Abstract
Research on microbial fatty acid metabolism started in the late 1960s, and till date, various developments have aided in elucidating the fatty acid metabolism in great depth. Over the years, synthesis of microbial fatty acid has drawn industrial attention due to its diverse applications. However, fatty acid overproduction imparts various stresses on its metabolic pathways causing a bottleneck to further increase the fatty acid yields. Numerous strategies to increase fatty acid titres in Escherichia coli by pathway modulation have already been published, but the stress generated during fatty acid overproduction is relatively less studied. Stresses like pH, osmolarity and oxidative stress, not only lower fatty acid titres, but also alter the cell membrane composition, protein expression and membrane fluidity. This review discusses an overview of fatty acid synthesis pathway and presents a panoramic view of various stresses caused due to fatty acid overproduction in E. coli. It also addresses how certain stresses like high temperature and nitrogen limitation can boost fatty acid production. This review paper also highlights the interconnections that exist between these stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial fatty acids enable a cell to form membranous components that are essential for its structural integrity. These fatty acids also serve as an alternative energy reservoir [1, 2]. Depending upon the carbon chain length, fatty acids can be broadly classified as Short-Chain Fatty Acids (SCFAs), Medium-Chain Fatty Acids (MCFAs) and Long-Chain Fatty Acids (LCFAs). Fatty acids with 1–6 carbon atoms (C) are considered as SCFAs whereas those between 7 and 12 C are categorised as MCFAs and fatty acid having more than 12 C are classified as LCFAs [3, 4]. Over the years, microbial fatty acids have grabbed immense attention on an industrial scale. SCFAs are required for synthesis of polymers and preservatives like volatile fatty acids (VFAs), whereas MCFAs are favoured as health supplements and LCFAs are preferred for biofuel production [5,6,7,8,9]. Thus, microbial fatty acids have diverse applications and E. coli serves as a classical paradigm for microbial fatty acid research.
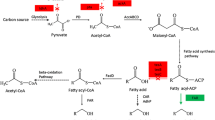
The basal ability of E. coli to produce fatty acids can be further increased by overexpressing/deleting few of the key genes or rate-limiting enzymes of fatty acid biosynthesis pathway [10,11,12,13]. Acetyl-CoA Carboxylase (ACC), a rate-limiting enzyme of fatty acid synthesis pathway, plays an important role in catalysing carboxylation of acetyl-CoA to form malonyl-CoA. The conversion of acetyl-CoA to malonyl-CoA is the committing step towards fatty acid synthesis. Hence, fatty acid titres can be increased by overexpressing acc gene along with other genetic alterations [13]. According to their results, a fatty acid producing strain yielded six times more fatty acids (691 mg/L) than the control strain (100 mg/L) by overexpressing an exogenous acc, pantothenate kinase (coaA) and fasA to supplement high levels of malonyl-CoA, coenzyme-A and enhancement of fatty acid pathway, respectively. Due to these genetic alterations, consequences like slow growth rate, high pH of the medium and high turbidity were observed. Factors like end-product toxicity and metabolic stress imparted during fatty acid overproduction could be the possible reasons for delayed cell growth whereas repressed acid generation by fatty acid producing strain led to increase in the pH of the medium. However, the study reported low level of confidence over high turbidity values. Turbidity indicates the presence of particulate matter in the medium (including dead and alive cells along with cell debris). Therefore, high turbidity implies increased cell number, but according to the transmission electron microscope (TEM) images, the observed cell number of fatty acid producing strain was lesser in comparison with the control strain. Also, the cell size of fatty acid producing strain was larger than the control strain due to intercalation of overproduced fatty acids in the inner membrane. Hence, the turbidity values do not co-relate well with its cell count.
Apart from overexpression, fatty acid titres can be increased further by deleting fadD gene, encoding FadD (fatty acid degrading enzyme) and thereby increasing the fatty acid pool. In this manner, a combinatorial strategy of ACC overexpression and fadD deletion will increase the flux of acetyl-CoA towards fatty acid synthesis and block the fatty acid degradation, respectively. A study by Xu et al. reported fatty acid yield that was increased to 8.6 g/L by combinatorial strategy along with overexpression of truncated thioesterase, tesA’, an enzyme responsible for cleaving fatty acids. In an attempt to increase fatty acid titres, researchers also tried to co-overexpress genes of glycolytic pathway and fatty acid pathway. However, sub-optimal production of fatty acid was observed due to metabolic imbalance. Therefore, a fine tuning of various metabolic network is necessary to uphold its maximum fatty acid production. Till date, 21.6 g/L is the highest fatty acid yield achieved with the help of advanced techniques like CRISPRi-Omics [14].
In these reports, though the ability of E. coli to overproduce fatty acid was fostered, several genetic alterations impart various stresses on its metabolic pathways. These stresses cause changes in cell membrane composition, thus, affecting the membrane fluidity, which thereby restricts the movement and orientation of transmembrane proteins [15]. Along with membrane integrity, expressions of various genes of fatty acid metabolism also lead to low fatty acid titres. This is due to the diversion of the cell’s resources to circumvent the stresses for survival, rather than increasing fatty acid titres.
Numerous strategies have been published for increasing the fatty acid titres; however, effect of stresses elicited during fatty acid overproduction is relatively unexplored. Understanding the stress mechanism and its effect on fatty acid synthesis can consequently assist in tackling the problem in a better manner. In the current review paper, we present a bird’s eye view of the effect of various stresses on fatty acid overproduction in E. coli. We discuss different stresses like pH, osmolarity and oxidative stress to give an insight on the interconnectedness between these stresses, and their cumulative negative impact on fatty acid synthesis pathway. This review also addresses a few of the stresses like temperature and nitrogen limitation (C/N ratio), which are known to enhance fatty acid production.
Overview of Fatty Acid Metabolism
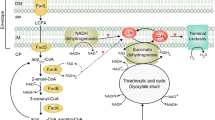
Fatty acid pathway is one of the important metabolic pathways, which is tightly regulated inside a cell, under different growth phases. Depending upon the environmental stimulus, the rate of its anabolic and catabolic reaction varies. Figure 1 shows an overview of fatty metabolism in E. coli. Fatty acid metabolism can be divided into four phases: (a) initiation/synthesis, (b) elongation, (c) termination and (d) degradation. Formation of acetyl-CoA via glycolysis is the nodal point for various pathways and has different fates. The acetyl-CoA is either channelised towards energy yielding pathways like TCA or to form acetate as a secretory by-product, or can be redirected towards fatty acid synthesis.
The initial step of fatty acid synthesis pathway is the carboxylation of acetyl-CoA to form malonyl-CoA under the influence of acetyl-CoA carboxylase (ACC) as discussed above. ACC is a rate-limiting multienzyme complex having multiple regulations by various regulators (FadR, GlnB), substrate (α-Ketoglutarate), feedback inhibition (palmitoyl-CoA), etc. [16, 17]. Malonyl Transacylase (FabD) and acetoacyl-ACP synthase (FabH) accompanies ACC in the initiation phase of the fatty acid synthesis pathway (Fig. 1). FabD converts malonyl-CoA to malonyl-ACP by capping Acyl Carrier Protein (ACP) whereas FabH condenses another molecule of acetyl-CoA with the pre-synthesised malonyl-ACP to form acetoacetyl-ACP [18]. The formation of acetoacyl-ACP serves as a primer for fatty acid chain elongation. Fatty acid chain is then elongated by various fab-cluster enzymes like FabH, FabI, FabA, FabB, etc. During every elongation cycle, a fresh acetyl-CoA molecule is added to the existing fatty acid backbone and thereby increasing the carbon chain length. At this point, the nature of the fatty acid chain like the degree of fatty acid saturation can be wired by regulating expression of FabA, FabB and FabF [19]. After the elongation phase, the growing fatty acid chain is cleaved and liberated from ACP by acyl CoA-thioesterase (TesA) releasing Free Fatty Acids (FFA) [20]. The TesA activity determines carbon chain length and, thus, terminates the reaction [21]. The cleaved FFA chain can be stored as an alternative energy reservoir by incorporating into cell membrane or can be exported across the cell membrane (Fig. 1). Depending upon the energy requirement, stored fatty acids or external fatty acid sources are degraded by Acyl-CoA synthetase (FadD) and other Fad cluster enzymes where fatty acids are used as a substrate (Fig. 1) [22, 23]. Degradation of fatty acid releases free acetyl-CoA, which can be reused for energy production via TCA cycle or can be consumed to synthesise fatty acids. All the four phases of fatty acid metabolism are tightly regulated by regulators like FadR and FabR. FadR is a global regulator which has a positive regulation on Fab cluster genes and a negative regulation on Fad cluster genes, whereas FabR has negative regulation on expression of fabA and fabB [24,25,26].
In case of SCFA, MCFA and LCFA overproduction, choice of thioesterase is a crucial aspect, due to its fatty acid chain length specificity, which is distinctive from other types of fatty acid synthesis strategies [20]. The native E. coli thioesterase (tesA) generates FFA distribution of 54% unsaturated fatty acids (UFA) and 46% saturated fatty acids (SFA) approximately, with broad chain length specificity [21, 27]. However, desired fatty acid chain length can be tailored by co-expressing exogenous thioesterase in E. coli. The exogenous source of thioesterase can be of bacterial origin or of plant origin. However, expression of exogenous TesA leads to impaired aerobic respiration, phage shock regulon activation, poor membrane integrity and loss of cell viability during fatty acid overproduction [21]. Therefore, it is imperative to understand the effect of various stresses on fatty acid overproduction before strain construction, in order to design a strategy that facilitates maximum yield.
Stresses Generated Due to Fatty Acid Overproduction
pH and Osmotic Stress
Maintaining optimum pH and osmolarity is a crucial task for the optimum functioning of the cell. Fluctuations in pH affect various cellular processes like protein folding and stability, enzyme activity, reaction conditions, thermodynamics, etc., whereas cellular osmolarity helps maintain the turgor pressure across cell membrane. Any drastic change in the pH and osmolarity of the medium can result in cell death. pH and osmotic stress are two of the stresses elicited concomitantly during fatty acid overproduction, prompting intracellular and extracellular damages [28,29,30].
A cell engineered for fatty acid overproduction, accumulates overproduced fatty acids in the membrane, or excretes it across the membrane in the form of FFA [13, 31]. Depending upon the pH of the medium, these fatty acids either exist in its acidic form (protonated) or in the form of its conjugate base (deprotonated) [32]. This can be expressed in terms of acid dissociation constant, pKa. pKa value determines the pH required for any chemical compound to donate/accept H+ ion. Fatty acid occurs in its protonated state if the environmental pH is lesser than the pKa value of fatty acid whereas if the environmental pH is higher than pKa value, fatty acid occurs in its deprotonated state [32]. Thus, deprotonation of fatty acid (R-COOH → R-COO− + H+) increases with increase in environmental pH [28, 29].
Unlike wild-type strain, engineered fatty acid producer increases the pH of the medium due to its poor acetate flux [1, 31]. Zhang and his colleagues observed an increase in the pH of the medium from 6.5 (16 h) to 7.5 (48 h) by the fatty acid producer, as compared to the control strain (pH 5.5 at 16 h, pH 5 at 48 h). Under such circumneutral conditions, fatty acids like hexanoic acid (C6), octanoic acid (C8) and decanoic acid (C10) will deprotonate and lead to increase in the H+ ion concentration in the medium during stationary phase. The accumulation of H+ ions leads to a drop in the pH of the medium imparting extracellular pH stress (Fig. 2) [33]. A study aiming at octanoic acid overproduction observed medium acidification up to pH 5.5 with fatty acid yields of 0.0385 g/g glucose, which is only about 11% of its theoretical value (~ 0.34 g/g glucose) [34]. The octanoic acid gets deprotonated after getting excreted into the medium, causing a drop in the pH of the medium. This disturbs the ion gradient across the membrane, challenging the cell survival and thereby lowering octanoic acid yield.
Fatty acid overproduction also imposes extracellular osmotic stress (Fig. 2). It is well known that the internal turgor pressure of a cell is higher than the environmental pressure [35, 36]. However, due to accumulation of fatty acids in the medium, the environmental osmotic pressure exceeds the internal turgor pressure imparting extracellular osmotic stress (Fig. 2) [37]. In such cases, medium components play an important role to maintain the external osmotic pressure. Industries prefer sustainable and cheap alternative carbon sources like lignocellulosic waste (LCW) as a growth medium. The hydrolysis and other pre-treatments of LCW includes alkali treatment using different kinds of caustic salts like KOH and NaOH [38]. These salts may get incorporated into the growth medium even after purification. Bacterial cells can tolerate these salts during initial phase of fatty acid fermentation; however, with the increase in product formation, these salts enhance the detrimental effects of osmotic stress elicited due to fatty acid accumulation [39]. So, the fatty acid overproduction is not only affected by its own synthesis but also by its growth medium composition. Other than salt-induced osmotic stress, synthesis of SCFAs as a by-product is a secondary effect of lignocellulosic fermentation. SCFAs like formic acid (C1) and levulic acid (C5) are few of the lignocellulosic hydrolysate (LCH) inhibitors formed during lignocellulosic fermentation. These LCH-derived inhibitors decrease intracellular ATP levels and inhibits DNA synthesis/repair, macromolecule biosynthesis and glycolytic enzymes [39]. Thus, extracellular pH-osmotic stress poses an obstacle to obtaining high secretory/extracellular fatty acid titres.
Overproduction of SCFAs and MCFAs can also induce intracellular pH-osmotic stress when an E. coli system is engineered to overproduce cellular fatty acids [28, 40, 41] (Fig. 2). These fatty acids, when overproduced, are excreted across the membrane via ArcAB-TolC complex, an efflux porin, but may re-enter the cell via OmpF, an outer membrane protein engaged in passive diffusion of SCFAs, MCFAs and other molecules [11, 42]. In E. coli, the normal intracellular pH is 7.13 whereas it has pH buffering capacity till pH 6.55 [43]. The neutral intracellular pH causes deprotonation of re-entered fatty acid leading to increase in the concentration of fatty acid anion and H+ ion. The accumulation of fatty acid anion further increases the internal turgor pressure and imparts intracellular osmotic stress whereas H+ ions accumulation leads to intracellular pH stress concomitantly [28, 29, 35, 44, 45]. These findings strongly suggest the interconnection between pH stress and osmotic stress during fatty acid overproduction in E. coli.
Other than these stresses, integration of fatty acid in the membrane is another challenge for maintaining the membrane integrity while overproducing fatty acids. As compared to other types of fatty acids, SCFAs and MCFAs have distinctive property of membrane intercalation [11, 20]. Due to their relatively small size and partial solubility in the membrane, SCFAs and MCFAs readily integrate into the inner membrane, leading to increase in the membrane fluidity. The integrated fatty acid molecule increases membrane fluidity by reducing hydrophobic interactions between fatty acid tails as they flow laterally within lipid bilayer [11, 46]. A cell with increased membrane fluidity is more prone to pH-osmotic stress. Thus, the cumulative effect of pH-osmotic stress and poor membrane integrity instigates bactericidal activity of SCFAs and MCFAs [28, 47]. This becomes a major concern when a system is engineered for overproduction of SCFA or MCFA. Volker et al. reported incomplete glucose consumption by a butyrate (C4) producer, due to end-product toxicity [48]. Thus, the max butyrate yield obtained was 1.77 g/L at 25% glucose consumption. Bactericidal activity of the overproduced butyrate led to reduction in the cell count, causing incomplete glucose consumption and ultimately, thus, restricting its own yield.
In response to the extracellular and intracellular pH-osmotic stress, RpoS, a master stress regulator, activates Cyclopropane Fatty Acid (CFA) synthase and GadBC complex (Fig. 2) [49,50,51,52]. When a cell is exposed to acidic or hyperosmotic environment, RpoS induces CFA synthase to cyclise UFA reservoirs into CFA [49, 52,53,54]. CFA synthesis decreases the unsaturation of membranous fatty acid, causing decrease in the membrane fluidity. This makes the cell membrane rigid, thus, blocking the re-entry of extracellular H+ ions and conferring tolerance to stress. During intracellular pH stress (pH 4–5), RpoS-induced GadB (Glutamate decarboxylase) and GadC (Gamma-Aminobutyric Acid (GABA) antiporter) help to mitigate the stress [55, 56]. By using intracellular accumulated H+ ions, amino acids like glutamate get decarboxylated by GadB leading to GABA formation. GABA is then transported outside the cell via GadC. This Gad system assists in restoring intracellular pH [31, 57].
One of the systems integrated with RpoS is the ppGpp alarmone signalling. The synthesis, degradation and activity of RpoS is ppGpp dependent [49]. During acid stress, ppGpp alarmone levels get raised leading to downregulation of various genes like fadR, plsB and upregulation of certain genes like cfa, fabA, fabB, etc. [1]. The ppGpp regulation on cfa and fadR gene expression is shown in Fig. 2. Downregulation of FadR by a more stringent response alarmone like ppGpp enables the cell to utilise its fatty acid synthesising and degrading machineries concomitantly. Along with CFA synthesis, ppGpp also promotes degradation of excess of fatty acids produced during acid stress. This disables the side reactions, which otherwise fall under the control of FadR. The acetyl-CoA generated during fatty acid degradation is then reused, either to synthesise CFA/other fatty acids, or to produce energy via TCA cycle. In this manner, ppGpp alarmone supervises fatty acid metabolism and energy conservation during acid stress.
The problem of re-entry of fatty acids and its intercalation in the cell membrane are controlled by blocking its entry via OmpF and FadL (an outer membrane protein engaged in LCFA influx) [11, 58,59,60]. Transmembrane proteins like OmpF and FadL are tightly regulated by the EnvZ-OmpR Two-Component System (TCS) (Fig. 2). EnvZ-OmpR TCS is involved in pH and osmoregulation by regulating approximately 1360 genes [61]. During pH-osmotic stress, the EnvZ-OmpR TCS recognises differences in the pH and osmolarity. Upon stress recognition, EnvZ (histidine kinase protein) gets self-phosphorylated and transfers the phosphate group to OmpR (DNA binding regulator) [43]. Phosphorylated OmpR regulates the transcription of various genes depending upon its degree of phosphorylation [62]. During pH-osmotic stress, OmpR lowers OmpF and FadL expression via upregulation of non-coding MicF RNA, a negative regulator of OmpF-FadL protein [60, 63,64,65,66]. MicF RNA controls OmpF-FadL expression by binding to their corresponding mRNAs, leading to formation of secondary structure. The secondary structure induces mRNA degradation and, thus, prevents their translation [67].
Oxidative Stress
Reactive oxygen species (ROS) are a type of highly reactive molecules derived from oxygen metabolism [68]. These unstable molecules cascade a chain reaction causing irreversible damage to various biomolecules leading to oxidative stress [69]. ROS like superoxide, peroxide, hydroxyl radicals etc. affect lipids, nucleic acids, proteins and other metabolic reactions which may even cause cell death [70]. Aerobic respiration has the potential to produce and scavenge ROS. A balance between ROS production and ROS detoxification is necessary for a cell to function properly. At steady state, the intracellular concentration of ROS like superoxide and hydrogen peroxide is ~ 0.2 and ~ 50 nM, respectively [71]. Overproduction of fatty acids with the help of gene-editing techniques increases respiration rate and thereby disturbs the steady state leading to oxidative stress [72].
Oxidative stress represents an imbalanced condition wherein oxidants outnumber antioxidants. During aerobic growth of E. coli, electron transport chain (ETC) is the major contributor of reactive species. Oxidation of ETC enzymes (NADH dehydrogenase, D-lactate dehydrogenase and succinate dehydrogenase) by molecular oxygen leads to formation of superoxide and hydrogen peroxide [73]. These reactive species at low concentration serve as signalling molecules; however, at higher concentrations, they can cause oxidative stress [69]. Genes of nuo and cyo operons (nuoH, nuoI, nuoJ, nuoN, nuoM, cyoD, cyoC, cyoE, etc.) encoding transmembrane proteins of ETC are upregulated during fatty acid overproduction [72]. These proteins are engaged in maintaining proton motive force (PMF) across the cell membrane. PMF is the flow of ions depending on the electrochemical gradient across the membrane. The protonated fatty acid anion acts as an uncoupling agent by interacting with the ATP synthase and disturbing the PMF. This is magnified in case of fatty acid overproducing strains. The impaired PMF induces upregulation of nuo and cyo operons, which increases the cellular respiration rate, thereby increasing the oxidation of ETC enzymes by molecular oxygen. This creates an imbalance wherein oxidants outnumber antioxidants leading to escape of ROS. Accidental auto-oxidation of flavoproteins by these ROS generates additional superoxide and flavosemiquinone [71]. High concentration of these ROS further oxidises nearby compounds imparting oxidative stress. Along with high respiration rate, the intracellular osmotic stress induced during fatty acid overproduction impels transmembrane proteins to transverse or “flip” fatty acids across the membrane. The pressure exerted on transmembrane proteins creates a localised spot for ROS production near inner membrane causing membranous oxidative stress as shown (Fig. 3) [21, 72]. Thus, membrane alterations in response to any other stress can also impart oxidative stress [72, 74].
Overproduction of MCFA led to phenotypic aberrations like loss of inner membrane integrity, heterologous cell size and 85% reduction in cell viability [72]. These anomalies were observed as early as the mid log phase and were prominent during early stationary phase. The bactericidal activity and membrane intercalation property of MCFA could be the possible reason for reduction in the cell count and for the loss of membrane integrity, respectively. Microarray analysis of the study indicates (1) downregulation of ompF, and (2) upregulation of marA/rob/soxS regulon and acid-resistance genes like gadABC and gadE (encoding glutamate decarboxylase-αβ, GABA antiporter and transcriptional regulator of gad-system, respectively). MarRAB and SoxRS regulons are activated in response to oxidative stress, whereas Rob protein is responsible for maintaining the cell viability. Mid-log phase induction of MarR leads to upregulation of several genes like marA, pqiA (encoding paraquat-inducible protein) and nfo (encoding DNA nuclease to repair oxidative damage) indicating production of ROS. Several oxidative stress genes (~ 70 genes) like ahpC (encoding alkyl hydroperoxide reductase), ahpF (encoding alkyl hydroperoxide reductase subunit F), dps (DNA protect during starvation protein), marR, marA and marB (encoding multiple antibiotic resistance proteins, MarR, MarA and MarB, resp.) have been reported to be upregulated during low pH stress [72, 75, 76]. The co-induction of acid stress-resistance genes and oxidative stress-resistance genes highlights the interconnectedness between these stresses. However, the exact mechanism remains unclear.
In response to oxidative stress, induction of SoxRS leads to downregulation of OmpF [72]. During oxidative stress, around 140 genes are upregulated, out of which, 30 genes belong to OxyR and SoxRS regulon [77,78,79]. SoxR is a homodimer (2Fe-2S cluster), which senses the presence of ROS and transforms from its inactive reduced state to active oxidised state (Fig. 3). The activated SoxR then upregulates soxS gene-transcribing SoxS protein. SoxS regulates expression of many genes, out of which, micF (repressor of OmpF) is upregulated [72, 80]. In this manner, SoxRS regulon downregulates OmpF expression via MicF RNA synthesis to reduce membranous oxidative stress. Hence, MCFA overproduction downregulates OmpF expression affecting MCFA efflux and thereby lowering MCFA yield.
Interestingly, downregulation of OmpF expression along with FadL deletion has reportedly enhanced the MCFA titres [40, 81]. In the earlier case, even if OmpF downregulation lowers the MCFA titres, it is anticipated that other transmembrane proteins like cmr-encoding multidrug translocase, MdfA may be involved in MCFA efflux, as cmr is often upregulated in an efficient fatty acid producer (Fig. 3) [82]. However, the cause for cmr induction during fatty acid overproduction remains unclear. MdfA is an efflux pump which can efflux broader range of molecules than OmpF [83]. Thus, it can be hypothesised that efflux through MdfA must be in a more controlled manner than OmpF causing lesser ROS production [40].
Oxidative stress also plays a role in the lipid composition of the membrane. The magnitude of oxidative stress is dependent on the ratio of saturated (SFA) to unsaturated (UFA) fatty acids. UFA poses an easy target for lipid peroxidation. This curtails membrane integrity and amplifies lipid derived-ROS production [84]. In conclusion, the lesser the UFA content, the higher the resistance to oxidative stress is. The lipid peroxidation can be managed by overexpressing fabA which is known to decrease UFA synthesis [85]. On the other hand, oxidative stress would be a bottleneck if an E. coli system is engineered for UFA overproduction. In such cases, increasing antioxidants like ubiquinone and/or expressing exogenous glutathione reductase can help the cell to combat oxidative stress [86,87,88]. Oxidative stress can also be managed by cyclising UFA reservoirs into CFA [89]. Like pH-osmotic stress, oxidative stress is sensed by stress regulator and alarmone like RpoS and ppGpp. These regulatory molecules induce CFA synthase in response to oxidative stress and cyclise UFA reservoirs into CFA (Fig. 3) [1]. Increase in CFA synthesis and decrease in the UFA content confers resistance to oxidative stress and also improves membrane integrity.
To provide a concise overview, a summary of the effects and cellular responses in pH, osmolarity, and oxidative stress generated due to fatty acid overproduction in E. coli is shown in Table 1.
Stresses Boosting Fatty Acid Overproduction
Stress generation due to fatty acid overproduction is an inherent part of the system yielding poor fatty acid titres than the expected yield. However, several studies suggest that sub-optimal level of stress can accelerate fatty acid overproduction. Sub-optimal level of stresses like temperature and nitrogen limitation will instigate the cell to rewire its metabolism towards fatty acid synthesis.
Temperature Stress
Optimum temperature promotes cell growth by impelling several temperature-dependent processes like activation/inactivation of enzymes, uptake of various biomolecules, etc. [90,91,92]. Unlike pH-osmolarity and oxidative stress, overproduction of fatty acid does not cause changes in the temperature. Instead, temperature is an external stress factor positively affecting fatty acid overproduction. Marr and Ingraham were the first to report changes in the fatty acid profile of E. coli in response to temperature variation [93].
Composition of bacterial membrane varies with changes in the temperature, which defines its lipid solidification point. Lipid solidification point is the temperature at which lipid exist in its solid or fluidic state. At higher temperature, lipids denature and liquefy, whereas at lower temperature, lipids solidify. Thus, a bacterial cell cannot grow below or above lipid solidification point of its cell membrane [93, 94]. The solid or fluidic state of membrane is maintained by regulating its SFA:UFA ratio. In E. coli fatty acid biosynthesis (Fab) cluster of enzymes, FabB and FabF are engaged in thermal regulation of fatty acid synthesis pathway [1]. High temperatures encourage SFA production by FabB, whereas low temperatures promote UFA production by FabF. Thus, the SFA/UFA ratio is dependent on temperature [19, 95]. High SFA content implies lowered UFA proportion and thereby improved membrane integrity in order to resist high temperature stress. Lee et al. engineered an E. coli strain to overproduce unsaturated LCFA by overexpressing FabF along with other Fab cluster enzymes [95]. FabB and FabF are the most common candidates favoured for LCFA overproduction due to their role in fatty acid chain elongation. Their study obtained fatty acid titres that were 2.7-fold higher than that of wild-type cells at lower temperature (20 °C) [95]. Thus, low-temperature stress can be used as a tool for increasing UFA production in E. coli.
Another study examined the effect of temperature (37 °C & 42 °C) on different parameters like gene expression levels, acetate accumulation, glucose consumption, in E. coli wild-type strain [96]. In response to high temperature, several genes like arcA, fadR, iclR, rpoS were upregulated [96]. The upregulated FadR regulates FabB and FabF expression to maintain high SFA:UFA ratio to resist the temperature up-shift (Fig. 4) [19, 93, 97]. The most common SFAs and UFAs getting altered in response to temperature variation are palmitic acid (C16), palmitoleic (cis-9-hexadecenoic) acid (C16), cis-vaccenic (C18), and CFA [89, 98]. One of the parameters assessed by Hasan and Shimizu at elevated temperature is acetate accumulation. According to their results, acetate accumulation up to 2.69 and 2.91 g/L was observed at 37 and 42 °C, respectively [96]. At high temperature, the upregulation of ArcA (Aerobic Respiration Control Protein A) and FadR caused more acetate formation by decreasing activity of TCA cycle and glyoxylate pathway (Fig. 4). ArcA regulator is a part of ArcBA TCS engaged in downregulating genes of aerobic respiration under microaerobic condition [99]. Due to high temperature, the solubility of dissolved oxygen decreases and thereby creating microaerobic condition. Depending on the aerobicity, ArcB (histidine kinase) activates ArcA (response regulator) by transphosphorylation. With the positive regulation of ArcA, the enzymes of TCA cycle are repressed and thereby redirecting acetyl-CoA towards acetate accumulation. Due to FadR upregulation, iclR gene gets activated, which supresses some of the glyoxylate pathway enzymes by repressing aceBAK operon [100, 101]. Since acetate accumulation in an engineered fatty acid producer is already diminished [1], the acetyl-CoA flux is redirected towards fatty acid synthesis. In this manner, the acetyl-CoA pool can be diverted to increased fatty acid production by increasing temperature and thus enhancing fatty acid overproduction. According to the literature, elevated temperature (45–48 °C) upregulates genes like soxS and sodA (SodA, superoxide dismutase A), conferring tolerance to oxidative stress [96, 102]. Also in other studies, temperature elevation led to downregulation of OmpF by EnvZ-OmpR TCS [103]. To summarise, temperature elevation results in (1) improved membrane integrity (2) tolerance/resistance to oxidative stress (3) lowered OmpF expression. These are the desirable alterations for combating pH-osmotic and oxidative stress induced during fatty acid overproduction. Hence, high temperature stress can be exploited as a strategy along with other genetic alterations to enhance fatty acid production.
Nitrogen Limitation Stress (High C/N Ratio)
Carbon is essential for the synthesis of various biomolecules like lipids, nucleic acids, proteins, etc. [104]. Other than carbon, nitrogen, sulphur, potassium, phosphorous, magnesium and calcium are also required for cell growth [105, 106]. Among these elements, carbon and nitrogen are important variables to propel fatty acid synthesis. Depletion of nitrogen has been shown to mimic the effect of high temperature on cellular growth [93].
High carbon to nitrogen ratio (C/N ratio) increases fatty acid titres [107]. In E. coli, the intracellular concentration of α-Ketoglutarate (α-KG) and GlnB, a regulatory protein, serves as a node for connecting C-N metabolism to fatty acid synthesis pathway. α-KG has dual roles: first, it serves as a signalling molecule indicating intracellular C and N status, and second, it regulates GlnB protein activity, a glutamine synthase regulatory protein [62]. GlnB is a part of NtrBC TCS (nitrogen assimilation control) and a negative regulator of acetyl-CoA carboxylase (ACC) [108, 109]. Under normal conditions, when nitrogen is abundant, ACC has a partial negative regulation by GlnB (Fig. 5). GlnB binds to Biotin Carboxyl Carrier Protein (BCCP) subunit of ACC and reduces its activity to 45% by reducing its turnover [17]. In this case, an engineered E. coli system will still not yield fatty acid titres as expected. On the other hand, during nitrogen limiting condition (high C/N ratio), the drop in the intracellular glutamine level induces uridylation of GlnB [62]. The uridylated GlnB cannot bind to ACC thereby relieving its partial negative regulation on ACC and increasing its activity (Fig. 5). GlnB can also sense intracellular concentration of α-KG [110]. At high C/N ratio, the intracellular α-KG concentration is high. α-KG binds GlnB allosterically and inhibits its binding to ACC in dose-dependent manner [17]. This provides an extra-regulation by α-KG other than uridylation of GlnB. In this manner, the flux of acetyl-CoA can be increased towards fatty acid synthesis pathway. One of the studies achieved more than 90% of octanoic acid yield than its control strain by tuning C/N ratio, medium pH and other genetic alterations [34].
As with other stresses, OmpF expression varies in response to high C/N ratio. OmpF acts like a portal for wide range of molecules like nitrogen, sugars, proteins, ions, antibiotics, etc. other than fatty acids [11, 111]. OmpF expression is lowered at high C/N ratio by SoxRS TCS in similar fashion as that of oxidative stress (Fig. 5) [112, 113]. Due to the absence of negative regulation of GlnB over ACC at high C/N ratio, the enhanced ACC activity propels fatty acid production and thereby impairing respiration rate (Fig. 5). The study also reported upregulation of soxRS, along with other respiratory genes like cyoA and cydB indicating oxidative stress caused due to increase in the respiration rate [113]. In this manner, SoxRS TCS downregulates OmpF expression via MicF RNA in response to oxidative stress at high C/N ratio.
To provide a concise overview, a summary of the effects and cellular responses in temperature and nitrogen limitation stress generated due to fatty acid overproduction in E. coli is shown in Table 2.
Conclusion and Future Prospect
Fatty acid is one of the valuable molecules which has attracted industrial attention due to its diverse applications. Till date, various algal, fungal, and vegetative sources of fatty acid have been explored, but microbial fatty acid has upper hand over other sources due to its cost effectiveness, ease with scale-up and down-streaming processes. The mycelial growth of the fungal source tends to clog filter during fatty acid extraction. Due to filter clogging, loss of product yield and heavy maintenance of instrument are few of the undesirable events during fatty acid overproduction. These shortcomings add economic burden on its down-streaming processes. Bacterial candidates being small in size and ease with their down-streaming processes often enable an industrialist to obtain maximum yield. Thus, many researchers are now trying to raise the potential of bacterial candidates in order to achieve these objectives.
Since last few decades, E. coli has served as an excellent model for overproducing fatty acids with the help of gene-editing techniques. However, various stresses like pH, osmolarity and oxidative stress elicited during fatty acid overproduction caused lowering of the final yield, whereas other stresses like temperature and nitrogen limitation have proved to enhance fatty acid production. Therefore, stress can exert positive and negative impacts on fatty acid production. It is evident that cellular machinery elicits a response at molecular level against these stresses, which include transporters, regulators like RpoS, the TCS system, and changes in the ppGpp levels. Although the cells use these measures for survival and thereby causes a change in the fatty acid production, these regulators, transporters, and pathways can be further explored to achieving the desired fatty acid levels.
Thus, it is imperative to understand the dynamics inside the cell during fatty acid overproduction, which we have presented in this review. There exists a complex interconnection between fatty acid metabolism and other metabolic as well as stress networks. Membrane integrity and activity of transmembrane porins are crucial to tackle these stresses. Various aspects of complex dynamics of fatty acid production and its correlation with stresses have definitely raised some interesting points, which demand further investigations. For instance, high tolerance for toxic metabolites, a property that is desirable during strain optimisation, can be achieved by changing the expression of important transmembrane porin proteins like FadL and OmpF, as they are majorly regulated in response to stress condition during fatty acid overproduction. There are other key players like RpoS, ppGpp and MicF RNA, which help in alleviating the stress produced during fatty acid overproduction. As discussed, the efflux pump, MdfA can efflux a wider range of molecules than OmpF, and studies indicate its role in fatty acid efflux. Thus, different pathways or regulators can be explored to enhance fatty acid overproduction. Using strategies on these lines can lead to the development of industrially relevant strains that can propel efficient fatty acid production.
References
Janßen, H. J., & Steinbüchel, A. (2014). Fatty acid synthesis in Escherichia coli and its applications towards the production of fatty acid based biofuels. Biotechnology for Biofuels, 7(7), 1–26.
Yao, J., & Rock, C. O. (2017). Bacterial fatty acid metabolism in modern antibiotic discovery. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1862(11), 1300–1309. https://doi.org/10.1016/j.bbalip.2016.09.014
Schönfeld, P., & Wojtczak, L. (2016). Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. Journal of Lipid Research, 57(6), 943–954. https://doi.org/10.1194/jlr.R067629
Campbell, J. W., Morgan-Kiss, R. M., & Cronan, J. E. (2003). A new Escherichia coli metabolic competency: Growth on fatty acids by a novel anaerobic b-oxidation pathway. Molecular Microbiology, 47(3), 793–805. https://doi.org/10.1046/j.1365-2958.2003.03341.x
Gupta, P. L., Rajput, M., Oza, T., Trivedi, U., & Sanghvi, G. (2019). Eminence of microbial products in cosmetic industry. Natural Products and Bioprospecting, 9(4), 267–278. https://doi.org/10.1007/s13659-019-0215-0
Rahman, Z., Rashid, N., Nawab, J., Ilyas, M., Sung, B. H., & Kim, S. C. (2016). Escherichia coli as a fatty acid and biodiesel factory: Current challenges and future directions. Environmental Science and Pollution Research, 23(12), 12007–12018. https://doi.org/10.1007/s11356-016-6367-0
Shinmen, Y., Shimizu, S., Akimoto, K., Kawashima, H., & Yamada, H. (1989). Production of arachidonic acid by Mortierella fungi: Selection of a potent producer and optimization of culture conditions for large-scale production. Applied Microbiology and Biotechnology, 31(1), 11–16. https://doi.org/10.1007/BF00252518
Baumann, I., & Westermann, P. (2016). Microbial production of short chain fatty acids from lignocellulosic biomass: Current processes and market. BioMed Research International. https://doi.org/10.1155/2016/8469357
Kassab, E., Fuchs, M., Haack, M., Mehlmer, N., & Brueck, T. B. (2019). Engineering Escherichia coli FAB system using synthetic plant genes for the production of long chain fatty acids. Microbial Cell Factories, 18(1), 1–10. https://doi.org/10.1186/s12934-019-1217-7
Bae, J. H., Park, B. G., Jung, E., Lee, P. G., & Kim, B. G. (2014). fadD deletion and fadL overexpression in Escherichia coli increase hydroxy long-chain fatty acid productivity. Applied Microbiology and Biotechnology, 98(21), 8917–8925. https://doi.org/10.1007/s00253-014-5974-2
Tan, Z., Black, W., Yoon, J. M., Shanks, J. V., & Jarboe, L. R. (2017). Improving Escherichia coli membrane integrity and fatty acid production by expression tuning of FadL and OmpF. Microbial Cell Factories, 16(1), 1–15. https://doi.org/10.1186/s12934-017-0650-8
Mi, L., Qin, D., Cheng, J., Wang, D., Li, S., & Wei, X. (2017). Efficient production of free fatty acids from ionic liquid-based acid- or enzyme-catalyzed bamboo hydrolysate. Journal of Industrial Microbiology and Biotechnology, 44(3), 419–430. https://doi.org/10.1007/s10295-016-1888-6
Satoh, S., Ozaki, M., Matsumoto, S., Nabatame, T., Kaku, M., Shudo, T., et al. (2020). Enhancement of fatty acid biosynthesis by exogenous acetyl-CoA carboxylase and pantothenate kinase in Escherichia coli. Biotechnology Letters, 42(12), 2595–2605. https://doi.org/10.1007/s10529-020-02996-w
Fang, L., Fan, J., Wang, C., Cao, Y., & Song, H. (2020). Genome-wide targets identification by CRISPRi-Omics for high-titer production of free fatty acids in Escherichia coli. Nature Communications. https://doi.org/10.1101/2020.03.03.974378
Huffer, S., Clark, M. E., Ning, J. C., Blanch, H. W., & Clark, D. S. (2011). Role of alcohols in growth, lipid composition, and membrane fluidity of yeasts, bacteria, and archaea. Applied and Environmental Microbiology, 77(18), 6400–6408. https://doi.org/10.1128/AEM.00694-11
My, L., Ghandour Achkar, N., Viala, J. P., & Bouveret, E. (2015). Reassessment of the genetic regulation of fatty acid synthesis in Escherichia coli: Global positive control by the functional dual regulator FadR. Journal of Bacteriology, 197(11), 1862–1872. https://doi.org/10.1128/JB.00064-15
Gerhardt, E. C. M., Rodrigues, T. E., Müller-Santos, M., Pedrosa, F. O., Souza, E. M., Forchhammer, K., & Huergo, L. F. (2015). The bacterial signal transduction protein GlnB regulates the committed step in fatty acid biosynthesis by acting as a dissociable regulatory subunit of acetyl-CoA carboxylase. Molecular Microbiology, 95(6), 1025–1035. https://doi.org/10.1111/mmi.12912
Zhang, Y. M., & Rock, C. O. (2016). Fatty acid and phospholipid biosynthesis in prokaryotes. Biochemistry of lipids, lipoproteins and membranes: Sixth Edition. Elsevier. https://doi.org/10.1016/B978-0-444-63438-2.00003-1
Cronan, J. E., & Thomas, J. (2009). Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways. Complex enzymes in microbial natural product biosynthesis, part B: Polyketides, aminocoumarins and carbohydrates (1st ed., Vol. 459). Elsevier Inc. https://doi.org/10.1016/S0076-6879(09)04617-5
Sherkhanov, S., Korman, T. P., & Bowie, J. U. (2014). Improving the tolerance of Escherichia coli to medium-chain fatty acid production. Metabolic Engineering, 25, 1–7. https://doi.org/10.1016/j.ymben.2014.06.003
Lennen, R. M., & Pfleger, B. F. (2012). Engineering Escherichia coli to synthesize free fatty acids. Trends in Biotechnology, 30(12), 659–667. https://doi.org/10.1016/j.tibtech.2012.09.006
Iram, S. H., & Cronan, J. E. (2006). The β-oxidation systems of Escherichia coli and Salmonella enterica are not functionally equivalent. Journal of Bacteriology, 188(2), 599–608. https://doi.org/10.1128/JB.188.2.599-608.2006
Overath, P., Pauli, G., & Schairer, H. U. (1969). Fatty acid degradation in Escherichia coli. European Journal of Biochemistry, 7(4), 559–574. https://doi.org/10.1111/j.1432-1033.1969.tb19644.x
Raman, N., Black, P. N., & DiRusso, C. C. (1997). Characterization of the fatty acid-responsive transcription factor FadR. Biochemical and genetic analyses of the native conformation and functional domains. Journal of Biological Chemistry, 272(49), 30645–30650. https://doi.org/10.1074/jbc.272.49.30645
Feng, Y., & Cronan, J. E. (2011). Complex binding of the FabR repressor of bacterial unsaturated fatty acid biosynthesis to its cognate promoters. Molecular Microbiology, 80(1), 195–218. https://doi.org/10.1111/j.1365-2958.2011.07564.x
DiRusso, C. O., Metzger, A. K., & Heimert, T. L. (1993). Regulation of transcription of genes required for fatty acid transport and unsaturated fatty acid biosynthesis in Escherichia coli by FadR. Molecular Microbiology, 7(2), 311–322. https://doi.org/10.1111/j.1365-2958.1993.tb01122.x
Naggert, J., Narasimhan, M. L., DeVeaux, L., Cho, H., Randhawa, Z. I., Cronan, J. E., et al. (1991). Cloning, sequencing, and characterization of Escherichia coli thioesterase II. Journal of Biological Chemistry, 266(17), 11044–11050. https://doi.org/10.1016/s0021-9258(18)99125-8
Sun, Y., & O’Riordan, M. X. D. (2013). Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Advances in applied microbiology (1st ed., Vol. 85). Elsevier Inc. https://doi.org/10.1016/B978-0-12-407672-3.00003-4
Roe, A. J., McLaggan, D., Davidson, I., O’Byrne, C., & Booth, I. R. (1998). Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. Journal of Bacteriology, 180(4), 767–772. https://doi.org/10.1128/jb.180.4.767-772.1998
Chung, H. J., Bang, W., & Drake, M. A. (2006). Stress response of Escherichia coli. Comprehensive Reviews in Food Science and Food Safety, 5(3), 52–64. https://doi.org/10.1111/j.1541-4337.2006.00002.x
Zhang, X., Li, M., Agrawal, A., & San, K. Y. (2011). Efficient free fatty acid production in Escherichia coli using plant acyl-ACP thioesterases. Metabolic Engineering, 13(6), 713–722. https://doi.org/10.1016/j.ymben.2011.09.007
Jin, Q., & Kirk, M. F. (2018). pH as a primary control in environmental microbiology: 1. Thermodynamic perspective. Frontiers in Environmental Science, 6, 1–15. https://doi.org/10.3389/fenvs.2018.00021
Scheel, R., Ho, T., Kageyama, Y., Masisak, J., Mckenney, S., Lundgren, B., & Nmoura, C. (2021). Optimizing a fed-batch high-density fermentation process for medium chain-length poly(3-hydroxyalkanoates) in Escherichia coli. Frontiers in Bioengineering and Biotechnology. https://doi.org/10.3389/fbioe.2021.618259
Tan, Z., Yoon, J. M., Chowdhury, A., Burdick, K., Jarboe, L. R., Maranas, C. D., & Shanks, J. V. (2018). Engineering of E. coli inherent fatty acid biosynthesis capacity to increase octanoic acid production. Biotechnology for Biofuels, 11(1), 1–15. https://doi.org/10.1186/s13068-018-1078-z
Bugg, T. D. H. (1999). Bacterial peptidoglycan biosynthesis and its inhibition. Comprehensive Natural Products Chemistry. https://doi.org/10.1016/b978-0-08-091283-7.00080-1
Guan, N., Li, J., Shin, H., & dong, Du, G., Chen, J., & Liu, L. (2017). Microbial response to environmental stresses: From fundamental mechanisms to practical applications. Applied Microbiology and Biotechnology, 101(10), 3991–4008. https://doi.org/10.1007/s00253-017-8264-y
Varela, C. A., Baez, M. E., & Agosin, E. (2004). Osmotic stress response: Quantification of cell maintenance and metabolic fluxes in a lysine-overproducing strain of Corynebacterium glutamicum. Applied and Environmental Microbiology, 70(7), 4222–4229. https://doi.org/10.1128/AEM.70.7.4222-4229.2004
Aftab, M. N., Iqbal, I., Riaz, F., Karadag, A., & Tabatabaei, M. (2019). Different pretreatment methods of lignocellulosic biomass for use in biofuel production. Biomass for bioenergy-recent trends and future challenges. Retrieved from https://www.intechopen.com/books/advanced-biometric-technologies/liveness-detection-in-biometrics
Piotrowski, J. S., Zhang, Y., Bates, D. M., Keating, D. H., Sato, T. K., Ong, I. M., & Landick, R. (2014). Death by a thousand cuts: The challenges and diverse landscape of lignocellulosic hydrolysate inhibitors. Frontiers in Microbiology, 5, 1–8. https://doi.org/10.3389/fmicb.2014.00090
Wu, J., Wang, Z., Zhang, X., Zhou, P., Xia, X., & Dong, M. (2019). Improving medium chain fatty acid production in Escherichia coli by multiple transporter engineering. Food Chemistry, 272, 628–634. https://doi.org/10.1016/j.foodchem.2018.08.102
He, L., Xiao, Y., Gebreselassie, N., Zhang, F., Antoniewicz, M. R., Tang, Y. J., & Peng, L. (2014). Central metabolic responses to the overproduction of fatty acids in Escherichia coli based on 13C-metabolic flux analysis. Biotechnology and Bioengineering, 111(3), 575–585. https://doi.org/10.1002/bit.25124
Fernández, L., & Hancock, R. E. W. (2012). Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clinical Microbiology Reviews, 25(4), 661–681. https://doi.org/10.1128/CMR.00043-12
Chakraborty, S., Winardhi, R. S., Morgan, L. K., Yan, J., & Kenney, L. J. (2017). Non-canonical activation of OmpR drives acid and osmotic stress responses in single bacterial cells. Nature Communications. https://doi.org/10.1038/s41467-017-02030-0
Sánchez-Clemente, R., Igeño, M. I., Población, A. G., Guijo, M. I., Merchán, F., & Blasco, R. (2018). Study of pH changes in media during bacterial growth of several environmental strains. Proceedings, 2(20), 1297. https://doi.org/10.3390/proceedings2201297
Guan, N., & Liu, L. (2019). Microbial response to acid stress: Mechanisms and applications. Applied Microbiology and Biotechnology, 104(1), 51–65. https://doi.org/10.1007/s00253-019-10226-1
Royce, L. A., Liu, P., Stebbins, M. J., Hanson, B. C., & Jarboe, L. R. (2013). The damaging effects of short chain fatty acids on Escherichia coli membranes. Applied Microbiology and Biotechnology, 97(18), 8317–8327. https://doi.org/10.1007/s00253-013-5113-5
Wilbanks, B., & Trinh, C. T. (2017). Comprehensive characterization of toxicity of fermentative metabolites on microbial growth Mike Himmel. Biotechnology for Biofuels, 10(1), 1–11. https://doi.org/10.1186/s13068-017-0952-4
Volker, A. R., Gogerty, D. S., Bartholomay, C., Hennen-Bierwagen, T., Zhu, H., & Bobik, T. A. (2014). Fermentative production of short-chain fatty acids in Escherichia coli. Microbiology (United Kingdom), 160, 1513–1522. https://doi.org/10.1099/mic.0.078329-0
Battesti, A., Majdalani, N., & Gottesman, S. (2011). The RpoS-mediated general stress response in Escherichia coli *. Annual Review of Microbiology, 65, 189–213. https://doi.org/10.1146/annurev-micro-090110-102946
Jung, I. L., & Kim, I. G. (2003). Polyamines and glutamate decarboxylase-based acid resistance in Escherichia coli. Journal of Biological Chemistry, 278(25), 22846–22852. https://doi.org/10.1074/jbc.M212055200
Shabala, L., & Ross, T. (2008). Cyclopropane fatty acids improve Escherichia coli survival in acidified minimal media by reducing membrane permeability to H+ and enhanced ability to extrude H+. Research in Microbiology, 159(6), 458–461. https://doi.org/10.1016/j.resmic.2008.04.011
Romantsov, T., Guan, Z., & Wood, J. M. (2009). Cardiolipin and the osmotic stress responses of bacteria. Biochimica et Biophysica Acta - Biomembranes, 1788(10), 2092–2100. https://doi.org/10.1016/j.bbamem.2009.06.010
Kanjee, U., & Houry, W. A. (2013). Mechanisms of acid resistance in Escherichia coli. Annual Review of Microbiology, 67(May), 65–81. https://doi.org/10.1146/annurev-micro-092412-155708
Imatoukene, N., Back, A., Nonus, M., Thomasset, B., Rossignol, T., & Nicaud, J. M. (2020). Fermentation process for producing CFAs using Yarrowia lipolytica. Journal of Industrial Microbiology and Biotechnology, 47(4–5), 403–412. https://doi.org/10.1007/s10295-020-02276-6
Capitani, G., De Biase, D., Aurizi, C., Gut, H., Bossa, F., & Grütter, M. G. (2003). Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO Journal, 22(16), 4027–4037. https://doi.org/10.1093/emboj/cdg403
Bearson, B. L., Lee, I. S., & Casey, T. A. (2009). Escherichia coli O157: H7 glutamate- and arginine-dependent acid-resistance systems protect against oxidative stress during extreme acid challenge. Microbiology, 155(3), 805–812. https://doi.org/10.1099/mic.0.022905-0
Xu, Y., Zhao, Z., Tong, W., Ding, Y., Liu, B., Shi, Y., et al. (2020). An acid-tolerance response system protecting exponentially growing Escherichia coli. Nature Communications, 11(1), 1–13. https://doi.org/10.1038/s41467-020-15350-5
Bekhit, A., Fukamachi, T., Saito, H., & Kobayashi, H. (2011). The role of OmpC and OmpF in acidic resistance in Escherichia coli. Biological and Pharmaceutical Bulletin, 34(3), 330–334. https://doi.org/10.1248/bpb.34.330
Kawaji, H., Mizuno, T., & Mizushima, S. (1979). Influence of molecular size and osmolarity of sugars and dextrans on the synthesis of outer membrane proteins O-8 and O-9 of Escherichia coli K-12. Journal of Bacteriology, 140(3), 843–847. https://doi.org/10.1128/jb.140.3.843-847.1979
Higashitani, A., Nishimura, Y., Hara, H., Aiba, H., Mizuno, T., & Horiuchi, K. (1993). Osmoregulation of the fatty acid receptor gene fadL in Escherichia coli. MGG Molecular & General Genetics, 240(3), 339–347. https://doi.org/10.1007/BF00280384
Chakraborty, S., & Kenney, L. J. (2018). A new role of OmpR in acid and osmotic stress in salmonella and E. coli. Frontiers in Microbiology, 9, 1–14. https://doi.org/10.3389/fmicb.2018.02656
Shimizu, K. (2013). Metabolic regulation of a bacterial cell system with emphasis on Escherichia coli metabolism. ISRN Biochemistry, 2013, 1–47. https://doi.org/10.1155/2013/645983
Ramani, N., Hedeshian, M., & Freundlich, M. (1994). micF antisense RNA has a major role in osmoregulation of OmpF in Escherichia coli. Journal of Bacteriology, 176(16), 5005–5010.
Lucht, J. M., & Bremer, E. (1994). Adaptation of Escherichia coli to high osmolarity environments: Osmoregulation of the high-affinity glycine betaine transport system ProU. FEMS Microbiology Reviews, 14(1), 3–20. https://doi.org/10.1111/j.1574-6976.1994.tb00067.x
Goh, E. B., Siino, D. F., & Igo, M. M. (2004). The Escherichia coli tppB (ydgR) gene represents a new class of OmpR-regulated genes. Journal of Bacteriology, 186(12), 4019–4024. https://doi.org/10.1128/JB.186.12.4019-4024.2004
Anderson, J., Delihas, N., Ikenaka, K., Green, P. J., Pines, O., Ilercil, O., & Inouye, M. (1987). The isolation and characterization of RNA coded by micF gene in E. coli. Nucleic Acids Research, 15(5), 2089–2101.
Inoue, K., Matsuzaki, H., Matsumoto, K., & Shibuya, I. (1997). Unbalanced membrane phospholipid compositions affect transcriptional expression of certain regulatory genes in Escherichia coli. Journal of Bacteriology, 179(9), 2872–2878. https://doi.org/10.1128/jb.179.9.2872-2878.1997
Gough, D. R., & Cotter, T. G. (2011). Hydrogen peroxide: A Jekyll and Hyde signalling molecule. Cell Death and Disease, 2(10), 1–8. https://doi.org/10.1038/cddis.2011.96
Kashmiri, Z. N., & Mankar, S. A. (2014). Free radicals and oxidative stress in bacteria. International Journal of Current Microbiology and Applied Sciences, 3(9), 34–40.
Avery, S. V. (2011). Molecular targets of oxidative stress. Biochemical Journal, 434(2), 201–210. https://doi.org/10.1042/BJ20101695
Imlay, J. A. (2013). The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nature Reviews Microbiology, 11(7), 443–454. https://doi.org/10.1038/nrmicro3032
Lennen, R. M., Kruziki, M. A., Kumar, K., Zinkel, R. A., Burnum, K. E., Lipton, M. S., et al. (2011). Membrane stresses induced by overproduction of free fatty acids in Escherichia coli. Applied and Environmental Microbiology, 77(22), 8114–8128. https://doi.org/10.1128/AEM.05421-11
Zhao, X., & Drlica, K. (2014). Reactive oxygen species and the bacterial response to lethal stress. Current Opinion in Microbiology, 21, 1–6. https://doi.org/10.1016/j.mib.2014.06.008
Rowlett, V. W., Mallampalli, V. K. P. S., Karlstaedt, A., Dowhan, W., Taegtmeyer, H., Margolin, W., & Vitrac, H. (2017). Impact of membrane phospholipid alterations in Escherichia coli on cellular function and bacterial stress adaptation. Journal of Bacteriology, 199(13), 10. https://doi.org/10.1128/JB.00849-16
Blankenhorn, D., Phillips, J., & Slonczewski, J. L. (1999). Acid- and base-induced proteins during aerobic and anaerobic growth of Escherichia coli revealed by two-dimensional gel electrophoresis. Journal of Bacteriology, 181(7), 2209–2216. https://doi.org/10.1128/jb.181.7.2209-2216.1999
Maurer, L. M., Yohannes, E., Bondurant, S. S., Radmacher, M., & Slonczewski, J. L. (2005). pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. Journal of Bacteriology, 187(1), 304–319. https://doi.org/10.1128/JB.187.1.304-319.2005
Kim, J. S., & Holmes, R. K. (2012). Characterization of OxyR as a negative transcriptional regulator that represses catalase production in corynebacterium diphtheriae. PLoS ONE. https://doi.org/10.1371/journal.pone.0031709
Farr, S. B., & Kogoma, T. (1991). Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiological Reviews, 55(4), 561–585. https://doi.org/10.1128/mmbr.55.4.561-585.1991
Semchyshyn, H. (2009). Hydrogen peroxide-induced response in E. coli and S. cerevisiae: Different stages of the flow of the genetic information. Central European Journal of Biology, 4(2), 142–153. https://doi.org/10.2478/s11535-009-0005-5
Pomposiello, P. J., Bennik, M. H. J., & Demple, B. (2001). Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. Journal of Bacteriology, 183(13), 3890–3902. https://doi.org/10.1128/JB.183.13.3890-3902.2001
Shin, K. S., & Lee, S. K. (2017). Increasing extracellular free fatty acid production in Escherichia coli by disrupting membrane transport systems. Journal of Agricultural and Food Chemistry, 65(51), 11243–11250. https://doi.org/10.1021/acs.jafc.7b04521
Lennen, R. M., Politz, M. G., Kruziki, M. A., & Pfleger, B. F. (2013). Identification of transport proteins involved in free fatty acid efflux in Escherichia coli. Journal of Bacteriology, 195(1), 135–144. https://doi.org/10.1128/JB.01477-12
Edgar, R., & Bibi, E. (1997). MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. Journal of Bacteriology, 179(7), 2274–2280. https://doi.org/10.1128/jb.179.7.2274-2280.1997
Cabiscol, E., Tamarit, J., & Ros, J. (2000). Oxidative stress in bacteria and protein damage by reactive oxygen species. International Microbiology, 3(1), 3–8. https://doi.org/10.2436/im.v3i1.9235
Pradenas, G. A., Paillavil, B. A., Reyes-Cerpa, S., Pérez-Donoso, J. M., & Vásquez, C. C. (2012). Reduction of the monounsaturated fatty acid content of Escherichia coli results in increased resistance to oxidative damage. Microbiology, 158(5), 1279–1283. https://doi.org/10.1099/mic.0.056903-0
Søballe, B., & Poole, R. K. (2000). Ubiquinone limits oxidative stress in Escherichia coli. Microbiology, 146(4), 787–796. https://doi.org/10.1099/00221287-146-4-787
Ji, M., Barnwell, C. V., & Grunden, A. M. (2015). Characterization of recombinant glutathione reductase from the psychrophilic Antarctic bacterium Colwellia psychrerythraea. Extremophiles, 19(4), 863–874. https://doi.org/10.1007/s00792-015-0762-1
Agrawal, S., Jaswal, K., Shiver, A. L., Balecha, H., Patra, T., & Chaba, R. (2017). A genome-wide screen in Escherichia coli reveals that ubiquinone is a key antioxidant for metabolism of long-chain fatty acids. Journal of Biological Chemistry, 292(49), 20086–20099. https://doi.org/10.1074/jbc.M117.806240
Chen, Y. Y., & Gänzle, M. G. (2016). Influence of cyclopropane fatty acids on heat, high pressure, acid and oxidative resistance in Escherichia coli. International Journal of Food Microbiology, 222, 16–22. https://doi.org/10.1016/j.ijfoodmicro.2016.01.017
Soini, J., Falschlehner, C., Mayer, C., Böhm, D., Weinel, S., Panula, J., et al. (2005). Transient increase of ATP as a response to temperature up-shift in Escherichia coli. Microbial Cell Factories, 4, 14. https://doi.org/10.1186/1475-2859-4-9
Clarkson, D. T., Earnshaw, M. J., White, P. J., & Cooper, H. D. (1988). Temperature dependent factors influencing nutrient uptake: An analysis of responses at different levels of organization. Symposia of the Society for Experimental Biology, 42, 281–309.
Liu, H., Yu, C., Feng, D., Cheng, T., Meng, X., Liu, W., et al. (2012). Production of extracellular fatty acid using engineered Escherichia coli. Microbial Cell Factories, 11(41), 1–13. https://doi.org/10.1186/1475-2859-11-41
Marr, A. G., & Ingraham, J. L. (1962). Effect of temperature on fatty acids in Escherichia coli. Transactions of the Indian Institute of Metals, 84(6), 1260–1267.
Osman, Y. A., Gbr, M. M., Abdelrazak, A., & Mowafy, A. M. (2018). Fatty acids and survival of bacteria in Hammam Pharaon springs Egypt. Egyptian Journal of Basic and Applied Sciences, 5(2), 165–170. https://doi.org/10.1016/j.ejbas.2018.04.003
Lee, S., Lee, S., Yoon, Y. J., & Lee, J. (2013). Enhancement of long-chain fatty acid production in Escherichia coli by coexpressing genes, including fabf, involved in the elongation cycle of fatty acid biosynthesis. Applied Biochemistry and Biotechnology, 169(2), 462–476. https://doi.org/10.1007/s12010-012-9987-y
Hasan, C. M. M., & Shimizu, K. (2008). Effect of temperature up-shift on fermentation and metabolic characteristics in view of gene expressions in Escherichia coli. Microbial Cell Factories, 7(1), 35. https://doi.org/10.1186/1475-2859-7-35
Sinensky, M. (1971). Temperature control of phospholipid biosynthesis in Escherichia coli. Journal of Bacteriology, 106(2), 449–455. https://doi.org/10.1128/jb.106.2.449-455.1971
Mansilla, M. C., Cybulski, L. E., Albanesi, D., & De Mendoza, D. (2004). Control of membrane lipid fluidity by molecular thermosensors. Journal of Bacteriology, 186(20), 6681–6688. https://doi.org/10.1128/JB.186.20.6681-6688.2004
van Beilen, J. W. A., & Hellingwerf, K. J. (2016). All three endogenous quinone species of Escherichia coli are involved in controlling the activity of the aerobic/anaerobic response regulator ArcA. Frontiers in Microbiology, 7, 1–11. https://doi.org/10.3389/fmicb.2016.01339
Gui, L., Sunnarborg, A., & Laporte, D. C. (1996). Regulated expression of a repressor protein: FadR activates iclR. Journal of Bacteriology, 178(15), 4704–4709. https://doi.org/10.1128/jb.178.15.4704-4709.1996
Park, D. M., Akhtar, M. S., Ansari, A. Z., Landick, R., & Kiley, P. J. (2013). The bacterial response regulator ArcA uses a diverse binding site architecture to regulate carbon oxidation globally. PLoS Genetics, 9(10), 15. https://doi.org/10.1371/journal.pgen.1003839
Privalle, C. T., & Fridovich, I. (1987). Induction of superoxide dismutase in Escherichia coli by heat shock. Proceedings of the National Academy of Sciences of the United States of America, 84(9), 2723–2726. https://doi.org/10.1073/pnas.84.9.2723
Utsumi, R., Horie, T., Katoh, A., Kaino, Y., Tanabe, H., & Noda, M. (1996). Isolation and characterization of the heat-responsive genes in Escherichia coli. Bioscience, Biotechnology and Biochemistry, 60(2), 309–315. https://doi.org/10.1271/bbb.60.309
Gutierrez-Ríos, R. M., Freyre-Gonzalez, J. A., Resendis, O., Collado-Vides, J., Saier, M., & Gosset, G. (2007). Identification of regulatory network topological units coordinating the genome-wide transcriptional response to glucose in Escherichia coli. BMC Microbiology, 7, 1–18. https://doi.org/10.1186/1471-2180-7-53
Nelson, S. M., Attwell, R. W., Dawson, M., & Smith, C. (1996). The effect of temperature on viability of carbon- and nitrogen-starved Escherichia coli. Microbial Ecology, 32(1), 11–21.
Zakaria Gomaa, E. (2014). Production of polyhydroxyalkanoates (PHAs) by Bacillus subtilis and Escherichia coli grown on cane molasses fortified with ethanol. Brazilian Archives of Biology and Technology, 57(1), 145–154.
Calvey, C. H., Su, Y. K., Willis, L. B., McGee, M., & Jeffries, T. W. (2016). Nitrogen limitation, oxygen limitation, and lipid accumulation in Lipomyces starkeyi. Bioresource Technology, 200, 780–788. https://doi.org/10.1016/j.biortech.2015.10.104
Mao, X. J., Huo, Y. X., Buck, M., Kolb, A., & Wang, Y. P. (2007). Interplay between CRP-cAMP and PII-Ntr systems forms novel regulatory network between carbon metabolism and nitrogen assimilation in Escherichia coli. Nucleic Acids Research, 35(5), 1432–1440. https://doi.org/10.1093/nar/gkl1142
Miranda-Rios, J., Sanchez-Pescador, R., Urdea, M., & Covarrubias, A. A. (1987). The complete nucleotide sequence of the glnALG operon of Escherichia coli K12. Nucleic Acids Research, 15(6), 14–19.
Huergo, L. F., & Dixon, R. (2015). The emergence of 2-oxoglutarate as a master regulator metabolite. Microbiology and Molecular Biology Reviews, 79(4), 419–435. https://doi.org/10.1128/mmbr.00038-15
Ziervogel, B. K., & Roux, B. (2013). The binding of antibiotics in OmpF porin. Structure, 21(1), 76–87. https://doi.org/10.1016/j.str.2012.10.014
Liu, X., & Ferenci, T. (1998). Regulation of porin-mediated outer membrane permeability by nutrient limitation in Escherichia coli. Journal of Bacteriology, 180(15), 3917–3922. https://doi.org/10.1128/jb.180.15.3917-3922.1998
Kumar, R., & Shimizu, K. (2010). Metabolic regulation of Escherichia coli and its gdhA, glnL, gltB, D mutants under different carbon and nitrogen limitations in the continuous culture. Microbial Cell Factories, 9, 1–17. https://doi.org/10.1186/1475-2859-9-8
Acknowledgements
None.
Funding
This research work was supported by WOS-A (Women Scientists Scheme-A) research grant (WOS-A/LS-438/2017) from DST (Department of Science and Technology), Govt. of India.
Author information
Authors and Affiliations
Contributions
NS: Writing, reviewing and editing the manuscript. HS: Reviewing and editing of manuscript. DA: Conceptualising, Supervising, writing, reviewing and editing of manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics Approval and Participant’s Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sawant, N., Singh, H. & Appukuttan, D. Overview of the Cellular Stress Responses Involved in Fatty Acid Overproduction in E. coli. Mol Biotechnol 64, 373–387 (2022). https://doi.org/10.1007/s12033-021-00426-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00426-4