Abstract
Growing concern about global warming and greenhouse effects has led to persistent demands for increased energy efficiency and reduced carbon dioxide emissions. As a result, energy-intensive processing of carbon dioxide separation became imperative. Accordingly, energy-efficient, economically viable carbon dioxide separation technologies are sought as carbon dioxide capture options for future industrial process schemes. The article provides an overview of current technology for the separation of carbon dioxide, specifically focusing on adsorption. In this study, amine-loaded Zeolite-Y adsorbents were evaluated to enhance carbon dioxide adsorption capacity through synthesis, characterization, and the adsorption of carbon dioxide, within the context of current trends in separation technology. This study aims to study the ability of amine-loaded Zeolite-Y to adsorb carbon dioxide using three different loadings ethanolamine, diethanolamine, and triethanolamine. The amine-loaded materials were characterized by various technologies, including X-ray diffraction pattern (XRD), Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), Brunauer–Emmett–Teller (BET), and field emission scanning electron microscope (FESEM) studies. The study suggests that monoethanolamine-loaded Zeolite-Y is a promising and cost-effective adsorbent for carbon dioxide adsorption in comparison to other synthesized amine-loaded adsorbents. The adsorbent has been able to adsorb carbon dioxide in the range of 1.14–2.26 mmol g−1 at 303 K and 1 bar for a loading of 1, 5, and 10 wt.% amine groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon dioxide (CO2), a greenhouse gas, has a serious issue in terms of global warming. The CO2 produced through point sources, such as fossil fuel-powered power plants, must be therefore captured, utilized, and stored (CCUS). For this purpose, oxygen-fuel-based capture, pre- and post-combustion capture, and industrial process streams have been suggested as large-scale viable methods (Herzog 2015). There have been intensive and long-term studies on the industrial usage of alkanolamine solutions for post-combustion CO2 capture (D’Alessandro et al. 2010). However, inherent limitations exist for such processes and these refer to potential environmental and health concerns due to volatile amine loss, corrosion problems, and high energy requirements for solvent regeneration (Nguyen et al. 2011). As an alternative to these limitations, solid CO2 adsorbents have been suggested due to their non-corrosive characteristics and lower energy requirements (Figueroa et al. 2008).
Several gas streams, including flue gasses, are well-suited for CO2 capture with different amine groups because of their reversible reactions with CO2. As a result, amine-functionalized adsorbents have been extensively studied for post-combustion CO2 capture as they can effectively adsorb CO2 even at its low concentrations (10–15%) and also in the presence of moisture (McDonald et al. 2012). The amine-functionalized adsorbents can be synthesized physically by impregnating amines onto porous supports (Siriwardane et al. 2005; Jadhav et al. 2007; Chatti et al. 2009), chemically by grafting amines onto the pore surface (Sayari and Belmabkhout 2010; Bollini et al. 2012; Heydari-Gorji and Sayari 2012), or through in situ polymerization of amine monomers within the pores (Qi et al. 2014; Newton Augustus et al. 2017).
The CO2 adsorption on amine-modified adsorbent involves the development of an adsorbent comprising a porous support and an amine attached to it. Various porous supports have been tested for amine heterogenization. These include mesoporous silica (Han et al. 2015), carbon-based adsorbents (Yang et al. 2012), zeolites (Shao et al. 2009), and MOFs (Dang et al. 2015; Ünveren et al. 2017; Yoo et al. 2018).
Among all studied adsorbents, zeolites possess a very good CO2 sorption capacity of 1.17–5.11 mmol g−1 at 25 °C and 1 bar (Akhtar and Bergström 2011; Espejel-Ayala et al. 2014; Nakrani et al. 2017; Khaleque et al. 2020). Thus, they are one of the most cost-effective CO2 capture adsorbents. A zeolite’s adsorptive affinity for CO2 molecules occurs due to the interaction between its electric field and CO2 molecules’ quadrupole moment. The unique properties of zeolites, such as ion exchange capability, high thermal stability, molecular sieving, shape selectivity, adsorption capability, and acidity or basicity based on cation types, received attention for a wider utility in petrochemical, fine chemical, and environmental protection fields. Further, their shape-selective catalytic properties are also well explored along with adsorption and separation properties. Zeolite-Y is one of the most used zeolites for catalytic cracking, adsorption, and separation processes. This is due to inherent structural and surface properties (Corma 1997, 2003; Zaarour et al. 2014; Sun et al. 2016). The crystalline frameworks of Zeolite-Y possess large internal and external surfaces and thereby create a major channel for substance transport (Camblor et al. 1989; Li et al. 2005; Lutz 2014).
Few articles were reported on sorption characteristics of amine-functionalized Zeolite-Y60 (AFZ). Su et al. (2010) reported AFZ adsorbent with 50 wt.% loading of TEPA on Zeolite-Y60. The highest adsorption capacity was recorded at 60 °C and in the range of 2.5–4.27 mmol g−1 for flue gas stream condition (15% CO2, 7% H2O, and balance N2). The AFZ exhibited a stable performance during prolonged cyclic operation. The CO2 adsorption capacity of hydrophilic adsorbents, like Zeolite-13X, may decrease in the presence of moisture, whereas it could potentially increase for hydrophobic sorbents, such as Y60-(TEPA).
Babaei et al. (2017) conducted CO2 adsorption studies for 50 wt.% TEPA-loaded NaY zeolite sorbent. The pure CO2 adsorption capacity of the adsorbents ranged from 1.77 to 2.11 mmol g−1 at 75 °C. The adsorption behavior could be attributed to a strong diffusion-controlled process. The mechanism of the CO2 adsorption on pure NaY is entirely based on a physical interaction process. However, its behavior changes to chemical interaction after amine modification. Thus, with increased temperature, chemical reactions occur between the amino groups and CO2 and thereby produce the carbamate species which facilitates better CO2 adsorption.
Tejavath et al. (2021) studied CO2 capture on three amine-modified zeolites (13X, 4A, and 5A) using 99.99% pure CO2 gas at different temperatures (25, 50, 75, and 100 °C). Zeolites were impregnated with four different amines (DETA, EDA, MEA, and TEA) to study CO2 absorption performance. The DETA-loaded Zeolite-13X adsorbent (40-DETA-13X) showed maximum CO2 adsorption capacity (1.054 mmol g−1) at 75 °C. It is mainly the diffusion-controlled mechanism of filling the pores of zeolites with DETA and other amines that limits the porosity of zeolites at temperatures up to 75 °C, thereby increasing the availability of CO2 adsorption sites.
Dinda et al. (2019) used fixed-bed flow reactors to study CO2 adsorption from simulated gas mixtures (15 vol.% CO2, balance N2). Zeolites ZSM-5, Zeolite-Y, and Zeolite-13X were used as support materials. To investigate the performance of the adsorbents for CO2 adsorption in the temperature range of 30–60 °C, four different types of amines (MEA, EDA, DETA, and TETA) were impregnated (5–40 wt.%) on various support materials. As amine loading increases up to 30 wt.%, the CO2 adsorption capacity increases, and afterward, a decreasing trend was observed. The CO2 uptake capacity of MEA-, EDA-, and DETA-loaded adsorbents decreased with increasing adsorption temperature. TETA-loaded adsorbents exhibit their maximum capacity at 50 °C, and the 30-TETA-ZSM-5 sorbent has a capture capacity of 1.20 mmol g−1. In the presence of amine compounds, more basic sites are available for the chemisorption of CO2, which is favored by surface area and pore volume analysis.
This article aims to develop amine-functionalized Zeolite-Y to enhance its carbon dioxide adsorption capacity. The impregnation method was used to functionalize the support material by using primary (monoethanolamine), secondary (diethanolamine), and tertiary (triethanolamine) amine groups. In addition, different characterization techniques were used to characterize the materials. The amine-modified adsorbents were tested for CO2 absorption at atmospheric pressure (1 bar) and low adsorption temperature (303 K).
Experimental procedure
Synthesis method
In this study, three different amine loadings were used to prepare amine-functionalized adsorbents (1 wt.%, 5 wt.%, and 10 wt.%) with monoethanolamine, diethanolamine, and triethanolamine using wet impregnation method. The materials used in this study were methanol (99.5%, C2H6O) from Sigma Aldrich, all three amine compounds (98%) were procured from Sigma Aldrich, and the support material Zeolite-Y (Si/Al ratio 5.1:1) was purchased from Alfa Aesar.
The support Zeolite-Y was calcinated at high temperatures for 4 h to remove volatile materials. Then, the Zeolite-Y powder was agitated in methanol for 60 min and at a solid-to-liquid ratio of 1:2. After this, the solution was air-dried for 180 min at the ambient temperature. The impregnation method was used to modify Zeolite-Y by adding amine solutions in methanol at 10 wt.% concentration for 20 min. Then, the amine solution was added to air-dried zeolite and stirred for 120 min. Consequently, the adsorbents were separated from the solution using filtration and were dried in an oven at 140 °C for 180 min (Babaei et al. 2017). As a result, the Zeolite-Y modified with 1 wt.%, 5 wt.%, and 10 wt.% of monoethanolamine loading were designated as MEOH1, MEOH5, and MEOH10. A similar process was carried out with diethanolamine (DEOH) and triethanolamine (TEOH) to realize amine-loaded adsorbents with similar designations (DEOH1, DEOH5, DEOH10, TEOH1, TEOH5, TEOH10).
Material characterization
Adsorbent thermal properties were measured using a thermogravimetric analyzer (model: STA449F3A00 by Netzsch) in a nitrogen atmosphere, with a temperature range of 20–600 °C and a heating rate of 10 °C min−1. The surface area of the adsorbents was characterized using nitrogen adsorption/desorption isotherms. Using the Brunauer–Emmett–Teller method, the specific surface area was evaluated for relative pressures (P/P0) ranging from 0.05 to 0.30. Before the analysis, the sample was degassed at 150 °C using a volumetric gas adsorption device (Quantachrome Instrument, Autosorb, IQ MP). X-ray powder diffraction analysis of the adsorbents was performed (Rigaku Technologies, Japan, model: Smartlab) with Johansson Kα1 optics scheme operated at a 9 kW rotating anode X-ray source in the range of 2° ≤ 2θ ≤ 40°. A field emission scanning electron microscope (make: Zeiss, model: Sigma 300) operated at 50k× and 5 kV was used to analyze the surface morphology. A Fourier transform infrared spectroscopy instrument (model: Spectrum Two) was used to confirm the presence of amine groups of the synthesized adsorbents in the 4000–400 cm−1 wavenumber range. This study collected X-ray photon spectroscopy (XPS) data using an AXIS Supra (make: Kratos Analytical Ltd) with a monochromatic X-ray source: Al Kα at 1486.7 eV. With this technique, the surface of a sample material is bombarded with high-energy X-rays (< 1 keV), and the kinetic energy of the emitted electrons is measured. In addition, the material to be investigated should be properly dried and remain stable in an ultrahigh vacuum environment.
Adsorption setup
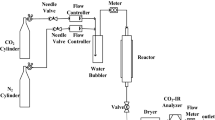
A volumetric gas adsorption analyzer (Quantachrome iSorbHP1-XKRLSPN100) at 303 K and a pressure range of 0.02–1 bar was used for the determination of CO2 adsorption of amine-functionalized adsorbents. Approximately, 200 mg of adsorbent was taken in a sample holder and it was fitted to the analysis port. The system was degassed at 423 K for 180 min to remove the pre-adsorbed gasses by filling the system with helium gas. In order to conduct the analysis, the sample was maintained at 303 K using a recirculating bath. The amount of carbon dioxide adsorbed was determined by measuring the change in gas volume.
Results and discussion
This study investigated the thermodynamic properties of different loadings of amine groups on Zeolite-Y.
Material characterization
Figure 1a shows the thermal behavior of all the amine-loaded zeolites. They were obtained with the TGA instrument under N2 conditions. The characterization was carried out in a temperature range of 25 to 600 °C and at a heating rate of 10 °C/min. It was observed that a two-step decomposition process occurred in the synthesized adsorbents (between 25 and 200 °C and 200 and 600 °C). The TGA profile indicated that the physically adsorbed water molecules got desorbed in the first step. The second region in the range of 200 °C and 600 °C can be attributed to the volatilization of the entrapped amine material. The heat treatment of materials from 160 to 600 °C did not result in significant weight loss. This confirmed the high thermal stability of all the synthesized samples. The mass loss profile showed a larger variation in the order of MEOH < DEOH < TEOH loaded zeolite adsorbents, which follows the boiling point order (MEOH < DEOH < TEOH).
X-ray diffraction was used for the determination of crystallinity in functionalized and commercial samples. Figure 1b shows the XRD patterns of Zeolite-Y and amine-functionalized Zeolite-Y. Based on the X-ray powder diffraction of amine-functionalized Zeolite-Y, it appears that the crystal structure of Zeolite-Y was retained even after the modification. This conveys that the impregnation process did not affect crystallinity in any of the synthesized samples. Also, the diffraction peaks located at 2θ values of 6.28°, 15.82°, and 23.86° can be seen in all the samples. This indicates the presence of an ordered hexagonal structure.
Figure 2 depicts nitrogen adsorption-desorption isotherms of amine-functionalized Zeolite-Y samples. For this case, a typical type-IV adsorption isotherm exists along with a hysteresis loop. This conveyed a uniform mesoporous structure since the high volume of nitrogen got adsorbed at very low relative pressures. Therefore, marginally higher nitrogen uptake observed at lower pressures, in the range of 0.0–0.1, can be probably attributed to the fact that amine groups added to the pure Zeolite-Y occupy the surface and pore openings of the support. The relative pressure in the range between 0.3 and 0.4 has a steep capillary condensation inflection. This was confirmed by the narrow pore size distribution in mesoporous materials. A sharp increase of relative pressures in the range of 0.9–1.0 was prevalent in some isotherms. This is probably due to multilayer adsorption. The Zeolite-Y being impregnated with other amine groups and at various amine loadings, affirmed progressive decline in the nitrogen uptake with the increasing loading.
Table 1 shows the surface properties of amine-functionalized Zeolite-Y. The surface area, pore volume, and pore size were determined using N2 isotherms. For enhanced amine loading, the specific surface area and pore volume are reduced at a progressive rate. This indicated that the amine groups filled the pores and prevented nitrogen from being adsorbed. Zeolite-Y loaded with the lowest amine wt.% and the commercial Zeolite-Y had similar surface characteristics.
Figure 3 shows the FTIR spectra of Zeolite-Y and the amine-functionalized Zeolite-Y adsorbents. All the samples exhibited symmetric T–O bands at 450 cm−1 (T: Si or Al) resulting from internal vibrations of the TO4 (SiO4 and AlO4) tetrahedron framework. Additionally, the spectrum suggests that the tetrahedral units are externally linked at 590 cm−1, and confirmed secondary building units (SBU) and external linkage. The IR spectrum of samples conveyed significant bands at 810 cm−1, which are related to T–O–T (Si–O–Si or Si–O–Al) symmetry stretching (external linkage to symmetric stretch reflecting structure). The strongest vibration at 1029 cm−1 occurred due to an asymmetric stretching mode O–T–O that involved motions primarily associated with oxygen atoms. The sample’s IR spectra affirmed a peak at 1633 cm−1 related to O–H stretching, which is due to the existence of water molecules. The peak intensities of synthesized samples corresponding to the Zeolite-Y structure reduced after amine loading. The peaks found in the region 3387 cm−1 provide important information on impregnated amine groups (Su et al. 2010). In comparison to Zeolite-Y and amine-functionalized samples, the presence of an additional peak can be observed for amine-loading with N–H stretching on the surfaces of adsorbents.
Figure 4 shows the SEM images of Zeolite-Y before and after amine loading. All the samples being characterized at 5 kV acceleration voltages have been presented at a magnification of 50kX. The primary tetrahedral and secondary hexagonal structures of Zeolite-Y were intact even in the amine-modified samples. This indicates that the morphology of Zeolite-Y support did not change even after amine loading, which is also in alignment with the literature (Tejavath et al. 2021).
Parameters effect for CO2 adsorption
During adsorption equilibrium measurements, 99.9% pure carbon dioxide gas was used. Using a volumetric adsorption system, the adsorption equilibrium of carbon dioxide gas on synthesized adsorbents was determined at 303 K. Before each isotherm measurement, the sample was degassed by heating to 423 K under vacuum before the measurement. Volumetric adsorption systems include a section of the system with a known volume, called the manifold. Using high-sensitivity pressure sensors, the exact amount of gas enclosed in these areas can be determined at any given time with the pneumatic valves connected to the system.
Effect of amine groups
In this study, the amine-loaded Zeolite-Y was used to adsorb carbon dioxide. During such sorption, some of the CO2 diffused into the adsorbent pores (physisorption), and the remainder reacted with the active amino sites. In empirical studies, it was affirmed that both Zeolite-Y and amine contribute to CO2 adsorption. FTIR spectra of the synthesized samples indicated the presence of amine groups and these functional amine groups act as active sites for CO2 adsorption. The Si–OH–Al clusters on Zeolite-Y surfaces serve as a link to connect amine molecules.
Figure 7 depicts the CO2 sorption capacities of different amines (MEOH, DEOH, and TEOH). Figure 5 illustrates the molecular structures of the amines and their reactions with CO2. MEOH exhibited good adsorption capacity due to its simple molecular structure and a terminal –NH2 group which easily reacted with CO2. The steric hindrance of DEOH and TEOH limits molecular contact with CO2. Hence, DEOH has lower adsorption capacity in comparison to MEOH due to the relatively larger molecules. In addition to steric hindrance, TEOH reacts with CO2 only in the presence of water resulting in its lowest adsorption capacity (Ko et al. 2011). The BET analysis of the samples discussed in the “Material characterization” section also supports the decreasing adsorption capacity of the synthesized samples. During the amine modification of the zeolite using wet impregnation method, the amine molecules occupy the surface and pores of the zeolite sample which could be confirmed from the decrease in surface area and average pore size of the sample. As a result of combined effect of steric hindrance and decreasing surface area, the CO2 adsorption capacities of the synthesized samples decreased in the order of MEOH < DEOH < TEOH. The CO2 adsorption tests carried out in this study were performed under anhydrous conditions. Under these circumstances, CO2 binding to tertiary amines is very difficult.
The reaction of primary and secondary amine groups with carbon dioxide results in the formation of carbamate ion as shown in Eqs. (1) and (2).
XPS measurements were performed on synthesized adsorbents after carbon dioxide adsorption to evaluate the adsorption mechanism of carbon dioxide on amine-loaded adsorbents. In the high-resolution XPS analysis of the C1s spectrum, three distinct peaks were observed at 284.49 eV, 286 eV, and 288.54 eV, as illustrated in Fig. 6a and b. These peaks are associated with the C–H, C–N, and O=C–N species, respectively (Liu et al. 2012; Zangmeister et al. 2013). However, in Fig. 6c, the C1s spectrum displays only two peaks at 284.49 eV and 286 eV, corresponding to C–H and C–N species, respectively. Therefore, both primary and secondary amine-loaded adsorbents exhibited adsorption mechanisms. Figure 6c shows a lack of adsorption peak for O=C–N, which may indicate that there is no adsorption between tertiary amines and carbon dioxide.
Effect of amine loading
Using a volumetric adsorption system, the adsorption capacity of CO2 for modified Zeolite-Y adsorbents at 303 K as a function of MEOH loadings (1 wt.%, 5 wt.%, and 10 wt.%) has been depicted in Fig. 7. According to the obtained results, CO2 adsorption capacity increased from 1 to 5 wt.% amine loading (2.13–2.26 mmol g−1) but decreased for 10 wt.% amine loading case (1.7 mmol g−1). A higher MEOH loading (< 5 wt.%) resulted in the maximum pore blocking of the zeolite structure. As a result of this, the zeolite surface available to adsorb CO2 decreased greatly and the diffusion barrier also increased. Therefore, support pore structure is not the only factor to influences CO2 adsorption capacity. However, MEOH supported on mesoporous Zeolite-Y barely adsorbs CO2 at room temperature at such a higher loading of the amine. Thus, adsorbents with low loadings will circumvent the adverse effect of diffusion limitation at room temperature. MEOH loading of 5 wt.% affirmed a maximum CO2 adsorption capacity of 2.26 mmol g−1 at 303 K and 1 bar pressure for pure CO2. The MEOH dispersion within the support cage-like structure can significantly enhance the CO2 adsorption capacity.
The CO2 adsorption capacities of the synthesized samples in the present work are compared with the existing literature data in Table 2. All the synthesized samples exhibited superior surface area, pore volume, and CO2 adsorption capacities. The MEOH modified zeolite (MEOH5) sample exhibited highest adsorption capacity and the effects of amine loading were discussed above.
Adsorption isotherm
A virial adsorption isotherm model was used to analyze the CO2 adsorption behavior of amine-functionalized adsorbents as shown in Fig. 8. The isotherm model has been used to determine the adsorbent’s affinity and thermodynamic properties for the design of an adsorption system. The virial isotherm model used for fitting experimental CO2 adsorption data is represented in Eq. 1. Accordingly, the isotherm plot illustrates the model data at 303 K and 0.1–1 bar pressure intervals.
where P stands for pressure (bar), and N stands for amount adsorption (mmol g−1). Apart from Henry’s constant (β) (mmol g−1 bar−1), two virial coefficients b (mmol−1 g) and c (mmol−2 g2) are also included in the above expression. Generally, these parameters depend upon temperature as:
In the above expressions, T represents the system temperature in Kelvin. The model was used to obtain type I CO2 isotherm with good fitting at ambient temperatures (303 K).
The expression revealed that the virial isotherm was independent of saturation capacity. In the literature (Barrer and Lee 1968), a different version of the equation exists that incorporates saturation capacity. Virial isotherms with a second or third truncated virial coefficient are capable of correlating gas–solid equilibrium data with high accuracy. Virial adsorption isotherms can very effectively describe heterogeneous surfaces of adsorbents which possess many types of adsorption sites.
The CO2 adsorption of all synthesized adsorbents increased gradually with increasing pressure. Adsorbents with a 5 wt.% amine loading affirmed the best CO2 adsorption capacity at 303 K and 1 bar. This is due to the large number of amine functionalities that act as CO2-affinity sites on the sorbent. The gradual increase in CO2 adsorption could be due to the initial packing of CO2 molecules in monolayers and then in multilayers. CO2 adsorption can also occur inside amine-loaded pores, or on their external surfaces. This is because most exposed amine sites are already occupied by CO2. The larger molecular structure of DEOH molecules possessing two alkyl groups connected to the central “N” may contribute less to the CO2 adsorption capacity of the sorbent and accordingly reported low CO2 uptake in comparison to MEOH functionalized adsorbents. The structure or type of an amine molecule influences CO2 adsorption on the adsorbent. In comparison to MEOH and DEOH, TEOH has a larger molecular structure with three alkyl groups on the central atom. CO2 adsorption capacity is lower for TEOH-functionalized adsorbents than for either MEOH- or DEOH-functionalized adsorbents. It could be due to the presence of three bulky alkyl groups on the central “N” atom causing steric hindrance for CO2 adsorption.
Adsorption enthalpy
Based on the virial adsorption isotherm, the following equation relates to the total amount of heat generated due to reactions between absorbent and CO2. For the heat of adsorption, the enthalpy of the standard state is computed with the determination of the heat of adsorption generated per mole of CO2. Amine structure is one of the main factors that affect heat adsorption (Kim and Svendsen 2007).
Figure 9 depicts the heat of absorption (−Δhads) generated by amine-functionalized adsorbent at 303 K. The heat adsorption at initial loading is extremely important as it represents the adsorption enthalpy at the strongest site and confirms its surface heterogeneity. At nearly zero loading, all graphs show relatively high adsorption enthalpy, which decreases with increasing amine loading. The negative values of −∆h0 confirm the exothermic adsorption of CO2. According to the thermodynamic analysis, the higher heat of adsorption value for MEOH-loaded adsorbents conveyed that they have a higher CO2 interaction potential.
While MEOH5 is considered to be the most efficient absorbent in the absorption process, it assured the highest heat of absorption value of 22.59 kJ mol−1 CO2. The lowest absorption rate was achieved for TEOH10, a tertiary amine (lowest heat of absorption value of 21.09 kJ mol−1 CO2). The heat of adsorption was observed to be as per the order of TEOH < DEOH < MEOH in the synthesized sorbents. Adsorption processes generally involve physical adsorption with enthalpy changes in the range of 20–40 kJ mol−1. Similarly, chemical adsorption assures enthalpy changes in the range of 40–400 kJ mol−1 (Zhou et al. 2012). Thus, the amine-loaded adsorbents are likely to strongly physisorbed carbon dioxide.
Cyclic study
A series of four continuous adsorption-desorption cycles have been performed in the adsorption setup to investigate the cyclic performance of the optimized adsorbent (MEOH5). During each cycle, the adsorption process is performed at 303 K and up to 1 bar with pure carbon dioxide gas. Regeneration of the adsorbent was performed at 423 K in a pure helium atmosphere under vacuum conditions to remove strongly adsorbed water molecules and make free adsorption sites available in small cages.
Figure 10 shows the CO2 adsorption capacity of the adsorbent in the first cycle at 2.26 mmol CO2/g. As a result, the CO2 adsorption capacity of MEOH5 decreased to 2.19 mmol CO2/g after four cycles, indicating that MEOH5 may be used for longer-term processes without major adsorption loss. All adsorption-desorption cycles had similar behavior with a small deviation over the four cycles studied. According to these results, it is mainly a physisorption process in which the adsorbent structure remains mechanically stable and there was oxidative degradation of the amine component to form amide, nitrite, and imine phases, which results in the deactivation of the adsorbent, resulting in a decrease in the CO2 adsorption capacity (Huang et al. 2023).
Conclusion
The effects of monoethanolamine (MEOH), diethanolamine (DEOH), and triethanolamine (TEOH) functionalization on highly ordered mesoporous Zeolite-Y adsorbent was compared for the pure CO2 case using a volumetric adsorption system. Various characterization techniques and CO2 adsorption thermodynamics models were used to verify the results. The amines were well distributed on the surface and also effectively filled the Zeolite-Y pore space. Amine loading higher than 5 wt.% was probably effective in coating the external surface of the support material. In this study, MEOH5, DEOH5, and TEOH5 showed maximum adsorption capacities of 2.26, 1.84, and 1.62 mmol of CO2 per g adsorbents, respectively. Hence, MEOH-loaded adsorbent was the suitable CO2 adsorbent. Physical adsorption was the primary mechanism for CO2 adsorption and the functional –NH2, –NH, and –N groups of the amines served as active sites. According to a thermodynamic perspective, the experimental results for CO2 adsorption were well concordant with the virial adsorption isotherm. Based on thermodynamic analysis, the corresponding heat of adsorption increased in the order MEOH > DEOH > TEOH. This is consistent with affirming a higher interaction potential between the adsorbate and adsorbent molecules. The monoethanolamine functionalized Zeolite-Y adsorbent can effectively mitigate well-known issues associated with high carbon capture costs, thus offering a promising application for future CO2 capture procedures.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Akhtar F, Bergström L (2011) Colloidal processing and thermal treatment of binderless hierarchically porous zeolite 13X monoliths for CO2 capture. J Am Ceram Soc 94:92–98. https://doi.org/10.1111/j.1551-2916.2010.04044.x
Babaei M, Anbia M, Kazemipour M (2017) Improving CO2 adsorption with new amine-functionalized Y-type zeolite. J Adv Env Heal Res 5:70–77. https://doi.org/10.22102/jaehr.2017.71674
Barrer RM, Lee JA (1968) Hydrocarbons in zeolite L II. Entropy, physical state and isotherm model. Surf Sci 12:354–368. https://doi.org/10.1016/0039-6028(68)90135-0
Bollini P, Brunelli NA, Didas SA, Jones CW (2012) Dynamics of CO2 adsorption on amine adsorbents. 2. Insights into adsorbent design. Ind Eng Chem Res 51:15153–15162. https://doi.org/10.1021/ie3017913
Camblor MA, Corma A, Martínez A et al (1989) Catalytic cracking of gasoil. Benefits in activity and selectivity of small Y zeolite crystallites stabilized by a higher silicon-to-aluminium ratio by synthesis. Appl Catal 55:65–74. https://doi.org/10.1016/S0166-9834(00)82317-9
Chatti R, Bansiwal AK, Thote JA et al (2009) Amine loaded zeolites for carbon dioxide capture: amine loading and adsorption studies. Microporous Mesoporous Mater 121:84–89. https://doi.org/10.1016/j.micromeso.2009.01.007
Corma A (1997) From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem Rev 97:2373–2419. https://doi.org/10.1021/cr960406n
Corma A (2003) State of the art and future challenges of zeolites as catalysts. J Catal 216:298–312. https://doi.org/10.1016/S0021-9517(02)00132-X
D’Alessandro DM, Smit B, Long JR (2010) Carbon dioxide capture: prospects for new materials. Angew Chemie - Int Ed 49:6058–6082. https://doi.org/10.1002/anie.201000431
Dang QQ, Zhan YF, Duan LN, Zhang XM (2015) A pyridyl-decorated MOF-505 analogue exhibiting hierarchical porosity, selective CO2 capture and catalytic capacity. Dalt Trans 44:20027–20031. https://doi.org/10.1039/c5dt01943e
Dinda S, Murge P, Chakravarthy Paruchuri B (2019) A study on zeolite-based adsorbents for CO2 capture. Bull Mater Sci 42:240. https://doi.org/10.1007/s12034-019-1936-8
Espejel-Ayala F, Corella RC, Pérez AM et al (2014) Carbon dioxide capture utilizing zeolites synthesized with paper sludge and scrap-glass. Waste Manag Res 32:1219–1226. https://doi.org/10.1177/0734242X14554643
Figueroa JD, Fout T, Plasynski S et al (2008) Advances in CO2 capture technology-the U.S. Department of Energy’s Carbon Sequestration Program. Int J Greenh Gas Control 2:9–20. https://doi.org/10.1016/S1750-5836(07)00094-1
Han Y, Hwang G, Kim H et al (2015) Amine-impregnated millimeter-sized spherical silica foams with hierarchical mesoporous-macroporous structure for CO2 capture. Chem Eng J 259:653–662. https://doi.org/10.1016/j.cej.2014.08.043
Herzog H (2015) Carbon Dioxide Capture and Storage. In: Dieter H and Cameron H (eds), The economics and politics of climate change, Oxford Academic, pp 263–283. https://doi.org/10.1093/acprof:osobl/9780199573288.003.0013
Heydari-Gorji A, Sayari A (2012) Thermal, oxidative, and CO2-induced degradation of supported polyethylenimine adsorbents. Ind Eng Chem Res 51:6887–6894. https://doi.org/10.1021/ie3003446
Huang P, Fu J, Qiu D et al (2023) Experimental and kinetic study on the cyclic removal of low concentration CO2 by amine adsorbents in confined spaces. Process Saf Environ Prot 180:417–427. https://doi.org/10.1016/j.psep.2023.10.032
Jadhav PD, Chatti RV, Biniwale RB et al (2007) Monoethanol amine modified zeolite 13X for CO2 adsorption at different temperatures. Energy and Fuels 21:3555–3559. https://doi.org/10.1021/ef070038y
Khaleque A, Alam M, Hoque M et al (2020) Zeolite synthesis from low-cost materials and environmental applications: a review. 2. https://doi.org/10.1016/j.envadv.2020.100019
Kim I, Svendsen HF (2007) Heat of absorption of carbon dioxide (CO2) in monoethanolamine (MEA) and 2-(aminoethyl)ethanolamine (AEEA) solutions. Ind Eng Chem Res 46:5803–5809. https://doi.org/10.1021/ie0616489
Ko YG, Shin SS, Choi US (2011) Primary, secondary, and tertiary amines for CO2 capture: designing for mesoporous CO2 adsorbents. J Colloid Interface Sci 361:594–602. https://doi.org/10.1016/j.jcis.2011.03.045
Li G, Jones CA, Grassian VH, Larsen SC (2005) Selective catalytic reduction of NO2 with urea in nanocrystalline NaY zeolite. J Catal 234:401–413. https://doi.org/10.1016/j.jcat.2005.06.025
Liu M, Li W, Rong J, Zhou C (2012) Novel polymer nanocomposite hydrogel with natural clay nanotubes. Colloid Polym Sci 290:895–905. https://doi.org/10.1007/s00396-012-2588-z
Lutz W (2014) Zeolite Y: synthesis, modification, and properties - a case revisited. Adv Mater Sci Eng 2014. https://doi.org/10.1155/2014/724248
McDonald TM, Lee WR, Mason JA et al (2012) Capture of carbon dioxide from air and flue gas in the alkylamine-appended metal-organic framework mmen-Mg 2(dobpdc). J Am Chem Soc 134:7056–7065. https://doi.org/10.1021/ja300034j
Nakrani D, Belani M, Bajaj HC et al (2017) Concentrated colloidal solution system for preparation of uniform Zeolite-Y nanocrystals and their gas adsorption properties. Microporous Mesoporous Mater 241:274–284. https://doi.org/10.1016/j.micromeso.2016.12.039
Newton Augustus E, Nimibofa A, AzibaolaKesiye I, Donbebe W (2017) Metal-organic frameworks as novel adsorbents: a preview. Am J Environ Prot 5:61–67. https://doi.org/10.12691/env-5-2-5
Nguyen T, Hilliard M, Rochelle G (2011) Volatility of aqueous amines in CO2 capture. Energy Procedia 4:1624–1630. https://doi.org/10.1016/j.egypro.2011.02.033
Qi G, Fu L, Giannelis EP (2014) Sponges with covalently tethered amines for high-efficiency carbon capture. Nat Commun 5:1–7. https://doi.org/10.1038/ncomms6796
Sayari A, Belmabkhout Y (2010) Stabilization of amine-containing CO2 adsorbents: dramatic effect of water vapor. J Am Chem Soc 132:6312–6314. https://doi.org/10.1021/ja1013773
Shao W, Zhang L, Li L, Lee RL (2009) Adsorption of CO2 and N2 on synthesized NaY zeolite at high temperatures. Adsorption 15:497–505. https://doi.org/10.1007/s10450-009-9200-y
Siriwardane RV, Shen MS, Fisher EP, Losch J (2005) Adsorption of CO2 on zeolites at moderate temperatures. Energy and Fuels 19:1153–1159. https://doi.org/10.1021/ef040059h
Su F, Lu C, Kuo SC, Zeng W (2010) Adsorption of CO2 on amine-functionalized y-type zeolites. Energy and Fuels 24:1441–1448. https://doi.org/10.1021/ef901077k
Sun MH, Huang SZ, Chen LH et al (2016) Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem Soc Rev 45:3479–3563. https://doi.org/10.1039/c6cs00135a
Tejavath V, Kasarabada V, Gonuguntla S et al (2021) Technoeconomic investigation of amine-grafted zeolites and their kinetics for CO2 capture. ACS Omega 6:6153–6162. https://doi.org/10.1021/acsomega.0c05397
Ünveren EE, Monkul BÖ, Sarıoğlan Ş et al (2017) Solid amine sorbents for CO2 capture by chemical adsorption: a review. Petroleum 3:37–50. https://doi.org/10.1016/j.petlm.2016.11.001
Yang H, Yuan Y, Tsang SCE (2012) Nitrogen-enriched carbonaceous materials with hierarchical micro-mesopore structures for efficient CO2 capture. Chem Eng J 185–186:374–379. https://doi.org/10.1016/j.cej.2012.01.083
Yoo DK, Abedin Khan N, Jhung SH (2018) Polyaniline-loaded metal-organic framework MIL-101(Cr): promising adsorbent for CO2 capture with increased capacity and selectivity by polyaniline introduction. J CO2 Util 28:319–325. https://doi.org/10.1016/j.jcou.2018.10.012
Zaarour M, Dong B, Naydenova I et al (2014) Progress in zeolite synthesis promotes advanced applications. Microporous Mesoporous Mater 189:11–21. https://doi.org/10.1016/j.micromeso.2013.08.014
Zangmeister RA, Morris TA, Tarlov MJ (2013) Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 29:8619–8628. https://doi.org/10.1021/la400587j
Zhou X, Yi H, Tang X et al (2012) Thermodynamics for the adsorption of SO2, NO and CO2 from flue gas on activated carbon fiber. Chem Eng J 200–202:399–404. https://doi.org/10.1016/j.cej.2012.06.013
Acknowledgements
The authors gratefully acknowledge the Central Instruments Facility (CIF), Analytical Lab of the Chemical Engineering Department, and Centre for Environment, Indian Institute of Technology Guwahati, for BET, XRD, CO2 adsorption/desorption, FESEM, and FTIR and Central Research Facility (CRF), Indian Institute of Technology Delhi, for XPS analysis.
Author information
Authors and Affiliations
Contributions
Geetanjali Bhati and Naga Phani Sai Kumar Dharanikota conducted experimental and modeling studies. The first draft of the manuscript was written by Geetanjali Bhati and Naga Phani Sai Kumar Dharanikota. Bishnupada Mandal and Ramgopal V. S. Uppaluri have supervised and contributed to the draft—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
We declare that all authors fully comply with ethical standards.
Consent to participate
All participants in this study consented to participate in the research.
Consent for publication
The manuscript has not been submitted nor is under consideration for publication by another journal and none of its contents have been previously published. All authors approved the final version for submission to your journal. All authors agreed to publication in the Journal of Environmental Science and Pollution Research.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhati, G., Dharanikota, N.P.S.K., Uppaluri, R.V.S. et al. Investigating the synergistic effects of various amine groups on Zeolite-Y for CO2 capture. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-33869-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11356-024-33869-8