Abstract
Telomerase plays a pivotal role in cellular immortality and tumorigenesis. Its activity is normally not detectable in most somatic cells while it is reactivated in the vast majority of cancer cells. Therefore, inhibition of telomerase has been viewed as a promising anticancer approach due to its specificity for cancer cells. Studies so far have shown that telomerase inhibition can inhibit the proliferation of cancer cells or cause apoptosis while it has no effect on most normal cells. Strategies currently being applied to induce telomerase inhibition target virtually all of the major components of the ribonucleoprotein holoenzyme and related cell signal pathways that regulate its activity. These strategies include inhibition of telomerase through targeting at the telomerase reverse transcriptase (TERT) catalytic subunit, the telomerase RNA (TR) component, and associated proteins. Other strategies have been developed to target the proteins associated with telomerase at the telomeric ends of chromosomes such as tankyrase. The specific mechanisms that mediate those inhibition effects include small molecules, antisense RNA, and ribozymes. Although the beneficial evidence of telomerase inhibition is obvious, limitations of strategies remain to be resolved to increase the feasibility of clinical application. This analysis will summarize recent developments of strategies in telomerase inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In human cells, a structure referred to as the telomere has been identified to cap the terminal regions of chromosomes which can protect the ends of DNA strands from degradation and fusion. A telomere is composed of telomeric DNA sequence, which is characterized by hexameric 5′-TTAGGG-3′ tandem repeats in humans, a G-rich overhang in its 3′ end, and a multiprotein complex termed telosome or shelterin which can bind to telomeric DNA sequences. Proteins found in this structure include the POT1/TPP1 heterodimer (which can bind to the G-strand overhang), TRF1 and TRF2 (which can bind to double-stranded repeats through their Myb-domain), RAP1 (repressor-activator protein 1) and TIN2 (TRF1-interacting nuclear factor 2, which can bind to TRF1 and TRF2). TANK1 and TANK2 poly(ADP)-ribosylases (also known as tankyrases) have also been indicated to interact with TRF1. Among those proteins, TRF1 and TRF1-associated proteins can negatively regulate telomere length by controlling the access of telomerase to telomeres, while TRF2 and POT1 can protect telomeres from end-to-end chromosome fusions by interacting with DNA-damage signaling and repair factors.
Telomeric DNA sequence will lose up to 150 bp after each cell division due to the end replication problem of linear chromosomes. However, some cells have evolved the telomerase enzyme to overcome this problem. This enzyme can add hexameric 5′-TTAGGG-3′ nucleotide repeats to the 3′ end of telomeric DNA strands to compensate for loss of sequence. Telomerase is a ribonucleoprotein which includes three major components: the telomerase reverse transcriptase (TERT) protein subunit that catalyzes the enzymatic reaction of DNA synthesis, the telomerase RNA (TR) component that serves as a template for TERT, and a protein termed dyskerin which binds to hTR. Although other proteins have been reported to associate with the holoenzyme (such as pontin and reptin which can help the assembly of telomerase), these three components are essential for telomerase activity and telomere lengthening [1–3].
Importance of Telomerase in Cancer and Aging
Telomerase exhibits a high activity in germline and embryonic stem cells which can maintain their immortality, while its activity is low or absent in other stem cells and somatic cells [4]. For those somatic cells and stem cells, the length of their telomeres gradually becomes shorter and shorter with each cellular division. When some of the telomeres reach a certain length, DNA damage signals will be produced by those telomeres and the cells will then undergo growth arrest and cease to divide. This process is referred to as cell replicative senescence [5]. Some cells, such as those with mutations of the p53 and pRB tumor suppressor genes, will continue to proliferate until their telomeres reach a critical length. Eventually, end-to-end chromosome fusions and apoptosis of some of the cells may occur along with genomic instability in a process referred to as cell crisis. Thus, the length of telomeres in normal stem cells and somatic cells has been considered to be a molecular clock important in the aging process [5]. Very few cells (~1 in 107) may escape the crisis by expressing telomerase to maintain telomeres, and this will increase the likelihood of tumorigenesis. Most cancer cells express a high level of telomerase which can satisfy their need for unlimited proliferative capacity while a few type of cancer cells employ alternate lengthening (ALT) of telomeres by recombination to maintain their immortality [4].
Due to the discrepancy of telomerase activity between cancer cells and normal somatic cells, analysis of telomerase activity has been considered as a potential diagnostic marker of cancer. The increase in telomerase in cancer cells generally occurs very early during tumorigenesis and sensitive techniques such as the Telomere Repeat Amplification Protocol or TRAP assay can detect trace levels of this enzyme which has obvious diagnostic potential in cancer [6].
Potential of Telomerase Inhibition in Cancer Therapy
Telomerase has been regarded as one of the most promising targets in cancer treatment. Many factors contribute to this such as its high expression level in cancer cells. More importantly, however, cancer cells generally have rather shorter telomeres than normal cells and a more rapid cellular proliferation rate, which means telomerase inhibition strategies would have a greater and more significant impact on the survival of cancer cells while having minimal effects on normal somatic cells. Numerous studies so far have indicated that telomerase inhibition leads to cellular proliferation inhibition or apoptosis of cancer cells [7, 8]. Anticancer approaches to telomerase inhibition are varied and methods ranging from RNA interference of the TERT catalytic subunit to inhibition of the proteins associated with telomerase at the telomeres have proven to have efficacy in cancer therapy. Since the telomeres of the rare normal cells that express telomerase are longer than in most cancer cells and the level of telomerase activity is generally lower in normal telomerase-positive cells as compared to cancer cells, the risks associated with possible telomere shortening in normal cells due to off-target telomere shortening are thought to be relatively minimal. Therefore, the efficacy of telomerase inhibition in inducing loss of viability or apoptosis of cancer cells combined with the relative low risk of inhibition of telomerase to normal cells have moved telomerase research to the forefront of anticancer approaches.
Strategies of Telomerase Inhibition
Methods for Inhibiting the TERT Catalytic Subunit of Telomerase
The hTERT catalytic subunit is the key catalytic component that regulates activity of telomerase. Its expression is tightly controlled which involves a complex transcription network. It cannot usually be detected in normal somatic cells, while it overexpresses in most cancer cells, and its expression level correlates very well with the activity of telomerase. Thus, telomerase inhibition targeted at hTERT has been considered as an ideal direction for cancer treatment.
Synthetic nucleic acids have been investigated widely to clarify their potential in telomerase inhibition. The principle of the mechanism is based on the specificity of the synthetic nucleic acids to bind to the mRNA of hTERT and then degrade it. It includes two strategies which are antisense oligodeoxynucleotides (AS-ODNs) and small-interfering RNAs (siRNA) (Fig. 1). Single-stranded AS-ODNs function by interfering with translation of hTERT which can lead to degradation of hTERT mRNA via RNase H-mediated cleavage [9]. Treatment of human bladder cancer cells through AS-ODNs targeted to hTERT in vitro leads to inhibition of the proliferation of these cells [10, 11]. siRNA is based on the ability of short double-stranded RNA molecules to form the RNA-induced silencing complex (RISC) which can then hybridize with specific mRNA and cleave it, thereby silencing expression [12]. siRNA can be used to generate an RNA interference or RNAi response in cells of embryonic origin such as human embryonic kidney (HEK) cells which is a popular cell type used in cancer research. This technique is especially effective for short-term analyses of TERT knockdown because the dsRNA is degraded in the cells in the long-term [13]. RNAi of TERT has also been successful with the use of plasmid constructs that exogenously express short hairpin RNA sequences to the TERT transcript. This technique allows analysis of downstream effects of TERT, serves as an alternative approach to gene therapy using viral vectors and allows long-term and permanent gene knockdown [14]. Also effective for long-term knockdown of TERT is the use of retroviral vectors that express short hairpin RNA specific to a segment of the TERT transcript. This RNAi-based technique can provide effective knockdown of hTERT and involves incorporation of the anti-telomerase sequence into the host genome [15].
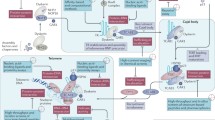
Telomerase reverse transcriptase (TERT) inhibition-based approaches are illustrated for their anticipated pharmaceutical potential. As illustrated, telomerase is a unique enzyme, mainly including TERT and the telomerase RNA (TR) component which serves as a template for reverse transcription. Three main strategies for TERT inhibition are RNAi-based TERT knockdown, small nucleosides, or non-nucleosides that inhibit the catalytic activity of TERT and immunotherapeutic approaches. The first approach, siRNA technique, has been applied in aimed gene silencing in vivo and in vitro by using sequence-specific short double-stranded RNA which can hybridize with specific mRNA and cleave it, thereby silencing its expression. The second approach is to repress TERT catalytic activities by introducing specific small nucleoside analogs or non-nucleosidic synthetic compounds, which target structural features of TERT. The third approach, telomerase immunotherapy, is designed to stimulate the patient’s immune system to attack and kill telomerase-positive tumor cells that express TERT. As indicated, the activated antigen-presenting cell elicits the expansion of memory TERT-specific T cells, which cooperate to kill tumor cells that display TERT peptides on their surface through the classical major histocompatibility complex (MHC) presentation
Nucleoside analogs can mediate telomerase inhibition by blocking the incorporation of dNTPs in telomerase’s reverse transcription (Fig. 1). For instance, AZT (3′-azido-2′,3′-dideoxythymine) can be effective in targeting the active site of TERT but this approach lacks the desired selectivity of many other approaches. Other examples include derivates of AZGTP (7-deaza-2′-deoxygunosine 5′-triphosphate) which may have a stronger inhibitory potential [16]. Small non-nucleosidic synthetic compounds can be quite effective in inhibiting the catalytic activity of the TERT protein component [17]. They can bind to the active site of telomerase and inhibit its function. One compound that has shown promise in this regard is BIBR1532 that inhibits the in vitro processivity of telomerase. The inhibition of TERT activity with BIBR1532 occurs in a dose-dependent manner and higher concentrations of this telomerase inhibitor can be cytotoxic to cancer cells of the hematopoietic system such as HL-60 cells while having little effect on normal cells.
Anticancer immuno-therapeutic approaches have also focused on TERT [18]. These methods involve the use of peptides derived from TERT. The peptides are presented by MHC class I alleles to T lymphocytes. The result is that CD8+ cytotoxic T lymphocytes specific for the TERT-derived antigenic peptides result in effective lysis of cancer cells that express TERT. These immunotherapy approaches directed against the TERT antigen can be carried out in the absence of toxicity and are showing great promise in anticancer research (Fig. 1).
It can be a challenge to identify small molecule compounds that affect the expression of TERT and the use of cell-based reporter systems for the analysis of TERT expression have been developed to enhance these endeavors [19]. For example, the hTERT promoter can be linked to two different reporter genes encoding green fluorescent protein (GFP) and secreted alkaline phosphatase (SEAP). The transfection of these reporter constructs result in stable clones that allow analysis of hTERT expression [19].
Inhibition of TERT is the goal of many anticancer approaches. Many of the most promising and effective methods for actively knocking down the TERT transcript have been developed, which include ablating its catalytic activity, directing the immune system to lyse telomerase-positive cancer cells, or using expression constructs to identify small molecule components that affect the expression of telomerase.
Telomerase RNA Inhibition as an Anticancer Approach
The RNA component of telomerase has also been a popular and effective mode of inhibiting telomerase activity to produce cytotoxicity of cancer cells. As in the case for hTERT transcript knockdown, antisense oligonucleotides against the human TR template can be employed to reduce or eliminate telomerase activity [20]. In this approach, a 2′,5′-oligoadenylate (2-5A) antisense system can be used as a mediator of interferon actions via RNase L activation. The result of this approach is that single-stranded templates, such as the TR component, are specifically cleaved. The anticancer utility of this approach has been proven not only in vitro, but also in vivo (Fig. 2).
Telomerase inhibition strategies aimed to the human telomerase RNA component (hTR) and telomerase-associated proteins. The hTR component inhibition involves three basic approaches, including antisense oligonucleotides, hammerhead ribozymes, and RNAi technique. These approaches can lead to hTR cleavage and are believed to be great promise in anti-telomerase approaches to cancer therapy. The strategy based on interfering target proteins that are associated with telomerase activity has attracted extensive interest for a new cancer therapeutic approach. Moreover, blocking signal pathways that could stimulate telomerase activity from transcription to post-translational modification can also obtain the goal of down-regulating the amount of functionally active telomerase in tumor cells
In addition to antisense sequences directed to the RNA component of telomerase, hammerhead ribozymes and RNAi can be used to inhibit the RNA of this ribonucleoprotein [21]. Both of these methods lead to degradation of the RNA component of telomerase. The effect is rapid cell growth inhibition both in vitro and in vivo independent of telomere length of the target cancer cell. The advantage of this technique is that it greatly reduces the lag period that is often encountered in approaches that are dependent upon the shortening of telomeres to result in cancer cell growth inhibition. Thus, methods to inhibit the RNA component of telomerase by using antisense oligonucleotides, hammerhead ribozymes or RNAi also show great promise in anti-telomerase approaches to cancer therapy (Fig. 2).
Targeting Proteins Associated with Telomerase Activity
More indirect approaches to telomerase inhibition have been developed that do not directly inhibit the TERT or TR components of telomerase, but rather, target proteins that are associated with telomerase activity. An example is tankyrase I, which is a telomeric poly(ADP-ribose) polymerase or PARP that can affect telomerase inhibition in cancer cells (Fig. 2) [22].
Signaling pathways such as those carried out by MAP kinase can result in stimulation of the hTERT gene. For example, Ets and AP-1 may play a role in MAP kinase signaling to the hTERT gene and inhibition of this pathway could be a novel approach to reducing TERT expression and telomerase activity (Fig. 2) [23]. It is apparent that many additional techniques will be developed to impact the proteins or pathways associated with telomerase activity in cancer cells.
Screening of Telomerase Inhibitors
Finding novel inhibitors of telomerase is an important aspect of increasing the tools that we have for anti-telomerase approaches to cancer therapeutics. A strategy for determining the therapeutic potential of telomerase inhibitors using a screening system in one cell type has been proposed [24]. For example, four completely different compounds, BRACO19, BIBR1532, 2′-O-methyl RNA, and peptide nucleic acids, were chosen for detailing the methods for screening of telomerase inhibitors. Additionally, TRAP assays or assessment of telomere lengths using Southern blot telomere restriction fragment analysis can be applied to determine the effectiveness of telomerase inhibition.
Telomerase Inhibition Combined with Other Chemotherapeutic Agents
Although a number of merits associated with telomerase-targeted treatment have been mentioned, there are concerns in using this strategy. For instance, the lag phase between the time when the telomerase of cancer cells has been inhibited and the shortening of the length of their telomeres may allow cancer cells to develop other mechanisms such as ALT to overcome telomere shortening caused by telomerase inhibition. Another concern is that long-term telomerase-targeted treatment may affect the function of those normal telomerase-positive cells. A solution to these problems is to combine telomerase inhibition with other chemotherapeutic reagents to enhance anticancer effects [25]. For instance, there is an indication that imatinib, a selective inhibitor of the BCR-ABL tyrosine kinase, can enhance the telomerase inhibition carried out using a dominant-negative form of human telomerase (DN-hTERT). In a completely different approach, telomestatin, a natural product and telomerase inhibitor isolated from Streptomyces anulatus, was combined with imatinib, daunorubicin, mitoxantrone, or vincristine and was shown to enhance the sensitivity of these chemotherapeutic agents [26]. Therefore, approaches to telomerase inhibition may also be merged with completely different anticancer approaches such as chemotherapeutic agents to render the most effective modes of cancer therapy.
Conclusion
Current strategies to inhibit proliferation of cancer cells targeted at telomerase have been reviewed which include inhibition of the TERT catalytic subunit of telomerase as well as the TR component of this ribonucleoprotein enzyme. Additional approaches involve intervention into the proteins that are associated with telomerase or pathways that modulate the TERT gene (Table 1). Methods for screening of telomerase inhibitors as well as the potential of merging telomerase inhibition with more conventional chemotherapy have also been reviewed. This last concept, that is, combination therapy, may be a promising approach in the future and it is likely that many new advances will develop that merge different types of anti-telomerase approaches or combine telomerase inhibition with other proven modes of anticancer therapy. However, there is still much progress needed for current research results to be incorporated into clinical treatment for cancer. Moreover, adverse effects of telomerase-targeted treatment on normal cells need to be evaluated urgently since this is actually the essence of this strategy.
References
Ishikawa, F. (1997). Regulation mechanisms of mammalian telomerase. A review. Biochemistry. Biokhimiia, 62, 1332–1337.
Weinrich, S., Pruzan, R., Ma, L., Ouellette, M., Tesmer, V., Holt, S., et al. (1997). Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTERT. Nature Genetics, 17, 498–502. doi:10.1038/ng1297-498.
Beattie, T., Zhou, W., Robinson, M., & Harrington, L. (1998). Reconstitution of human telomerase activity in vitro. Current Biology, 8, 177–180. doi:10.1016/S0960-9822(98)70067-3.
Shay, J., & Bacchetti, S. (1997). A survey of telomerase activity in human cancer. European Journal of Cancer, 33, 787–791. doi:10.1016/S0959-8049(97)00062-2.
Ahmed, A., & Tollefsbol, T. (2001). Telomeres and telomerase: Basic science implications for aging. Journal of the American Geriatrics Society, 49, 1105–1109. doi:10.1046/j.1532-5415.2001.49217.x.
Saldanha, S., Andrews, L., & Tollefsbol, T. (2003). Analysis of telomerase activity and detection of its catalytic subunit, hTERT. Analytical Biochemistry, 315, 1–21. doi:10.1016/S0003-2697(02)00663-2.
Hodes, R. (2001). Molecular targeting of cancer: Telomeres as targets. Proceedings of the National Academy of Sciences of the United States of America, 98, 7649–7651. doi:10.1073/pnas.151267698.
Ahmed, A., & Tollefsbol, T. (2003). Telomeres, telomerase, and telomerase inhibition: Clinical implications for cancer. Journal of the American Geriatrics Society, 51, 116–122. doi:10.1034/j.1601-5215.2002.51019.x.
Crooke, S. (1999). Molecular mechanisms of action of antisense drugs. Biochimica et Biophysica Acta, 1489, 31–44.
Kraemer, K., Fuessel, S., Schmidt, U., Kotzsch, M., Schwenzer, B., Wirth, M., et al. (2003). Antisense-mediated hTERT inhibition specifically reduces the growth of human bladder cancer cells. Clinical Cancer Research, 9, 3794–3800.
Kraemer, K., Fuessel, S., & Meye, A. (2007). Telomerase inhibition by synthetic nucleic acids and chemosensitization in human bladder cancer cell lines. Methods in Molecular Biology (Clifton, N.J.), 405, 9–22. doi:10.1007/978-1-60327-070-0_2.
Agrawal, N., Dasaradhi, P., Mohmmed, A., Malhotra, P., Bhatnagar, R., & Mukherjee, S. (2003). RNA interference: Biology, mechanism, and applications. Microbiology and Molecular Biology Reviews, 67, 657–685. doi:10.1128/MMBR.67.4.657-685.2003.
Lai, S., Andrews, L., & Tollefsbol, T. (2007). hTERT knockdown in human embryonic kidney cells using double-stranded RNA. Methods in Molecular Biology (Clifton, N.J.), 405, 23–29. doi:10.1007/978-1-60327-070-0_3.
Lai, S., Andrews, L., & Tollefsbol, T. (2007). RNA interference using a plasmid construct expressing short-hairpin RNA. Methods in Molecular Biology (Clifton, N.J.), 405, 31–37. doi:10.1007/978-1-60327-070-0_4.
Cunningham, A., Andrews, L., & Tollefsbol, T. (2007). Retrovirus-mediated RNA interference. Targeting hTERT through stable expression of short-hairpin RNA. Methods in Molecular Biology (Clifton, N.J.), 405, 39–46. doi:10.1007/978-1-60327-070-0_5.
Fletcher, T., Cathers, B., Ravikumar, K., Mamiya, B., & Kerwin, S. (2001). Inhibition of human telomerase by 7-deaza-2’-deoxyguanosine nucleoside triphosphate analogs: Potent inhibition by 6-thio-7-deaza-2’-deoxyguanosine 5’-triphosphate. Bioorganic Chemistry, 29, 36–55. doi:10.1006/bioo.2000.1194.
El Daly, H., & Martens, U. (2007). Telomerase inhibition and telomere targeting in hematopoietic cancer cell lines with small non-nucleosidic synthetic compounds (BIBR1532). Methods in Molecular Biology (Clifton, N.J.), 405, 47–60. doi:10.1007/978-1-60327-070-0_6.
Li, H., Katik, I., & Liu, J. (2007). Uses of telomerase peptides in anti-tumor immune therapy. Methods in Molecular Biology (Clifton, N.J.), 405, 61–86. doi:10.1007/978-1-60327-070-0_7.
Huang, Y., Shih, J., & Lin, J. (2007). Establishing cell-based reporter systems for the analysis of hTERT expression. Methods in Molecular Biology (Clifton, N.J.), 405, 87–96. doi:10.1007/978-1-60327-070-0_8.
Kondo, Y., & Kondo, S. (2007). Telomerase RNA inhibition using antisense oligonucleotide against human telomerase RNA linked to a 2’,5’-oligoadenylate. Methods in Molecular Biology (Clifton, N.J.), 405, 97–112. doi:10.1007/978-1-60327-070-0_9.
Li, S., Nosrati, M., & Kashani-Sabet, M. (2007). Knockdown of telomerase RNA using hammerhead ribozymes and RNA interference. Methods in Molecular Biology (Clifton, N.J.), 405, 113–131. doi:10.1007/978-1-60327-070-0_10.
Ohishi, T., Tsuruo, T., & Seimiya, H. (2007). Evaluation of tankyrase inhibition in whole cells. Methods in Molecular Biology (Clifton, N.J.), 405, 133–146. doi:10.1007/978-1-60327-070-0_11.
Xu, D., Li, H., & Liu, J. (2007). Inhibition of telomerase by targeting MAP kinase signaling. Methods in Molecular Biology (Clifton, N.J.), 405, 147–165. doi:10.1007/978-1-60327-070-0_12.
Kleideiter, E., Piotrowska, K., & Klotz, U. (2007). Screening of telomerase inhibitors. Methods in Molecular Biology (Clifton, N.J.), 405, 167–180. doi:10.1007/978-1-60327-070-0_13.
Tauchi, T., Ohyashiki, J., & Ohyashiki, K. (2007). Telomerase inhibition combined with other chemotherapeutic reagents to enhance anti-cancer effect. Methods in Molecular Biology (Clifton, N.J.), 405, 181–189. doi:10.1007/978-1-60327-070-0_14.
Tauchi, T., Shin-Ya, K., Sashida, G., Sumi, M., Nakajima, A., Shimamoto, T., et al. (2003). Activity of a novel G-quadruplex-interactive telomerase inhibitor, telomestatin (SOT-095), against human leukemia cells: Involvement of ATM-dependent DNA damage response pathways. Oncogene, 22, 5338–5347. doi:10.1038/sj.onc.1206833.
Acknowledgments
We apologize to those scientists whose work has not been cited in this article due to limited space. This work was supported by grants from the National Cancer Institute (R01 CA129415) and the Susan G. Komen for the Cure.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Li, Y. & Tollefsbol, T.O. Strategies Targeting Telomerase Inhibition. Mol Biotechnol 41, 194–199 (2009). https://doi.org/10.1007/s12033-008-9117-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-008-9117-9