Abstract
Improving the productivity of a biopharmaceutical Chinese hamster ovary (CHO) fed-batch cell culture can enable cost savings and more efficient manufacturing capacity utilization. One method for increasing CHO cell productivity is the addition of histone deacetylase (HDAC) inhibitors to the cell culture process. In this study, we examined the effect of valproic acid (VPA, 2-propylpentanoic acid), a branched-chain carboxylic acid HDAC inhibitor, on the productivity of three of our CHO cell lines that stably express monoclonal antibodies. Fed-batch shake flask VPA titrations on the three different CHO cell lines yielded cell line-specific results. Cell line A responded highly positively, cell line B responded mildly positively, and cell line C did not respond. We then performed factorial experiments to identify the optimal VPA concentration and day of addition for cell line A. After identifying the optimal conditions for cell line A, we performed verification experiments in fed-batch bioreactors for cell lines A and B. These experiments confirmed that a high dose of VPA late in the culture can increase harvest titer >20 % without greatly changing antibody aggregation, charge heterogeneity, and N-linked glycosylation profiles. Our results suggest that VPA is an attractive and viable small molecule enhancer of protein production for biopharmaceutical CHO cell culture processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of today’s biopharmaceutical products are made using Chinese hamster ovary (CHO) cell culture with host cells that stably express a recombinant therapeutic protein-of-interest. Since cell culture is the first step in any biologic manufacturing process, improving the productivity of the upstream process plays a key role in reducing the cost of and increasing the manufacturing capacity for bringing biologic drugs to market.
In addition to cell line engineering [1, 2] and media and process optimization [3] approaches for increasing productivity, small molecule enhancers can also be used. One small molecule approach for rapidly increasing CHO cell productivity is the administration of carboxylic acid histone deacetylase (HDAC) inhibitors such as hydroxamic acids [4] and alkanoic acids [5] to CHO cell cultures, the most widely used of which is sodium butyrate [6–10]. Mammalian cells use epigenetic modifications such as histone deacetylation and DNA methylation as defense mechanisms for preventing the expression of undesired or foreign genes, such as those involved in cancer or viral integration [11]. When expressing heterologous proteins such as antibodies in CHO cells, these defense mechanisms can act as a hindrance to obtaining high producers; methylation is one of many reasons why cell line selection must be performed. HDAC inhibitors can interfere with these defense mechanisms; not only do they inhibit the HDACs that compact chromatin to transcription [12], but they also reverse DNA methylation by downregulating methyltransferase expression [13]. Indeed, the administration of HDAC inhibitors like butyrate to CHO cell cultures has resulted in higher levels of recombinant mRNA and protein production [7, 8].
In this study, we focused on the effects of valproic acid (VPA, 2-propylpentanoic acid) on the productivity of stably transfected CHO cells. VPA is a branched-chain carboxylic acid and, like butyrate, a HDAC inhibitor. VPA has been shown to improve recombinant protein expression in transiently transfected CHO [14] and HEK293 [15] cells. The hypothesized mechanism of action is similar to that of butyrate; it increases synthesis of the mRNA of the recombinant protein-of-interest [16]. Since the majority of VPA usage has been confined to transient gene expression applications, we sought to evaluate and characterize the effect of VPA on biopharmaceutical CHO cell culture processes that use stable gene expression. Furthermore, we wanted to examine its effects on antibody product quality features such as aggregation, charge heterogeneity, and N-linked glycosylation. While all drug manufacturers seek to increase product yields and reduce production costs, they must at the same time deliver consistent product quality to ensure safe and reproducible clinical performance [17].
Thus, we used sodium valproate, the salt form of VPA, to increase the antibody harvest titers in our CHO cell lines. We first screened for cell-specific effects of VPA in a fed-batch shake flask model using three CHO cell lines, identifying two out of the three as responsive to VPA. We then used the shake flask model in a factorial experiment to determine the optimal concentration and timing of VPA administration for the most responsive cell line. Finally, we verified the optimal VPA concentration and day of addition in a fed-batch bioreactor model and showed that VPA can increase antibody harvest titers over 20 % in bioreactors without compromising product quality.
Materials and Methods
Cell Lines and Media
Three different CHO cell lines producing three different monoclonal antibodies were used in this study. The stable expression system used by cell lines A, B, and C was based on dihydrofolate reductase (DHFR) amplification [18, 19]. Cells in this study were all grown using proprietary chemically defined basal and feed media [20, 21]. Dextran sulfate (DS) with an average molecular weight of 5,000 Da and sodium valproate (Sigma-Aldrich, St. Louis, MO, USA) were used as cell culture media supplements in this study.
Seed Cultures
All three cell lines were thawed and grown as previously described [20, 21]. Cells were passaged in 1- or 3-L shake flasks (Corning, NY, USA) every 3–4 days using chemically defined basal media with incubator settings of 36 °C and 5 % CO2.
Fed-Batch Shake Flask Cultures
The fed-batch shake flask cultures for cell lines A, B, and C were performed in 500-mL shake flasks (Corning, NY, USA) with 75-mL working volumes in a humidified INFORS incubator (INFORS AG, Bottmingen, Switzerland) at 35 °C and 5 % CO2. Agitation was set at 150 rpm. All cells were seeded at 4 × 105 vc/mL in chemically defined basal media containing 1 g/L DS. Feed media were administered on day 3 and day 5 onwards daily until the day before harvest and culture termination. The feed amount was calculated as a pre-determined fixed percentage based on current culture volume. Glucose stock solution was added as necessary. 1, 2, or 3 mM VPA was added on day 8 of the culture.
A rotatable central composite design fed-batch shake flask experiment to optimize VPA concentration and timing of addition for cell line A was designed using Design-Expert 8 statistical software (Stat-Ease, Minneapolis, MN, USA). The concentration of VPA ranged from 0 to 4 mM, and the timing of VPA addition ranged from day 4 to day 10. Since the VPA concentration and timing affected cell growth, growth-based feeding was implemented for this particular experiment. Feeding was proportional to the integral of viable cells (IVC), which was determined from the area under the viable cell density (VCD) curve and is estimated using a sum of trapezoids approximation across the desired time interval.
Fed-Batch Bioreactor Cultures
The fed-batch production bioreactors for cell lines A and B were performed in 5-L Applikon bioreactors with 2.5-L initial working volumes. Like the fed-batch shake flask experiments, all cells were seeded at 4 × 105 vc/mL in chemically defined basal media with or without 1 g/L DS. For cell line A, 1 g/L DS was present on day 0 for conditions containing DS. For cell line B, 1 g/L DS was added on day 9 for conditions containing DS. Feed media with or without 1 g/L DS were administered on day 3 and day 5 onwards daily until the day before harvest and culture termination for conditions containing DS. The feed amount was calculated as a pre-determined fixed percentage based on culture volume. Glucose stock solution was added as necessary. Bioreactor temperature was controlled at 35 °C, pH was controlled between 6.9 and 7.3 using 1 M sodium carbonate and CO2 sparging, and agitation varied between 200 and 400 rpm. 3.5 mM VPA was added to the appropriate bioreactors on day 9 (cell line A) and day 12 (cell line B).
Offline Analytical Methods
Daily bioreactor sampling was performed to monitor metabolic parameters including glucose, glutamine, glutamate, lactate, ammonium, sodium, potassium, and calcium using the NOVA Flex (NOVA Biomedical, Waltham, MA, USA). VCD and viability were measured using Trypan blue exclusion via an automatic Cedex Cell Counter (Roche, Penzberg, Germany). For bioreactor experiments, pH and pCO2 were measured using a NOVA pHOX Analyzer (NOVA Biomedical, Waltham, MA, USA). Supernatant samples were sterile filtered and stored at 2–8 °C for further titer and product quality analyses.
Analysis of Protein Concentration and Product Quality
Protein concentrations for fed-batch shake flask experiments involving cell lines A, B, and C were measured using the IgG module of a Cedex Bio HT Analyzer (Roche, Penzberg, Germany). Protein concentrations of the cell lines A and B fed-batch bioreactor experiments were measured using an HPLC system (Waters, MA, USA) with a UV detector and a Protein G affinity column (Applied Biosystems, CA, USA).
The effect of VPA on antibody product quality was examined for cell lines A and B. Small scale purification of the harvest supernatant was performed using Protein A affinity chromatography. The percentage aggregation (purity) of the Protein A eluate was measured using the LabChip GXIIs protein profiling assay under non-reducing conditions. Imaging capillary isoelectric focusing (ICIEF) was used to measure the charge heterogeneity. Hydrophilic interaction chromatography using ultra performance liquid chromatography (HILIC-UPLC) was used to measure the N-linked glycosylation profile. N-glycans were enzymatically liberated using peptidase N-glycosidase F, and the released glycan structures were labeled with 2-aminobenzamide (2-AB). Labeled glycans were then analyzed using HILIC-UPLC.
Results and Discussion
Enhancement of CHO Cell Productivity by VPA Varies From Cell Line to Cell Line
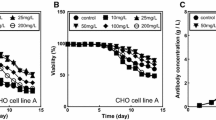
VPA titrations were performed on cell lines A, B, and C. 1, 2, or 3 mM VPA was added to fed-batch shake flask cultures on day 8. A slightly toxic effect of VPA on cell growth was observed on all cell lines; VCD and cell viability declined in the days following VPA addition (Figs. 1a, b, 2a, b). VPA increased the titer and specific productivity of our stably transfected CHO cells, confirming the results observed in transiently transfected CHO cells [14]. However, the effect of VPA on productivity varied from cell line A to C. VPA improved the harvest titer of cell line A by 15 % (Fig. 1c) and harvest titer of cell line B by 10 % (Fig. 2c). There was also an increase in specific productivity due to the depressed growth (Figs. 1d, 2d). However, VPA did not universally increase harvest titer as it had no effect on the harvest titer of cell line C (data not shown).
Effect of VPA on cell line A growth (a), viability (b), harvest titer (c), and titer v. IVC (d). 1 g/L DS was added on day 0, and varying concentrations of VPA were added on day 8. Addition of VPA increases cell line A harvest titer >15 % over the control condition. Experiments were performed using fed-batch shake flasks containing 1 g/L DS. ● control, ■ 1 mM VPA, ▲ 2 mM VPA, ▼ 3 mM VPA
Effect of VPA on cell line B growth (a), viability (b), harvest titer (c), and titer v. IVC (d). 1 g/L DS was added on day 0, and varying concentrations of VPA were added on day 8. Addition of VPA increases cell line B harvest titer >10 % over the control condition and has a dose-dependent effect on specific productivity. Experiments were performed using fed-batch shake flasks containing 1 g/L DS. ● control, ■ 1 mM VPA, ▲ 2 mM VPA, ▼ 3 mM VPA
The cell-specific effect of VPA could be a manifestation of its mechanism of action as a HDAC inhibitor. Since different antibody sequences insert in different regions of the CHO cell genome, it is possible that the regions of the chromosomes surrounding the coding sequence in one cell line (i.e., cell line A) are more methylated and compacted than those in other cell lines (i.e., cell line C). Thus, VPA would not have an effect on cells that do not have high levels of epigenetic modifications. The ideal application for VPA and other epigenetic modifiers such as butyrate and 5-azacytidine would be their use on cell lines that experience an epigenetic-induced decline in specific productivity as the culture duration progresses [22]. If VPA can reverse a drop in specific productivity or prevent cell line instability, it would not only make the process for that particular product more efficient, but it would also greatly increase the value and utility of VPA as a titer enhancer.
We observed cell-specific results of VPA using three of our DHFR-knockout cell lines from the DG44 lineage. The wider biopharmaceutical industry employs a variety of CHO cell lines ranging from DHFR-knockout to glutamine synthetase (GS) knockout cells made from CHO-K1 and CHO-S cells [23]. The cytotoxic and titer-enhancing effects of VPA should not be confined to DHFR-knockout cells, because HDAC inhibitors do not specifically target the DHFR pathway. The genomes of the cell lines from the various CHO expression systems and lineages have been shown to contain numerous single nucleotide polymorphisms (SNPs), deletions, insertions, and copy number variations (CNVs) [24]. With such a large degree of variation in the global genome landscape, we would expect to correspondingly observe a greater degree of variation in the response if cell lines from the different CHO lineages and expression systems were surveyed for VPA effects, as VPA affects global methylation and, therefore, protein expression. Thus, the cell-specific increases in titer that we see in our DHFR-knockout cells would likely be mirrored by those of CHO cells from other backgrounds.
Enhancement of CHO Cell Productivity by VPA is a Function of Concentration and Timing
Since we observed that VPA was a cytotoxic compound in the titration experiments for cell lines A, B, and C, we sought to determine the optimum concentration and timing of VPA administration. We chose cell line A as the model cell line since it had the largest response in magnitude to VPA.
We used a 2-factor central composite design examining the interaction of VPA concentration (0–4 mM) and addition timing (day 4 to day 10). We evaluated the response of VCD, viability, IVC, harvest titer, and specific productivity as a function of VPA concentration and day of addition. In agreement with the titration experiments, we observed toxicity with increased concentrations of VPA. Though adding low concentrations of VPA early in the culture increased specific productivity, it also led to growth inhibition. This growth inhibition reduced the cell mass available to make protein. Thus, the harvest titer on day 15 for the early VPA addition condition was similar to that of the control. The best time to minimize the effects of a growth-inhibiting, cytotoxic compound would be later in the culture. Indeed, this was observed in the factorial experiment as there is a reduction in toxicity if VPA is added later in the culture duration (Fig. 3). The majority of the models are one-factor effects, except that of harvest titer. VPA concentration and day of addition were insignificant as single factors in determining harvest titer, but their two-factor interaction was statistically significant. The results suggested that the maximal harvest titer could be obtained by adding a high dose of VPA later in the culture.
Synergism between VPA concentration and day of addition for cell line A. Concentration and day of addition were not statistically significant individually accordingly to the factorial experiment, but the two-factor interaction was significant (p < 0.03). The model shows that higher concentrations of VPA added later in the culture duration yield higher titers (Color figure online)
VPA Addition in Bioreactors Increases Antibody Titers Without Changing Product Quality
Based on the results of the fed-batch shake flask design-of-experiment study, we performed confirmatory fed-batch bioreactor experiments where we examined the effect of VPA on the growth, viability, and productivity of cell line A. Addition of VPA was again slightly cytotoxic, reducing growth and viability (Fig. 4a, b). The bioreactor experiments confirmed the results of the shake flask experiments, showing that VPA increases the harvest titer compared to the control (Fig. 4c). VPA also improved the specific productivity of cell line A in bioreactors (Fig. 4).
VPA increases harvest titer in cell line A fed-batch bioreactors. Effect of VPA on cell line A growth (a), viability (b), harvest titer (c), and titer v. IVC (d). 1 g/L DS was added on day 0, and 3.5 mM VPA was added on day 9. Addition of VPA increases cell line A harvest titer >20 % over the control condition, but the increase is only observed in the presence of 1 g/L DS. ● control, ■ DS, ▲ VPA, ▼ VPA + DS
Interestingly, the VPA effect in bioreactors was completely dependent on the presence of DS. VPA alone has little effect on harvest titer, but when combined with DS, it increases harvest titer by >20 % (Fig. 4c). We tested the effect of VPA on bioreactors with or without DS, because DS is present by default in our shake flask model as a means to reduce cell aggregation. DS is not present in our bioreactor model, because automated cell counting data show that the agitation supplied by the impeller is sufficient to break apart cell aggregates. Perhaps, the DS is interacting with the VPA and the cells in such a way that it enhances VPA uptake or dislodges a pool of protein that is adsorbed on the surface of the cell.
We also performed confirmatory bioreactor experiments with cell line B. In these experiments, we delayed the addition of VPA from day 9 to day 12 in cultures containing DS. The DOE experiment suggested that higher titer could be obtained with a later VPA addition as the shorter exposure time may minimize cytotoxic effects. Thus, we hoped that the later VPA addition would yield higher titer. Addition of VPA and DS again reduced growth and viability (Fig. 5a, b). However, the delayed addition strategy yielded a 20 % increase in cell line B’s harvest titer with little difference in the integrated cell mass (Fig. 5c, d). The titer increase was higher than that observed in cell line B shake flasks, which suggests that delaying the time of VPA addition can generate higher titers.
The most encouraging result from the bioreactor experiments was discovering that the increase in titer obtained through VPA addition did not come at the expense of antibody product quality for cell lines A and B (Table 1). The ability to generate a product quality profile that is comparable to an existing process greatly increases the commercial attractiveness of VPA. Process changes that increase the productivity of clinical or commercial program must result in critical quality attribute profiles that ultimately do not change the protein-of-interest’s efficacy and safety, such as antibody aggregation, charge heterogeneity, and glycosylation. VPA did not change the aggregation, charge heterogeneity, or the glycosylation profile of the antibodies produced by cell line A or B. However, this effect may be cell- or product-specific, as butyrate has been shown to both change [25–27] and maintain [6] recombinant protein product quality.
Thus, sodium butyrate may not be suitable for applications where product quality comparability is desired, as there are more reported instances of butyrate changing product quality than instances of butyrate maintaining it. In our hands, VPA maintained product quality for two cell lines. However, this could also be a cell-specific effect. An additional benefit of VPA is that it may be more cost effective than butyrate [14]. The combination of VPA’s cost and its ability to maintain key product quality attributes of the corresponding control process makes it an attractive and flexible cell culture lever for not only improving the titer of new products in early stage development, but also that of legacy products and products in late stage development where product quality comparability is important.
Conclusion
We have demonstrated that addition of VPA and DS to CHO cells stably expressing monoclonal antibodies can increase harvest titers by up to 20 % without compromising antibody product quality, though the magnitude of the increase is dependent on the cell line. We performed factorial experiments to identify the optimal concentration and timing of VPA addition for one CHO cell line in a shake flask model and then verified the results in a bioreactor model. In doing this, we also discovered that the addition of DS may further improve overall cell culture performance in our bioreactor model. These results suggest that VPA is a straightforward and attractive method for rapidly improving the productivity of biopharmaceutical cell culture processes.
References
Kramer, O., Klausing, S., & Noll, T. (2010). Methods in mammalian cell line engineering: From random mutagenesis to sequence-specific approaches. Applied Microbiology and Biotechnology, 88(2), 425–436.
Kim, J. Y., Kim, Y. G., & Lee, G. M. (2012). CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Applied Microbiology and Biotechnology, 93(3), 917–930.
Li, F., Vijayasankaran, N., Shen, A. Y., Kiss, R., & Amanullah, A. (2010). Cell culture processes for monoclonal antibody production. mAbs, 2(5), 466–479.
Allen, M. J., Boyce, J. P., Trentalange, M. T., Treiber, D. L., Rasmussen, B., Tillotson, B., et al. (2008). Identification of novel small molecule enhancers of protein production by cultured mammalian cells. Biotechnology and Bioengineering, 100(6), 1193–1204.
Liu, C., Chu, I., & Hwang, S. (2001). Pentanoic acid, a novel protein synthesis stimulant for Chinese Hamster Ovary (CHO) cells. Journal of Bioscience and Bioengineering, 91(1), 71–75.
Mimura, Y., Lund, J., Church, S., Dong, S., Li, J., Goodall, M., et al. (2001). Butyrate increases production of human chimeric IgG in CHO-K1 cells whilst maintaining function and glycoform profile. Journal of Immunological Methods, 247(1–2), 205–216.
Jiang, Z., & Sharfstein, S. T. (2008). Sodium butyrate stimulates monoclonal antibody over-expression in CHO cells by improving gene accessibility. Biotechnology and Bioengineering, 100(1), 189–194.
Jeon, M. K., & Lee, G. M. (2007). Correlation between enhancing effect of sodium butyrate on specific productivity and mRNA transcription level in recombinant Chinese hamster ovary cells producing antibody. Journal of Microbiology and Biotechnology, 17(6), 1036–1040.
Hendrick, V., Winnepenninckx, P., Abdelkafi, C., Vandeputte, O., Cherlet, M., Marique, T., et al. (2001). Increased productivity of recombinant tissular plasminogen activator (t-PA) by butyrate and shift of temperature: A cell cycle phases analysis. Cytotechnology, 36(1–3), 71–83.
Kantardjieff, A., Jacob, N. M., Yee, J. C., Epstein, E., Kok, Y. J., Philp, R., et al. (2010). Transcriptome and proteome analysis of Chinese hamster ovary cells under low temperature and butyrate treatment. Journal of Biotechnology, 145(2), 143–159.
Doerfler, W. (2006). De novo methylation, long-term promoter silencing, methylation patterns in the human genome, and consequences of foreign DNA insertion. Current Topics in Microbiology and Immunology, 301, 125–175.
Rountree, M. R., Bachman, K. E., Herman, J. G., & Baylin, S. B. (2001). DNA methylation, chromatin inheritance, and cancer. Oncogene, 20(24), 3156–3165.
Sarkar, S., Abujamra, A. L., Loew, J. E., Forman, L. W., Perrine, S. P., & Faller, D. V. (2011). Histone deacetylase inhibitors reverse CpG methylation by regulating DNMT1 through ERK signaling. Anticancer Research, 31(9), 2723–2732.
Backliwal, G., Hildinger, M., Kuettel, I., Delegrange, F., Hacker, D. L., & Wurm, F. M. (2008). Valproic acid: A viable alternative to sodium butyrate for enhancing protein expression in mammalian cell cultures. Biotechnology and Bioengineering, 101(1), 182–189.
Backliwal, G., Hildinger, M., Chenuet, S., Wulhfard, S., De Jesus, M., & Wurm, F. M. (2008). Rational vector design and multi-pathway modulation of HEK 293E cells yield recombinant antibody titers exceeding 1 g/L by transient transfection under serum-free conditions. Nucleic Acids Research, 36(15), e96.
Wulhfard, S., Baldi, L., Hacker, D. L., & Wurm, F. (2010). Valproic acid enhances recombinant mRNA and protein levels in transiently transfected Chinese hamster ovary cells. Journal of Biotechnology, 148(2–3), 128–132.
Chirino, A. J., & Mire-Sluis, A. (2004). Characterizing biological products and assessing comparability following manufacturing changes. Nature Biotechnology, 22(11), 1383–1391.
Pallavicini, M. G., DeTeresa, P. S., Rosette, C., Gray, J. W., & Wurm, F. M. (1990). Effects of methotrexate on transfected DNA stability in mammalian cells. Molecular and Cellular Biology, 10(1), 401–404.
Gandor, C., Leist, C., Fiechter, A., & Asselbergs, F. A. (1995). Amplification and expression of recombinant genes in serum-independent Chinese hamster ovary cells. FEBS Letters, 377(3), 290–294.
Huang, Y. M., Hu, W., Rustandi, E., Chang, K., Yusuf-Makagiansar, H., & Ryll, T. (2010). Maximizing productivity of CHO cell-based fed-batch culture using chemically defined media conditions and typical manufacturing equipment. Biotechnology Progress, 26(5), 1400–1410.
Kshirsagar, R., McElearney, K., Gilbert, A., Sinacore, M., & Ryll, T. (2012). Controlling trisulfide modification in recombinant monoclonal antibody produced in fed-batch cell culture. Biotechnology and Bioengineering, 109(10), 2523–2532.
Yang, Y., Mariati, Chusainow, J., & Yap, M. G. (2010). DNA methylation contributes to loss in productivity of monoclonal antibody-producing CHO cell lines. Journal of Biotechnology, 147(3–4), 180–185.
Wurm, F. (2013). CHO quasispecies: Implications for manufacturing processes. Processes, 1(3), 296–311.
Lewis, N. E., Liu, X., Li, Y., Nagarajan, H., Yerganian, G., O’Brien, E., et al. (2013). Genomic landscapes of Chinese hamster ovary cell lines as revealed by the Cricetulus griseus draft genome. Nature Biotechnology, 31(8), 759–765.
Gawlitzek, M., Estacio, M., Furch, T., & Kiss, R. (2009). Identification of cell culture conditions to control N-glycosylation site-occupancy of recombinant glycoproteins expressed in CHO cells. Biotechnology and Bioengineering, 103(6), 1164–1175.
Lamotte, D., Buckberry, L., Monaco, L., Soria, M., Jenkins, N., Engasser, J. M., et al. (1999). Na-butyrate increases the production and alpha2,6-sialylation of recombinant interferon-gamma expressed by alpha2,6-sialyltransferase engineered CHO cells. Cytotechnology, 29(1), 55–64.
Andersen, D. C., Bridges, T., Gawlitzek, M., & Hoy, C. (2000). Multiple cell culture factors can affect the glycosylation of Asn-184 in CHO-produced tissue-type plasminogen activator. Biotechnology and Bioengineering, 70(1), 25–31.
Acknowledgments
The authors would like to thank Biogen Idec Analytical Development and the Biogen Idec High Throughput Analytical Group for performing the Protein G and product quality analyses. We would also like to thank Kevin Ramer for review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, W.C., Lu, J., Nguyen, N.B. et al. Addition of Valproic Acid to CHO Cell Fed-Batch Cultures Improves Monoclonal Antibody Titers. Mol Biotechnol 56, 421–428 (2014). https://doi.org/10.1007/s12033-013-9725-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-013-9725-x