of operating parameters are not explicitly reported in many literature studies. Therefore, the aim of this work is to develop amine-impregnated zeolite-supported solid adsorbents and to investigate their \(\hbox {CO}_{2}\) capture capacity from a simulated (14% \(\hbox {CO}_{2})\) gas stream employing a fixed-bed reactor. For the synthesis of sorbents, different types of amine such as MEA, ethylenediamine (EDA), diethylenetriamine (DETA) and TETA were impregnated on various support materials such as ZSM-5, zeolite-13X, zeolite-Y and alumina materials. The other purpose of this work is to measure the effects of various parameters on \(\hbox {CO}_{2}\) capture capacity of the developed adsorbents. Also, an effort has been made to characterize the adsorbent properties in detail. This study will be useful to compare the performance of various amine-impregnated zeolite adsorbents in a single window evaluated under similar conditions.
2 Experimental
2.1 Materials
Chemicals such as MEA, anhydrous ethanol, DETA, TETA, aluminium nitrate and urea were obtained from S D Fine Chem Ltd., India. EDA was purchased from Avra Synthesis Pvt. Ltd., India. ZSM-5 zeolite (with a silica-to-alumina ratio of 30) was procured from Sud-Chemicals Pvt. Ltd., India. Zeolite-13X was procured from Sisco Research Laboratories Pvt. Ltd., India. Zeolite-Y was purchased from Hychem Laboratories, India. A 99.5% purity nitrogen and carbon dioxide gases are procured from G. M. Tech., India. Chemix Specialty Gases and Equipment, India, supplied calibration gas (15% \(\hbox {CO}_{2}\) and rest \(\hbox {N}_{2})\) for the \(\hbox {CO}_{2}\)-IR analyser.
2.2 Experimental setup and procedure
The adsorption experiments were executed using a fixed-bed reactor of dimensions 2.5 cm diameter and 30 cm length. The reactor was fitted with band-heaters, temperature indicators and proportional-integral-derivative controllers to maintain the reactor temperature precisely within \(\pm 1^{\circ }\hbox {C}\). A schematic of the experimental setup is shown in figure 1. Approximately 10 g of synthesized sorbent was mixed with 5 g of quartz sand and was filled into the reactor. Sand was added to increase the porosity of the bed. The reactor was heated to a desired temperature before allowing the simulated flue gas (mixture of \(\hbox {CO}_{2}\) and N\(_{2 }\) saturated with water vapour) to flow through the reactor. In each adsorption experiment, the inlet concentration (\(C_{\mathrm{o}})\) of \(\hbox {CO}_{2}\) and total flow rate were maintained at 15 ± 1 vol% and 50 ± 3 cc per min, respectively. To measure the outlet concentration (\(C_{\mathrm{e}})\) of \(\hbox {CO}_{2}\), the exit gas stream was passed through a dryer followed by a \(\hbox {CO}_{2}\)-IR analyser (SR-2016, Technovation Analytical Instruments Pvt. Ltd., India). After each adsorption cycle, the reactor temperature was increased in the presence of \(\hbox {N}_{2}\) flow to regenerate the adsorbent. In the present work, all the adsorption and desorption experiments were performed under atmospheric pressure. For each adsorbent, two cycles of adsorption–desorption experiments were carried out under identical conditions, and the average value of the two runs has been mentioned in this paper.
2.3 Preparation of amine-impregnated adsorbents
In this study, different types of zeolites were used as support materials to prepare the adsorbents for \(\hbox {CO}_{2}\) capture. Commercially available zeolites were dried at 103 ± \(1^{\circ }\hbox {C}\) to remove the free moisture. The wet impregnation method was adopted to prepare the amine-impregnated adsorbents. The measured amount of amine was mixed in anhydrous ethanol in a glass beaker. A calculated quantity of zeolite was mixed to the amine solution and stirred for around 20 min using a magnetic stirrer. The material was placed in a vacuum oven maintained at 103 ± \(1^{\circ }\hbox {C}\) for around 5 h to remove the ethanol solvent. The amine loaded sorbents were cooled and labelled accordingly. An adsorbent with label 5-MEA-ZSM-5 means 5 wt% (with respect to the support material) MEA impregnated on the ZSM-5 material.
2.4 Characterization of in-house synthesized adsorbents
The Brunauer–Emmett–Teller (BET) method was used to measure the pore volume, surface area and pore size of the unmodified zeolite material and the synthesized sorbents were characterized using a BELSORP-mini-II instrument (Micromeritics Instrument Corporation, USA). The X-ray diffraction (XRD) study was carried out to know the crystallinity of adsorbents using an X-ray diffractometer (Bruker D8 Advance diffractometer, Japan). For the XRD analysis, monochromatic \(\hbox {CuK}{\upalpha }\) radiation (\(\uplambda = 1.54\) Å) was used and the diffraction angle (2\(\theta \)) was varied from 5 to \(60^{\circ }\) at a rate of \(5^{\circ }\,\hbox {min}^{-1}\). A (Apreo FESEM, USA) scanning electron microscope (SEM) instrument with an Everhart-Thornley detector was used to explore the shape or structure of adsorbents. To investigate the degradation characteristics of the adsorbents, thermogravimetric analysis was carried out using a Shimadzu make DTG-60 thermogravimetric analyser.
3 Results and discussion
The adsorbents were synthesized and the effects of parameters like temperature, types of support materials, nature of amines and amine concentration on \(\hbox {CO}_{2}\) adsorption were examined. Simulated gas was sent through the reactor bed to find the \(\hbox {CO}_{2}\) sorption capacity of the adsorbents. The reactor temperature was varied between 30 and \(60^{\circ }\hbox {C}\) during the adsorption process, and the regeneration was carried out in the temperature range of 105–\(120^{\circ }\hbox {C}\). The \(\hbox {CO}_{2}\) adsorption capacity of the adsorbent is expressed as grams of \(\hbox {CO}_{2}\) adsorbed per kilogram of adsorbent. Experiments were examined repeatedly under identical conditions to evaluate the deviation of the results. 1 to 4% deviation was observed.
3.1 Characterization
3.1.1 Surface area, pore volume analysis and adsorption isotherms
The qualitative information on the pores present in the material was obtained from adsorption isotherms. To investigate the pore volume and surface area of the support materials, a nitrogen adsorption isotherm study was carried out and the isotherms are shown in figure 2. A typical feature of Type-I adsorption isotherm was observed for ZSM-5, zeolite-Y and zeolite-13X materials. A higher amount of adsorption at lower pressures and nearly a constant value at higher pressure indicate the presence of microporous structure of the materials. However, the adsorption isotherm of in-house synthesized alumina shows a type-IV isotherm. The measured properties from the BET instrument for the support materials and selected sorbents are given in table 1. The average pore diameters of ZSM-5, zeolite-Y, zeolite-13X and alumina are 3, 1.5, 7.2 and 4.3 nm, respectively. The analysis shows that both the pore volume and surface area of the material decreased with the enhancement of amine loading.
3.1.2 XRD analysis
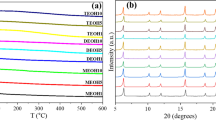
The nature of the sorbents was analysed by XRD. The diffraction patterns of pristine ZSM-5 and amine-impregnated ZSM-5 are shown in figure 3. The XRD patterns indicate the crystalline nature of the ZSM-5 material. In the case of amine-impregnated adsorbents, a group of sharp and intense peaks at a 2\(\theta \) value of around \(23^{\circ }\) in the XRD pattern confirmed the presence of amines on the ZSM-5 support.
3.1.3 SEM analysis
A SEM study was carried out to analyse the shape and structure of the adsorbent materials. The SEM images of four supports and 30% amine-impregnated adsorbent materials are shown in figure 4. The figure depicts that the morphologies of the four materials are different from each other. Cuboid shapes are observed for ZSM-5, and zeolite-Y shows mostly pyramidal geometry. Zeolite-13X shows an agglomerated structure, and a very irregular and relatively polished surface was observed for \(\hbox {Al}_{2}\hbox {O}_{3}\) materials. The image shows that the surface become relatively smooth after amine impregnation particularly for zeolite-13X.
3.2 Breakthrough curve
A breakthrough curve is very much useful to characterize the nature of an adsorbent. To estimate the \(\hbox {CO}_{2}\) capture capacity of the synthesized adsorbents, breakthrough curves were generated at a fixed temperature. Typical breakthrough curves for the virgin ZSM-5 and 30 wt% amine-impregnated ZSM-5 are shown in figure 5. The breakthrough time is noted when the outlet concentration (\(C_{\mathrm{e}})\) of \(\hbox {CO}_{2}\) reached 5% of the feed \(\hbox {CO}_{2}\) concentration (\(C_{\mathrm{o}})\). The plots show that the breakthrough time of the 30% amine-loaded sorbents is around 2 to 3 times higher than that of the virgin ZSM-5. A longer breakthrough time indicates the higher capture capacity of the adsorbents. The \(\hbox {CO}_{2}\) capture capacity of the adsorbents up to breakthrough (at \(C_{\mathrm{e}}/C_{\mathrm{o}} = 0.05)\) and exhaustion point (at \(C_{\mathrm{e}}/C_{\mathrm{o}} = 0.95)\) is tabulated in table 2 along with the breakthrough time. Among the adsorbents studied in the present work, the 30-TETA-ZSM-5 material shows the maximum capture capacity at \(30^{\circ }\hbox {C}\). The variation of \(\hbox {CO}_{2}\) capture capacity of the support and amine-impregnated materials is explained in the subsequent sections.
3.3 Performance of the support material
To understand the relative position of support materials in terms of \(\hbox {CO}_{2}\) capture capacity, four different types of mesoporous materials namely ZSM-5, zeolite-13X, zeolite-Y and alumina were considered in the present study. The data (table 2) depict that the adsorption capacity of \(\hbox {CO}_{2}\) in unmodified-zeolite-Y was significantly higher than that in other three pristine materials at \(30^{\circ }\hbox {C}\). This may be due to the high-surface area of zeolite-Y compared to other materials. To understand the relative performance of the amine-impregnated materials, a fixed percentage of TETA was impregnated on each of the support materials. The \(\hbox {CO}_{2}\) adsorption study was performed under identical conditions and the outcomes are shown in figure 6. The order of \(\hbox {CO}_{2}\) adsorption capacity of the TETA-impregnated adsorbents is ZSM-5 > zeolite-Y > \(\hbox {Al}_{2}\hbox {O}_{3}\,>\) zeolite-13X over the temperature range of 30–\(60^{\circ }\hbox {C}\). The result indicates that the \(\hbox {CO}_{2}\) capacity enhanced with an increase in the Si/Al ratio for the amine-impregnated zeolite materials. The Si/Al ratios of the ZSM-5, zeolite-Y and zeolite-13X are 15.6, 5.2 and 1.5, respectively. From the present study, it can be said that the adsorption capacity of a material depends on several parameters like total surface area, microporous surface area, pore volume, pore size distribution, pore geometry, etc.
3.4 Effect of temperature
To analyse the temperature effect on \(\hbox {CO}_{2}\) adsorption, the analysis was conducted at different temperature values ranging from 25–\(60^{\circ }\hbox {C}\) at a fixed concentration of the amine (30 wt%) and the observations are shown in figure 7. It was seen that the \(\hbox {CO}_{2}\) uptake capacity decreased gradually with the increasing bed temperature from 25–\(50^{\circ }\hbox {C}\) for the EDA-impregnated adsorbent. No significant change in adsorption capacity was observed for the MEA-loaded adsorbent. For the TETA impregnated adsorbent, an increasing pattern in \(\hbox {CO}_{2}\) uptake capacity was observed with the temperature up to around \(50^{\circ }\hbox {C}\). However, when the bed temperature was further increased to \(60^{\circ }\hbox {C}\), a depreciating trend in adsorption capacity was found. For the DETA-impregnated adsorbent, the maximum adsorption capacity was observed at \(30^{\circ }\hbox {C} \) under similar conditions of the study. A decrease in \(\hbox {CO}_{2}\) uptake capacity at higher temperature indicates that the desorption of \(\hbox {CO}_{2}\) is relatively more compared to the adsorption rate. The result also shows that the maxima of \(\hbox {CO}_{2}\) capture capacity shifted towards the higher temperatures with an increase in the number of amine groups for a homologous series (EDA, DETA and TETA contain two, three and four amine groups, respectively).
3.5 Effects of various amines and its concentration on \(\hbox {CO}_{2}\) capture capacity
Amines are used to enhance the \(\hbox {CO}_{2}\) adsorption from a gas stream. Primary and secondary amines react with \(\hbox {CO}_{2}\) to form a zwitterion species (\(\hbox {CO}_2 +\hbox {RNH}_2 \rightleftarrows \hbox {RNH}_2^+ \hbox {CO}_2^- )\). In the present study, four-different amines namely, MEA, EDA, DETA and TETA were considered to find the relative performance for \(\hbox {CO}_{2}\) capture under similar operating conditions. Also, to study the impact on \(\hbox {CO}_{2}\) capture capacity during amine loading, the experiments were conducted at four-different amine concentrations ranging between 5 and 40 wt% at a temperature of \(30^{\circ }\hbox {C}\) and the outcomes are shown in figure 8. The result shows that the \(\hbox {CO}_{2}\) capture capacity of the materials increased up to around 30% amine loading. However, a decrease in \(\hbox {CO}_{2}\) capture capacity was noted when the amine impregnation increased beyond 30% for the present experimental conditions.
An increase in \(\hbox {CO}_{2}\) capture capacity with amine loading in-spite of the decreased surface area and pore volume shows that the chemisorption dominated over physisorption. The reduction in \(\hbox {CO}_{2}\) capture capacity beyond 30% amine impregnation may be due to blockage of pores, which hinders \(\mathrm{CO}_2\) molecules to reach near to the active sites for adsorption. The adsorption capacity of the TETA-modified zeolite increased rapidly compared to other three-amine-impregnated sorbents. This may be due to the availability of more number of basic sites for chemisorption of \(\hbox {CO}_{2}\) with increased loading. TETA has four amino groups (two primary and two secondary amino groups). The result also shows that the capture capacity of \(\hbox {CO}_{2}\) with the MEA-loaded absorbent is slightly higher than that with the EDA-loaded adsorbent. This may be due to the presence of the hydroxyl group in MEA, and which have the ability to form hydrogen bonding with the adsorbate.
3.6 Cyclic stability of the sorbent
To evaluate the steadiness of the synthesized materials for \(\hbox {CO}_{2}\) capture, multiple cycles of the adsorption–desorption study were conducted with the 30-TETA-ZSM-5 adsorbent. The adsorption of \(\hbox {CO}_{2}\) was performed at \(30^{\circ }\hbox {C}\) and regeneration was performed at \(120^{\circ }\hbox {C}\). The adsorption process was continued for around 30 min for each cycle. After the adsorption experiment, nitrogen gas was sent to the reactor for 3 min to evacuate the gas mixture from the reactor and then the regeneration process was started. The concentration profiles of \(\hbox {CO}_{2}\) for five continuous cycles are shown in figure 9.
The profile shows that the \(\hbox {CO}_{2}\) concentration was nearly zero for 2.5 min during the adsorption process and then increased gradually to the feed level. However, during the regeneration process, the concentration of \(\hbox {CO}_{2}\) increased slowly for the first 3 min, and then increased suddenly to a peak value of around 15 vol%. The calculated adsorption capacities of the sorbent for the 1st, 2nd, 3rd, 4th and 5th cycles are 47.1, 47.2, 45.9, 44.4 and 44.1 g of \(\hbox {CO}_{2}/\hbox {kg}\) of sorbent, respectively. Similarly, the estimated amounts of desorbed \(\hbox {CO}_{2}\) during regeneration cycles for the 1st, 2nd, 3rd, 4th and 5th cycles are 45.2, 44.7, 45.3, 42.1 and 42.7, respectively. The difference in the \(\hbox {CO}_{2}\) amount between the adsorption and the corresponding desorption cycle lies in the range of 1.5–5.5%. It was also observed that for a particular cycle, the amount of \(\hbox {CO}_{2}\) released during the regeneration process is less than the corresponding value of adsorption capacity. This shortfall may be due to the removal/elimination of the some amounts of adsorbed \(\hbox {CO}_{2}\) during \(\hbox {N}_{2}\) flashing before starting the regeneration cycle. The reduction in the capture capacity with the number of cycles may be due to the combined effect of loss in the active surface area, incomplete regeneration or degradation of impregnated amine. Therefore, based on the study, it is observed that the materials are quite stable and capable of adsorbing \(\hbox {CO}_{2}\) in multiple cycles.
3.7 Analysis of thermal stability of the TETA-impregnated sorbent
To investigate the thermal stability of the adsorbents, thermogravimetric analysis was performed using a DTG-60 instrument. The accurately measured (5 ± 0.1 mg) quantity of the adsorbent sample was taken into the sample pan and heated it gradually from room temperature 30–\(400^{\circ }\hbox {C}\) at a rate of \(10^{\circ }\hbox {C}\) \(\hbox {min}^{-1}\) under the nitrogen gas flow. The weight loss percentages estimated from the TGA-thermograms for TETA-impregnated and pristine ZSM-5 samples are shown in figure 10. The plot shows that around 4–7% loss in weights occurred within \(120^{\circ }\hbox {C}\). The initial loss in weight was mainly due to the removal of water molecules and volatile matters from the materials. Among the two samples, virgin ZSM-5 shows a higher (\(\sim \)8%) loss in weight up to \(200^{\circ }\hbox {C}\), and beyond \(400^{\circ }\hbox {C}\), no significant loss in weight was observed. However, for the TETA-doped sample, the loss percentage increased sharply after \(150^{\circ }\hbox {C}\) and which may be due to the partial decomposition of impregnated-anime molecules. The reduction in weight above \(200^{\circ }\hbox {C}\) may be due to the loss of water molecules from hydration and dihydroxylation complexes from zeolite structures in addition to the decomposition of amine molecules. Therefore, from the present investigation, it can be said that, though the amine-doped sample shows some initial weight loss due to the elimination of unbound moisture, the materials are thermally stable up to around \(160^{\circ }\hbox {C}\).
4 Conclusions
In this study, zeolite-based sorbents were prepared and tested for \(\hbox {CO}_{2}\) adsorption from simulated gas mixtures using a fixed-bed flow reactor. Four-different types of amines were impregnated on various support materials to investigate the performance of the adsorbents for \(\hbox {CO}_{2}\) adsorption. Also, the effects of operating conditions on \(\hbox {CO}_{2}\) adsorption have been examined in this work. Further, an effort has been made to characterize various properties of the developed adsorbents. It was found that the \(\hbox {CO}_{2}\) adsorption capacity enhanced with an increase in amine loading up to 30 wt% and beyond that a decreasing trend in adsorption capacity was observed. For MEA-, EDA- and DETA-loaded adsorbents, the \(\hbox {CO}_{2}\) uptake capacity decreased with an increase in the adsorption temperature. In the case of the TETA-loaded adsorbent, the maximum capacity was observed at \(50^{\circ }\hbox {C}\), and the capture capacity of the 30-TETA-ZSM-5 sorbent is 53 g \(\hbox {kg}^{-1}\). Based on the surface area and pore volume analysis, it can be concluded that the adsorption was preferably chemisorption in the presence of amine compounds. The study shows that the newly developed adsorbent can be used multiple times without much compromise on the capture capacity.
References
Alhwaige A A, Agag T, Ishida H and Qutubuddin S 2013 RSC Adv. 3 16011
Stangeland A 2007 Int. J. Greenhouse. Gas Control. 1 418
Pirouzmand M, Nikzad-Kojanag B and Hosseini-Yazdi S A 2016 J. Braz. Chem. Soc. 27 2354
Belmabkhout Y and Sayari A 2009 Adsorption 15 318
Sarfraz M and Ba-Shammakh M 2018 Braz. J. Chem. Eng. 35 217
Ye S, Jiang X, Ruan L W, Liu B, Wang Y M, Zhu J F et al 2013 Micropor. Mesopor. Mater. 179 191
Sanz R, Calleja G, Arencibia A and Sanz-Pérez E S 2012 Micropor. Mesopor. Mater. 158 309
Knowles G P, Liang Z and Chaffee A L 2017 Micropor. Mesopor. Mater. 238 14
Castellazzi P, Notaro M, Busca G and Finocchio E 2016 Micropor. Mesopor. Mater. 226 444
Chen C, Park D W and Ahn W S 2014 Appl. Surf. Sci. 292 63
Dantas T L P, Luna F M T, Silva I J Jr, Torres A E B, de Azevedo D C S, Rodrigues A E et al 2011 Braz. J. Chem. Eng. 28 533
Madden D and Curtin T 2016 Micropor. Mesopor. Mater. 228 310
Girimonte R, Formisani B and Testa F 2017 Powder Technol. 311 9
Bezerra D P, Silva F W M D, Moura P A S D, Sousa A G S, Vieira R S, Rodriguez-Castellon E et al 2014 Appl. Surf. Sci. 314 314
Chatti R, Bansiwal A K, Thote J A, Kumar V, Jadhav P, Lokhande S K et al 2009 Micropor. Mesopor. Mater. 121 84
Frantz T S, Ruiz W A, Da Rosa C A and Mortola V B 2016 Micropor. Mesopor. Mater. 222 209
Lee C H, Hyeon D H, Jung H, Chung W, Jo D H, Shin D K et al 2015 J. Ind. Eng. Chem. 23 251
Dinda S 2013 Sep. Purif. Technol. 109 64
Murge P, Dinda S and Roy S 2018 Energy Fuels 32 10786
Qi G, Wang Y, Estevez L, Duan X, Anako N, Park A H A et al 2011 Energy Environ. Sci. 4 444
Sanz R, Calleja G, Arencibia A and Sanz-Pérez E S 2010 Appl. Surf. Sci. 256 5323
Xu X, Song C, Miller B G and Scaroni A W 2005 Ind. Eng. Chem. Res. 44 8113
Aruldoss D, Saigoanker R, Das Savarimuthu J and Jagannathan R 2014 J. Ceram. Int. 40 7583
Acknowledgements
The authors express their gratitude to the Council of Scientific and Industrial Research (CSIR), India, for funding (CSIR No: 22(0694)/15/EMR-II) the present research work and also grateful to the BITS–Pilani Hyderabad Campus for extending the necessary support for the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dinda, S., Murge, P. & Chakravarthy Paruchuri, B. A study on zeolite-based adsorbents for \(\hbox {CO}_{2}\) capture. Bull Mater Sci 42, 240 (2019). https://doi.org/10.1007/s12034-019-1936-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12034-019-1936-8