Abstract
Over the past decade, global interest in the development of therapeutic monoclonal antibodies (mAbs) has risen rapidly. As therapeutic agents, antibodies have shown marked efficacy in combatting a range of cancers and immune diseases with high target specificity and low toxicity (Carla Lucia et al. in PLoS ONE 6:e24071, 2011; Donaghy in MAbs 8:659–671, 2016; Nasiri et al. in J Cell Physiol 9:6441–6457, 2018; Teo et al. in Cancer Immunol Immunother 61:2295–2309, 2012). Recent advances in cell culture technology, such as high-throughput clone screening, have facilitated antibody production at concentrations exceeding 10 g/L (Chen et al. in BMC Immunol 19:35, 2018; Huang et al. in Biotechnol Prog 26:1400–1410, 2010; Lu et al. in Biotechnol Bioeng 110:191–205, 2013; Singh et al. in Biotechnol Bioeng 113:698–716, 2016). As titers have improved, the industry has begun to focus on the adjustment of target antibody quality profiles to improve efficacy. Cell lines, culture media, and culture conditions impact protein quality (Van Beers and Bardor in Biotechnol J 7:1473–1484, 2012). Optimization of critical quality attributes (CQAs), such as charge variants, can be achieved through bioprocess development and is the preferred approach as changes to the cell line or growth media used is considered unfavorable by regulatory bodies (Gawlitzek et al. in Biotechnol Bioeng 103:1164–1175, 2009; Jordan et al. in Cytotechnology 65:31–40, 2013; Pan et al. in Cytotechnology 69:39–56, 2016). In this study, the effect of process control and ion supplementation on charge variants of mAbs produced by Chinese hamster ovary (CHO) cells was investigated. Results of this study demonstrated that the concentration of Zn2+, duration of culturing, and temperature affect charge variants of a given mAb. Under the optimum conditions of 3L bioreactors, the most significant was that Zn2 + and temperature shift could further improve the quality of antibody. The main peak increased by 12%, and the acid peak decreased by 16%. At the same time, there was no significant loss of titer. This study provided supporting evidence for methods to improve charge variants arising during mAb production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pharmaceutical industry benefits from robust market demand with global prescription drug sales exceeding 1 trillion USD in 2017. Furthermore, global monoclonal antibody (mAb) market size exceeded $100 billion USD in 2017, and investment into the development of biologics is expected to continue. Following years of research and development, CHO cells are most commonly used host cell line for antibody production due to their ability to execute appropriate post-translational modifications of mAbs necessary for therapeutic utility (Chu and Robinson 2001; Lim et al. 2010). Monoclonal antibodies are complex heterogeneous glycoproteins with multiple post-translational modifications; therefore a number of different variants exists for a particular mAb (Brorson and Jia 2014; Higel et al. 2016; Houde et al. 2010). Each variant of a mAb has unique biochemical and biophysical properties associated with their specific post-translational modifications (Higel et al. 2016; Mueller 2017; Zheng et al. 2014). The majority of mAbs developed and tested by Chinese biopharma company pipelines are biosimilar, therefore mAbs should have molecular properties similar to those of the original targets (Li et al. 2015; Silva et al. 2014). However, controlling post-translational modifications both in and outside of cells is difficult as the underlying mechanisms are complex and often poorly understood (Begona and Leyre 2012; Walsh 2010). Common issues in the development of biosimilar mAb include aggregates, fragments, and differentially glycosylated mAbs (Berkowitz et al. 2012; Read et al. 2011; Wei et al. 2006). Charge variants can be identified by use of cation exchange chromatography (IEC), whereby the acidic mAb peak elutes before the major peak and the basic mAb peak elutes later (Khawli et al. 2015; Lingg et al. 2013). For example, the IEC elution profile of the mAb assessed in this study is shown in Fig. 1, where the early- and late-eluting peaks are indicative of the acidic and basic charge variants, respectively. These charge variants have the potential to alter molecular properties of mAbs, and therefore their safety and efficacy profiles, thus presenting significant regulatory concern (Beck et al. 2013; Chirino and Mire-Sluis 2004; Dada et al. 2015; Hintersteiner et al. 2016; Rea et al. 2011; Shukla and Thömmes 2010). To date, the relationship between charge variant production and presence of impurities during mAb production has been elucidated (Khawli et al. 2015; Kishishita et al. 2015; Prabhu and Gadgil 2019). For biosimilar mAb production, the frequency of charge variants must be adjusted to correspond to the original drugs (Begona and Leyre ; Vázquez-Rey and Lang 2011). Traditionally, IEC has been used in the purification of mAbs. Unfortunately, this approach greatly reduces product yield. Optimization of cell culture processes in the production of mAbs can alter charge variant frequencies (Kishishita et al. 2015; Seo et al. 2016). Therefore, in the present study we investigated methods to optimize the production of charge variants and to improve mAb production quality.
Cation exchange chromatography elution profile. The acidic mAb peaks elute before the main peak and the basic mAb peaks elute after the main peak. As shown in this figure, the peaks of approximately 18 min are the acid charge peaks, the largest peak being the main one followed by the basic charge peaks. The horizontal axis represents the retention time of HPLC and the vertical axis represents the absorbance unit (mAU)
Materials and methods
Cell lines
The cell line used in this paper was developed by Sunshine Guojian (A Member of 3SBio). According to the published amino acid information of Trastuzumab, the recombinant anti-HER2 humanized mAb DNA sequence was synthesized and inserted into the pUC57 intermediate vector. The PCR method was utilized to amplify the antibody heavy chain and light chain sequence from the pUC57 plasmid and a signal peptide was added to the N-terminus of the corresponding sequence. Meanwhile, AvrII/BstZ17I restriction site was added to the ends of heavy chain Open Reading Frames (ORFs), EcoRV/PacI restriction site was added to the ends of light chain ORFs. Freedom pCHO1.0 plasmid (Gibco, ThermoFisher, USA) was selected as the expression vector. The heavy chain ORFs and the light chain ORFs were cloned into the multiple cloning site AvrII/BstZ17I and EcoRV/PacI of pCHO1.0 respectively. The recombinant plasmid was named pCHO-VHL. See Supplementary information Figure 1 for detail plasmid map information. After the antibody gene sequence accuracy was confirmed, the plasmid was transfected into host cell CHO-DG44 cell (Thermo Fisher, USA). The transfected cells were screened by pressure screening, clonal screening, single cell subcloning screening and engineered cell strain producing trastuzumab was obtained. These cells were finally stored in liquid nitrogen tanks.
Cell culture media
The basic medium used during the cultivation was 300F (Sunshine GuoJian, CHINA), and the nutrient medium 300S (Sunshine GuoJian, CHINA) was added with 0.008 mol/L glutamine(Gln) during the cultivation, and the solution used was 30% Glucose solution, 10% AF(antifoam) and 2 mol/L Na2CO3 solution. 300F and 300S produced by Sunshine Guojian was chemically defined media. Dissolved the medium powder in water for injection (WFI) and kept agitating for 3 h and weighed the medium to a final volume. The media were filtered through sterilizing grade filter in biosafety cabinet(Esco, Germany). The sterile media should be stored at 4–8 °C avoiding light. ZnSO4·7H2O, MnCl2 and CuSO4 metal salts were purchased from Sigma.
Cell culture method
One vial of working cell bank (WCB) was thawed in a water bath at 37 °C, Shook the vial until thawed completely and inoculated into a 125 mL shake flask. The CHO cells in shaking flask were cultured in CO2 shaking incubators (INFORS Multitron, Switzerland) incubated at 36.5 °C, 5% CO2, 145 rpm. During scale-up operations, cells were cultivated in fed-bath mode in 3L bioreactors (Eppendorf DASGIP, Germany) at an inoculation density of 0.9 × 106 cells/mL and set the other parameters as following: 35–37 °C, pH 6.8–7.1, 40% dissolved oxygen, 200–400 rpm. All experiments were maintained under the same conditions except when concentration of Zn2+, Cu2+, Mn2+ ions or temperature were altered (Ex: 36.5 °C to 35 °C on Day 0 /Day 2/Day 3/Day 5). Nutrient solutions were added daily between Day 3 and 13. Culture media were sampled for analysis daily between Day 11 and 15.
Online measurements and offline analysis
CHO cells density was counted by use of an automated cell counter (Countstar, ALIT, China), Glucose and lactate levels were measured by use of a biochemical analyzer (Roche, Switzerland), pH and Dissolved oxygen (DO) were measured by use of sensors (Mettler Toledo, Switzerland), Charge variants and titer were analyzed by use of high performance liquid chromatography (Agilent 1260, USA).
Results
Influence of culture temperature shift
Temperature impacts cell growth and can affect metabolism by altering the effective rate of energy transfer within cells (Sengupta et al. 2011; Si et al. 2014). During the later stages of antibody production, lower temperatures can improve cell survival (Yoon et al. 2003). To study the effects of temperature variation on mAb production, a series of experiments were conducted. Culture parameters were as follows: flasks were incubated at 5% CO2 with the shaking speed of 145 rpm, an initial cell density of 0.9 × 106 cells/mL and a final pH of 6.8–7.1. As shown in Fig. 2 and Supplementary Table 1, in order to evaluate the influence of temperature shift, the three shake flasks were shifted from 36.5 to 35 °C on day 2, day 3 or day 5 respectively. The other two flasks were cultured at 36.5 °C and 35 °C respectively. All batches were harvested on Day 14.
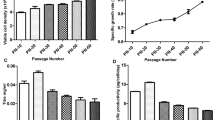
The different effect of temperature shift on tilter and charge variants. The three shake flasks were shifted from 36.5 to 35 °C on day 2, day 3 or day 5 respectively. The other two flasks were cultured at 36.5 °C and 35 °C respectively. All batches were harvested on Day 14. Tilter and charge variants of Day 14 as shown in this figure were the averages of triplicates of three experiments
Previous studies have demonstrated that lower temperatures yield a higher proportion of cells in the G1 phase, and thereby improve antibody titer (Fogolín et al. 2004; Kaufmann et al. 1999; Oguchi et al. 2006; Schatz et al. 2003). In contrast, the temperature is reduced, the decrease in protein titers always occurs when the temperature does not significantly affect the viability of the cells. some other studies have similar results to the manuscript (Bloemkolk et al. 1992; Fogolín et al. 2004; Reuveny et al. 1986; Sureshkumar and Mutharasan 1991), lower temperatures reduce titers and impact the glycoprotein charge heterogeneity (Fig. 2). It is likely that a shift in temperature extended the transit time of antibodies through the endoplasmic reticulum and Golgi apparatus, thereby promoting glycosylation (Bollati-Fogolín et al. 2005; Gomez et al. 2012; Si et al. 2014), similar to previous reports where it was demonstrated that lower temperatures reduce erythropoietin (EPO) sialic acid content (Ahn et al. 2008; Kaneko et al. 2010). Post-translational modifications such as sialylation can alter the charge distribution of mAbs, and might explain results of this study (Clark et al. 2004). As demonstrated in Fig. 2, a shift in cell culture temperature on Day 3 resulted in an acceptable mAb titer and fewer charge variants, thus indicating the utility of these culture conditions.
Influence of culture duration
Following inoculation, cell culture growth proceeded through a number of phases; latent phase (Day 0–Day 1), logarithmic growth phase (Day 2–Day 6), and stagnant phase (Day 7–Day 15). In this study, the effect of culture duration on the growth of CHO cells, mAb titer, and charge variant frequency were investigated. Parameters of cell cultivation were as described above and cell culture media was sampled daily on day 11–15.
The effect of culture duration on charge variants and titer. The mAb titer increased with the extension of culture time. Acidic variants increased and main peak decreased with the extension of culture time. a mAb titer. b Distribution of charge variants. All data points were the averages of triplicates of three experiments
Results demonstrate that antibody titer and size of acidic charge variant peak increased significantly with incubation time (Fig. 3). In contrast, main peak size decreased significantly and the peak associated with the acidic species between Day 11 and 15 reached a maxima of approximately 20%. The peak size of the basic charge variant remained constant over time. According to the previous research find, increase in sialic acid content or deamidation of asparagine residues was likely associated with an increase in the presence of acidic charge variants (Du et al. 2012; Leblanc et al. 2017). Thus, incubation time is a critical parameter which can be adjusted according to desired outcomes in order to maintain appropriate mAb product quality. Results of this experiment demonstrate an optimal harvest times on day 14.
Influence of metal ion concentration
The impact of ion concentrations on cell growth, mAb titer, and charge variant was investigated. Ions tested included Zn2+, Cu2+ and Mn2+ in various concentrations. Culture conditions were as described in the previous two studies and temperature were shifted from 36.5 to 35 °C on day 3, all cell batches were harvested on Day 14.
The effect of metal ions on cell growth, titer and charge variants. High Cu2+ concentration (400 µM) negatively impacted cell density, viability, and mAb titers. In contrast, high concentrations of Zn2+ (40 µM) promote expression and reduce acidic peaks. All batches were harvested on Day 14. a Cell growth. b Viable cell density (VCD). c mAb titer. d Distribution of charge variants. All data points were the averages of triplicates of three experiments
Mn2+ and Zn2+ function to catalyze enzymatic reactions, whereas, Cu2+ often functions to inhibit the activity of enzymes (Luo et al. 2012; Radhakrishnan et al. 2018). By varying concentration of the selected ions, effects on enzymatic reactions involved in mAb production by CHO cells was investigated. As presented in Fig. 4, It was demonstrated that higher concentrations of Zn2+ (40 µM) reduced the size of acidic charge variant peaks and increased the size of main peaks relative to lower concentration of Zn2+ (4 µM) (Fig. 4d). At the same time, cultures with Zn2+ expressed higher protein than cultures with Mn2+ and Cu2+. Compared with no additive culture and 4 µM, found that Zn2+ concentration increased the protein expression significantly at 40 µM almost 12.24% and 5.45% (Fig. 4c). Mn2+ and Cu2+ had little effect on the acidic charge variant peak, whereas Cu2+ somewhat reduced the size of the main peak and increased the size of basic charge variant peaks (Fig. 4d). A high Cu2+ concentration (400 µM) negatively impacted cell density, viability, and mAb titer(Fig. 4a–c).
CPB is an exocrine protease, each of which contains an ion of zinc, which hydrolyzes the C-terminal residues of peptide chains, such as lysine, glycine or arginine. Previous work has shown that CPB activity is enhanced by maintaining a proper Zn2+ concentration, eventually leading to charge variations in mAb production (Luo et al. 2012; Yoshimoto et al. 2013).
Optimization of cell culture processes
To investigate the effect of varying Zn2+ concentrations on cell growth, mAb titer, and charge variants frequencies, cells were grown by use of media containing 30 µM to 150 µM of Zn2+. Cells were initially seeded at a density of 1.0 × 106 cells/mL, and growth conditions were as described in the preceding section.
The effect of Zn2+ on cell growth, mAb titer, charge variants. A certain range of Zn2+ concentrations promote expression and reduce acidic peaks. In contrast, high Zn2+ concentration (150 µM) negatively impacted cell density and viability. All batches were harvested on Day 14. a Viability. b Viable cell density (VCD). c mAb titer. d Distribution of charge variants. All data points were the averages of triplicates of three experiments
As presented in Fig. 5, a certain range of Zn2+ concentrations promote expression and reduce acidic peaks. In contrast, high Zn2+ concentration (150 µM) negatively impacted cell density and viability (Fig. 5a, b). The size of the main mAb peak increased as Zn2+ concentration increased from 30 to 110 µM. However, when zinc concentration was equal to 150 µM, main peak size decreased (Fig. 5d). The experiment showed that when the expression of protein reached the highest peak, Zn2+ concentrations were 70 µM (Fig. 5c), and when the concentration reached 110 µM, the main mAb peak peaked (Fig. 5d). Therefore, we focus on 100 µM, with a range of plus or minus 30 µM, the concentrations of Zn2+ between 70 and 130 µM were selected as optimal.
Based on results of our experiments, Zn2+ concentration and cell culture temperature were deemed critical process parameters impacting the quality of produced mAb. Therefore, experiments were designed to assess the effect of temperature and Zn2+ concentration on cell growth, mAb titer, and charge variant frequencies. Culture conditions were as described in the preceding two sections, with varying Zn2+ concentration and culture temperature as outlined in Table 1.
As mentioned previously, the frequency of basic variants processed by CPB remained stable across culture conditions. Therefore, focus was given to the main peak (++++), acidic charge variant peak (+++), and titer (++) as the key outcomes of this study, whereby the “+” symbol represents relative weight. Following response surface modeling (Fig. 6), the DoE software indicated that the best control point to minimize the frequency of charge variants was to culture cells at a temperature of 35.13 °C on day 3 with media Zn2+ concentration equal to 79.82 µM.
Process validation (3L)
To validate finding of this study at a larger scale, the above mentioned temperature (temperature shift from 36.5 to 35 °C on day 3), harvest time (day 14), and metal ion concentration results (80 µM Zn2+) were used in the culturing of cells in large 3L bioreactors in duplicate (reactor 1 and reactor 2). A third reactor was used as a negative control with temperature maintained at 36.5 °C and a Zn2+ concentration equal to 80 µM. As presented in Fig. 7, it was found that a shift in temperature changed glycoprotein charge heterogeneity and increased main peak size relative to the negative control reactor(Fig. 7a–c). Addition of 80 µM Zn2+ further reduced the frequency of charge variants and increased main peak by 12% and a concomitant decrease in the acidic peak of 16%(Fig. 7d).
The effect of Zn2+ on cell growth, mAb titer, charge variants. The parameters of two parallel reactors as following: temperature shift from 36.5 °C to 35 °C on day 3 and 80 µM Zn2+, the third reactor was used as a control with temperature maintained at 36.5 °C and 80 µM Zn2+. All batches were harvested on Day 14. a Viable cell density (VCD). b Viability. c mAb titer. d Distribution of charge variants. All data points were the averages of triplicates of three experiments
Discussion
Many factors including cell line, media, and culture processes significantly influence antibody quality (Rouiller et al. 2014). Under current regulatory guidelines or in consideration of other limitations, cell lines or media cannot be changed, therefore optimization of other CQAs such as culture duration, temperature, and metal ion concentrations can be used to improve enzyme activity and production of mAb. Enzymes are vital to mAb maturation and in vivo occurs in the endoplasmic reticulum or Golgi apparatus which are the major site of glycosylation in mammalian cells.
Post-translational modifications arising in vitro or in vivo can affect antibody quality. Following secretion from cells, mAbs are subject to additional modifications mediated by enzymes released from host cells. During later stages of cell culture, cells begin to die leading to a significant release of active enzymes into cell culture media. High enzyme concentrations and longer culture durations can thus significantly alter the frequency of particular variants of proteins of interest. Therefore, process parameters can be optimized to elicit desired quality standards for a given mAb. Previous studies have demonstrated that sialic acid content, deamination of asparagine residues, presence of non-classical disulfide linkages, and high mannose content are post-translational modifications leading to the development of acidic charge variants (Du et al. 2012; Leblanc et al. 2017). In contrast, the presence of C-terminal lysine, tryptophan oxidation, methionine and glycine amidation can all contribute to the presence of basic charge variant species (Khawli et al. 2015).
As demonstrated harvest time, culture temperature, and Zn2+ concentration impact charge variant frequency of mAbs produced in CHO cells and impact the frequency of acidic charge variant species. In this study, it was demonstrated that optimization of critical process parameters (CPPS) and critical material attributes (CMAs), can be achieved by optimization of CQA for robust manufacturing. It is vital that future work expand upon these results in order to define broadly acceptable operational ranges in which mAb production can be robustly optimized in order to facilitate biosimilar production workflows.
References
Ahn WS, Jeon JJ, Jeong YR, Lee SJ, Yoon SK (2008) Effect of culture temperature on erythropoietin production and glycosylation in a perfusion culture of recombinant CHO cells. Biotechnol Bioeng 101:1234–1244
Beck A et al (2013) Analytical characterization of biosimilar antibodies and Fc-fusion proteins. TrAC Trends Anal Chem 48:81–95
Begona C, Leyre Z (2012) Therapeutic monoclonal antibodies: strategies and challenges for biosimilars development. Curr Med Chem 19:4445–4450
Berkowitz SA, Engen JR, Mazzeo JR, Jones GB (2012) Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nat Rev Drug Discov 11:527–540
Bloemkolk JW, Gray MR, Merchant F, Mosmann TR (1992) Effect of temperature on hybridoma cell cycle and MAb production. Biotechnol Bioeng 40:427–431
Bollati-Fogolín M, Forno G, Nimtz M, Conradt HS, Etcheverrigaray M, Kratje R (2005) Temperature reduction in cultures of hGM-CSF-expressing CHO cells: effect on productivity and product quality. Biotechnol Prog 21:17–21
Brorson K, Jia AY (2014) Therapeutic monoclonal antibodies and consistent ends: terminal heterogeneity, detection, and impact on quality. Curr Opin Biotechnol 30:140–146
Carla Lucia E et al (2011) A neutralizing RNA aptamer against EGFR causes selective apoptotic cell death. PLoS ONE 6:e24071
Chen Q et al (2018) A novel approach for rapid high-throughput selection of recombinant functional rat monoclonal antibodies. BMC Immunol 19:35
Chirino AJ, Mire-Sluis A (2004) Characterizing biological products and assessing comparability following manufacturing changes. Nat Biotechnol 22:1383–1391
Chu L, Robinson DK (2001) Industrial choices for protein production by large-scale cell culture. Curr Opin Biotechnol 12:180–187
Clark KJR, Chaplin FWR, Harcum SW (2004) Temperature Effects on Product-Quality-Related Enzymes in Batch CHO Cell Cultures Producing Recombinant tPA. Biotechnol Prog 20:1888–1892
Dada OO, Jaya N, Valliere-Douglass J, Salas-Solano O (2015) Characterization of acidic and basic variants of IgG1 therapeutic monoclonal antibodies based on non-denaturing IEF fractionation. Electrophoresis 36:2695–2702
Donaghy H (2016) Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody-drug conjugates. MAbs 8:659–671
Du Y, Walsh A, Ehrick R, Xu W, May K, Liu H (2012) Chromatographic analysis of the acidic and basic species of recombinant monoclonal antibodies. MAbs 4:578–585
Fogolín MB, Wagner R, Etcheverrigaray M, Kratje R (2004) Impact of temperature reduction and expression of yeast pyruvate carboxylase on hGM-CSF-producing CHO cells. J Biotechnol 109:179–191
Gawlitzek M, Estacio M, Fürch T, Kiss R (2009) Identification of cell culture conditions to control N-glycosylation site-occupancy of recombinant glycoproteins expressed in CHO cells. Biotechnol Bioeng 103:1164–1175
Gomez N et al (2012) Culture temperature modulates aggregation of recombinant antibody in cho cells. Biotechnol Bioeng 109:125–136
Higel F, Seidl A, Sorgel F, Friess W (2016) N-Glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur J Pharm Biopharm 100:94–100
Hintersteiner B et al (2016) Charge heterogeneity: Basic antibody charge variants with increased binding to Fc receptors. mAbs 8:1548–1560
Houde D, Peng Y, Berkowitz SA, Engen JR (2010) Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol Cell Proteomics 9:1716–1728
Huang YM, Hu W, Rustandi E, Chang K, Yusuf-Makagiansar H, Ryll T (2010) Maximizing productivity of CHO cell-based fed-batch culture using chemically defined media conditions and typical manufacturing equipment. Biotechnol Prog 26:1400–1410
Jordan M, Voisard D, Berthoud A, Tercier L, Kleuser B, Baer G, Broly H (2013) Cell culture medium improvement by rigorous shuffling of components using media blending. Cytotechnology 65:31–40
Kaneko Y, Sato R, Aoyagi HJJoB (2010) Changes in the quality of antibodies produced by Chinese hamster ovary cells during the death phase of cell culture. J Biosci Bioeng 109:281–287
Kaufmann H, Mazur X, Fussenegger M, Bailey JE (1999) Influence of low temperature on productivity, proteome and protein phosphorylation of CHO cells. Biotechnol Bioeng 63:573–582
Khawli LA et al. (2015) Charge variants in IgG1: isolation, characterization, in vitro binding properties and pharmacokinetics in rats. Mabs 2:613–624
Kishishita S, Nishikawa T, Shinoda Y, Nagashima H, Okamoto H, Takuma S, Aoyagi H (2015) Effect of temperature shift on levels of acidic charge variants in IgG monoclonal antibodies in Chinese hamster ovary cell culture. J Biosci Bioeng 119:700–705
Leblanc Y, Ramon C, Bihoreau N, Chevreux G (2017) Charge variants characterization of a monoclonal antibody by ion exchange chromatography coupled on-line to native mass spectrometry: case study after a long-term storage at + 5 °C. J Chromatogr B 1048:130–139
Li W, Kerwin JL, Schiel J, Formolo T, Davis D, Mahan A, Benchaar SA (2015) Structural Elucidation of post-translational modifications in monoclonal antibodies. In: State-of-the-art and emerging technologies for therapeutic monoclonal antibody characterization vol 2. Biopharmaceutical characterization: the NISTmAb case study, vol 1201. ACS symposium series, vol 1201. American Chemical Society, Washington, pp 119–183. https://doi.org/10.1021/bk-2015-1201.ch003
Lim Y, Wong NS, Lee YY, Ku SC, Wong DC, Yap MG (2010) Engineering mammalian cells in bioprocessing - current achievements and future perspectives. Biotechnol Appl Chem 55:175–189
Lingg N, Tan E, Hintersteiner B, Bardor M, Jungbauer A (2013) Highly linear pH gradients for analyzing monoclonal antibody charge heterogeneity in the alkaline range. J Chromatogr A 1319:65–71
Lu F, Toh PC, Burnett I, Li F, Li J (2013) Automated dynamic fed-batch process and media optimization for high productivity cell culture process development. Biotechnol Bioeng 110:191–205
Luo J, Zhang J, Ren D, Tsai W-L, Li F, Amanullah A, Hudson T (2012) Probing of C-terminal lysine variation in a recombinant monoclonal antibody production using Chinese hamster ovary cells with chemically defined media. Biotechnol Bioeng 109:2306–2315
Mueller MM (2017) Post-translational modifications of protein backbones: Unique functions, mechanisms and challenges. Biochemistry 57:177–185
Nasiri H, Valedkarimi Z, Aghebati-Maleki L, Majidi J (2018) Antibody-drug conjugates: promising and efficient tools for targeted cancer therapy. J Cell Physiol 9:6441–6457
Oguchi S, Saito H, Tsukahara M, Tsumura H (2006) pH Condition in temperature shift cultivation enhances cell longevity and specific hMab productivity in CHO culture. Cytotechnology 52:199–207
Pan X, Streefland M, Dalm C, Wijffels RH, Martens DE (2016) Selection of chemically defined media for CHO cell fed-batch culture processes. Cytotechnology 69:39–56
Prabhu A, Gadgil M (2019) Nickel and cobalt affect galactosylation of recombinant IgG expressed in CHO cells. Biometals 32:11–19
Radhakrishnan D, Robinson AS, Ogunnaike BA (2018) Controlling the glycosylation profile in mAbs using time-dependent media supplementation. Antibodies 7:1
Rea JC, Moreno GT, Lou Y, Farnan D (2011) Validation of a pH gradient-based ion-exchange chromatography method for high-resolution monoclonal antibody charge variant separations. J Pharm Biomed Anal 54:317–323
Read EK, Park JT, Brorson KA (2011) Industry and regulatory experience of the glycosylation of monoclonal antibodies. Biotechnol Appl Biochem 58:213–219
Reuveny S, Velez D, Macmillan JD, Miller L (1986) Factors affecting cell growth and monoclonal antibody production in stirred reactors. J Immunol Methods 86:53–59
Rouiller Y, Périlleux A, Vesin M-N, Stettler M, Jordan M, Broly H (2014) Modulation of mAb quality attributes using microliter scale fed-batch cultures. Biotechnol Prog 30:571–583
Schatz SM, Kerschbaumer RJ, Gerstenbauer G, Kral M, Dorner F, Scheiflinger F (2003) Higher expression of fab antibody fragments in a CHO cell line at reduced temperature. Biotechnol Bioeng 84:433–438
Sengupta N, Rose ST, Morgan JA (2011) Metabolic flux analysis of CHO cell metabolism in the late non-growth phase. Biotechnol Bioeng 108:82–92
Seo JS et al (2016) Characteristics of human cell line, F2N78, for the production of recombinant antibody in fed-batch and perfusion cultures. J Biosci Bioeng 121:317–324
Shukla AA, Thömmes J (2010) Recent advances in large-scale production of monoclonal antibodies and related proteins. Trends Biotechnol 28:253–261
Si NS, Sellick C, Lee K, Mason A, Kontoravdi C (2014) How does mild hypothermia affect monoclonal antibody glycosylation? Biotechnol Bioeng 112:1165–1176
Silva Ad et al (2014) Target-directed development and preclinical characterization of the proposed biosimilar rituximab GP2013. Leuk Lymphoma 55:1609–1617
Singh N, Arunkumar A, Chollangi S, Tan ZG, Borys M, Li ZJ (2016) Clarification technologies for monoclonal antibody manufacturing processes: Current state and future perspectives. Biotechnol Bioeng 113:698–716
Sureshkumar GK, Mutharasan R (1991) The influence of temperature on a mouse–mouse hybridoma growth and monoclonal antibody production. Biotechnol Bioeng 37:292–295
Teo PZ, Utz PJ, Mollick JA (2012) Using the allergic immune system to target cancer: activity of IgE antibodies specific for human CD20 and MUC1. Cancer Immunol Immunother 61:2295–2309
Van Beers MMC, Bardor M (2012) Minimizing immunogenicity of biopharmaceuticals by controlling critical quality attributes of proteins. Biotechnol J 7:1473–1484
Vázquez-Rey M, Lang DA (2011) Aggregates in monoclonal antibody manufacturing processes. Biotechnol Bioeng 108:1494–1508
Walsh G (2010) Post-translational modification of protein biopharmaceuticals. Drug Discov Today 15:773–780
Wei W, Singh S, Zeng DL, King K, Nema S (2006) Antibody structure, instability, and formulation. J Pharm Sci 96:1–26
Yoon SK, Song JY, Lee GM (2003) Effect of low culture temperature on specific productivity, transcription level, and heterogeneity of erythropoietin in Chinese hamster ovary cells. Biotechnol Bioeng 82:289–298
Yoshimoto N, Itoh T, Inaba Y, Ishii H, Yamamoto K (2013) Structural basis for inhibition of carboxypeptidase B by selenium-containing inhibitor: selenium coordinates to zinc in enzyme. J Med Chem 56:7527–7535
Zheng K, Yarmarkovich M, Bantog C, Bayer R, Patapoff TW (2014) Influence of glycosylation pattern on the molecular properties of monoclonal antibodies. MAbs 6:649–658
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest in this work.
Ethics approval
Gibco Freedom CHO-DG44 cell and freedom pCHO1.0 mammalian cell expression vector in this paper were purchased commercially from ThermoFisher scientific and institutional approval was not needed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Weng, Z., Jin, J., Shao, C. et al. Reduction of charge variants by CHO cell culture process optimization. Cytotechnology 72, 259–269 (2020). https://doi.org/10.1007/s10616-020-00375-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-020-00375-x