Abstract
l-Asparaginase is an antileukemic drug long approved for clinical use to treat childhood acute lymphoblastic leukemia, the most common cancer in this population worldwide. However, the efficacy and its use as a drug have been subject to debate due to the variety of adverse effects that patients treated with it present, as well as the prompt elimination in plasma, the need for multiple administrations, and high rates of allergic reactions. For this reason, the search for new, less immunogenic variants has long been the subject of study. This review presents the main aspects of the l-asparaginase enzyme from a structural, pharmacological, and clinical point of view, from the perspective of its use in chemotherapy protocols in conjunction with other drugs in the different treatment phases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lymphoblastic leukemia ALL is the most common childhood cancer, with a high incidence in children aged 2 to 4 years [1]. It is defined as a hematological malignancy of the bone marrow, in which lymphoblasts blocked at a point of early cell differentiation, proliferate rapidly, accumulate and supplant hematopoietic cells; competition then arises between the rapidly growing immature cells and healthy cells, causing great damage to the body and giving rise to cytopenia, leukopenia, thrombocytopenia, anemia, fatigue, lethargy, bone and joint pain, respiratory problems and increased susceptibility of the body to infections [2, 3].
Asparaginase (ASNase) is an enzyme used to treat ALL. It is obtained from the culture of bacteria such as Escherichia coli and is a highly effective product in the treatment of these leukemias. It is administered intramuscularly or intravenously presenting half-life times that vary according to the formulation used in a treatment [4]. In ALL, malignant cells depend on an external source of asparagine, an essential nutrient for their survival. ASNase catalyzes the hydrolysis of asparagine (ASN) and degrades it until it is depleted in the blood, depriving the neoplastic cell of this amino acid, and causing its death [5, 6].
Some contraindications should be considered when using ASNase, such as hypersensitivity to the enzyme, skin rashes, mild allergic and anaphylactic reactions, pancreatitis, thrombosis, fever, hepatic insufficiency, hyperammonemia, coagulation abnormalities, as well as its administration generating increases in blood glucose and uric acid concentrations [4]. A hypersensitivity reaction is one of the most significant risks during the administration of this enzyme, being uncommon with the first application, but with increasing frequency with subsequent doses [7].
Despite the disadvantages of its use, l-asparaginase has been the treatment of choice for ALL for several decades, which indicates the importance of its study and the search for improvements in its formulations, some of which have been developed over time, since the incidence of side effects leads to the early withdrawal of the enzyme in a treatment protocol.
E. coli l-asparaginase characterization
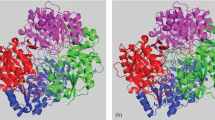
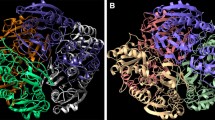
Escherichia coli l-asparaginase (EcA) was the first bacterial ASNase where the high-resolution crystallographic structure was determined55, asparaginase exists in active form as a well-organized tetramer and both E. coli and Erwinia sp. l-asparaginase have similar structures [8, 9].
However, the two enzymes have somewhat different properties; E. coli l-asparaginase (EcA) is an acidic protein with an isoelectric point (pI) of 5.0 while Erwinia chrysanthemi l-asparaginase (ErA) is an acidic protein [5]. E. coli ASNase is made up of four equal subunits or monomers A and C, D and B. Each EcA subunit has a pI of 8.7 C1377H2208N382O442S17 and molecular weight for each of 35.6 kDac [6, 10] according to data reported by X-ray crystallography X [11]. For ErA of 43KDa, each monomer in EcA contains 330 amino acids arranged in two domains (N- and C-terminal) of the class α/β. The N-terminal domain is made up of the residues 1–190 and binds to the C-terminal domain (residue 213–326) by a loop (waste 191–212). This enzyme presents an active site characterized by the presence of the residues Thr12, Tyr25,Thr89, Asp90, Lys162, where Tyr25,Thr12 and Thr89 are involved in their interaction with the substrate, so the homotetramers in the structure are best described as intimately related dimers characterized by an extensive interface between the subunits that are held together by various interactions, mainly van der Waals and electrostatic interactions [12, 13].
The pure enzyme has a specific activity of 280–400 IU/mg protein and an optimal pH range for its activity between 7.0 and 8.0, with an optimal temperature of 37 °C and KM value indicative of its Michaelis–Menten kinetic behavior and related to its affinity for the substrate as well as an effective antitumor activity [14], a good therapeutic l-asparaginase should have a low KM value and a high kcat value, which is sufficient to reduce endogenous asparagine levels from 40 to 80 µM to less than 0.2 µM ideally 65 according to current treatments on the market [15, 16].
Asparaginase can come from sources such as plants, bacteria and fungi [12], being the best bacterial source for obtaining it due to its ability to grow on simple substrates, the ease of optimizing culture conditions in the production of large quantities of the enzyme, the possibility of making genetic modifications to increase yields, the feasibility of its extraction and purification in a more economical way, and the possibility of using the enzyme in the production of large quantities [17].
Biological role and mode of action of asparaginase
Asparagine is an essential amino acid used by the leukemic cell for proliferation. The tumor cell shows a reduction in asparagine synthetase expression levels due to epigenetic regulations, such as hypermethylation of the CpG islets of the promoter, or by methylation and acetylation of histones [18] which prevents it from synthesizing ASN in the amount required for its growth and proliferation, which is why it seeks to incorporate this amino acid from blood plasma [19].
Normal cells perform ASN biosynthesis by employing transaminase that converts oxaloacetate to an aspartate intermediate, which then transfers an amino group from glutamate to oxaloacetate generating α-ketoglutarate and aspartate. Finally, in the healthy cell, aspartate is converted to ASN asparagine by the action of ASNs.21 For the metabolic functioning of leukemic cells, the supply of ASN is important and these cells require high demand for this amino acid, so that the growth of these cells is suppressed since they become dependent on an exogenous source of ASN because they do not have asparagine synthetase [20] to perform the biosynthesis of the same.
The antineoplastic activity of asparaginase is because it causes a reduction in the levels of asparagine, exerting its action continuously until the available reserves in the blood are exhausted, thus making it impossible to nourish the cell. This depletion has a negative impact [21], as asparagine is a crucial amino acid for DNA and RNA protein synthesis, and is a specific requirement for the G1 phase of cell division. It therefore suppresses the growth of malignant cells that are more dependent on an exogenous source of asparagine and glutamine than healthy cells and inducing apoptosis of the neoplastic cell population [22].
L-ASNase catalyzes the hydrolysis of asparagine, causing the formation of an unstable intermediate which, by hydrolysis, leads to the formation of aspartic acid and ammonia, so that the ASN available in the blood is degraded until its levels are exhausted [13, 23] (Fig. 1).
l-Asparaginase in the treatment of acute lymphoblastic leukemia
The treatment of ALL lasts several years and is divided into phases. The first phase, the induction phase, is the initial stage of treatment, with a duration of 4–6 weeks of therapy. The goal is to reduce the load of leukemic cells (blasts) in the bone marrow to normal levels according to the patient's age, eliminate them in the blood, achieve complete remission and restore hematopoiesis. Complete remission is the basis of treatment and a requirement for prolonged survival [24]; with the improvement of supportive care and chemotherapeutic agents, the rate of complete remission achieved is 96–99% [25].
At this stage, l-asparaginase is usually used in its 3 available preparations and in combination therapy with OV or IV chemotherapy drugs as appropriate such as vincristine, dexamethasone, prednisone or prednisolone, doxorubicin or daunorubicin. Some regimens may include cyclophosphamide and/or high doses of methotrexate or cytarabine as part of the induction phase, depending on the patient's prognostic factors. Leukemia usually goes into remission; however, additional treatment is provided to prevent leukemic cells from spreading to the central nervous system (CNS prophylaxis), which may include intrathecal or IV chemotherapy, radiation therapy to the brain and marrow. Treatment with imatinib (a tyrosine kinase inhibitor) and newer inhibitors, such as dasatinib or nilotinib, have also increased the remission rate in patients with Philadelphia chromosome-positive ALL [24].
The second and third phases are consolidation and reinduction, where intensive treatment is administered immediately after induction. If the leukemia goes into remission at this stage, the aim is to eradicate residual leukemic cells and reduce the risk of recurrence; the focus is on protecting the central nervous system. It can last several months and 6MP and methotrexate are administered [25]. Asparaginase administration is uninterrupted for 20–30 weeks and reinduction therapy employs drugs similar to those used during the previous stage.
Finally, during maintenance or continuation therapy as the final stage of treatment, patients with ALL require prolonged maintenance treatment for 2 years or more, with frequent re-evaluation for relapse, using weekly methotrexate and 6-mercaptopurine (6-MP) used daily [24].
The administration of l-asparaginase in conjunction with other drugs has been considered prominent for the treatment of this disease; however, its use causes adverse effects in the body [19]. For this reason, science has sought new forms of the enzyme for testing and implementation, it being important considered some aspects, such as their high affinity for L-ASN, low percentage of activity on L-glutamine, high stability, extended half-life in blood plasma and mainly anti-leukemic activity when a low dose is applied, as well as lower immunogenesis and a better toxicological profile.
Chemotherapy protocols that include asparaginase
There are a variety of drugs which have been used in combination with l-asparaginase in first and second-line protocols to treat ALL, their activities, adverse effects, indications, routes of administration are summarized [26,27,28] then in the next table (Table 1).
Therapeutic application of l-asparaginase
At present, the enzymes of E. coli and Erwinia chrysanthemi and their derivatives are the only preparations available for medical use [29]. For the treatment of acute lymphoblastic leukemia (ALL), FDA-approved ASNase formulations are used: native forms of the enzyme, obtained from Escherichia coli [30] (EcAII) Elspar® and Dickeya chrysanthemi [7] (ErAII) Erwinase®, a chemically modified form of the ASNase from E. coli native [31] (PEG-asparaginase) Oncaspar® (Pegaspargase®), which has been modified to covalently link it to molecules of monomethoxy polyethylene glycol, a recombinant form of ASNase from E.coli Spectrila® [32]. Asparaginases from Escherichia coli have also been known as Kidrolase®, Medac®, Crasnitin®, being part in its different formulations of the treatments in ALL [33]. The different formulations used share the same mode of action, but have different pharmacological properties (plasma half-life, dose periodicity and specific doses) preventing ASNases from being interchangeable at the same doses and administration frequencies [34].
In general terms, E. coli asparaginase is the first-line formulation in most protocols, but its availability varies from country to country; it has been considered that each dose of 10,000 UI/m2 of E. coli l-asparaginase should be replaced by 20,000–25,000 UI/m2 of Erwinia l-asparaginase. The replacement dose of Erwinia l-asparaginase in patients suffering from hypersensitivity to PEG-asparaginase would be 25,000 UI/m2 administered IV or IM (3 days every other day) for 2 weeks, for each dose of PEG-asparaginase. Regarding asparaginase from Erwinia chrysanthemi, it is a formulation indicated in Spain, as well as in many other countries, as second- or third-line treatment in cases of hypersensitivity to the derivative forms of E. coli.98 According to the protocols, in case of allergic reactions to E. coli the second-line formulation can be Erwinia asparaginase or PEG-asparaginase. In the latter case, Erwinia asparaginase can be used as a second-line formulation and Erwinia would go to third-line, as the half-life in this enzyme is shorter than those of E. coli. Therefore, higher, and more frequent doses are necessary to achieve a complete serum depletion of asparagine. The recommended doses are 20,000 IU/m2, 3 times/week [29]. As for the asparaginase from PEGylated E. coli, this is a modified form of the enzyme obtained from native E. coli, through a covalent conjugation with monomethoxy polyethylene glycol units (PEG). The commercial preparation, Oncaspar®, is available in most countries, although in the US it has been approved as a first-line treatment and in Europe as a second or third-line treatment in cases of hypersensitivity to native forms of E. coli. The objective of this formulation is to reduce immunogenicity, but also to reduce the frequency of administration, which includes the incidence of allergies and the development of antibodies, both of which are lower than with native E. coli asparaginase. The recommended doses are 1000–2500 IU/m2 each, or 2 weeks [21, 35].
Studies with native l-asparaginases of Erwinia and E. coli origin have been numerous and extensively discussed over the last 3 decades. However, no data have been presented for clinical trials of the enzyme from different sources such as fungi, yeasts, actinomycetes and plants as they have not been extensively characterized from these sources [14]. Given this and the association of adverse effects such as hypersensitivity in the native forms of asparaginase, modifications of the drug have been gaining importance in clinical applications; thus, the PEGylated enzyme is preferred over any of the available native preparations, and its administration has been found to be safe in patients mostly allergic to the l-asparaginases of asparagine. E. coli y Erwinia; is also known to be eliminated from plasma in a delayed mode, decreasing the frequency of medication [36].
Escalating doses in a phase I study, PEGylated asparaginase was administered to 31 adult patients by IV (doses between 500 and 8000 IU/m2) for 1 h at 2-week intervals before repeating the dose and 3/31 patients developed symptoms and anaphylactic reactions. Other important associated toxicities were hyperglycemia and dysfunction. As a result, of this study, no correlation was found between drug dose and toxicity; there were even responses in patients with ALL and lymphoma. These results provided the basis for further trials, using a similar dose range between 2000 and 2500 IU/m2 for clinical studies [37].
It is known that other investigators, using 500 IU/m2 of PEGylated asparaginase in children with relapsed ALL, achieved good plasma asparaginase activity, being efficient at depleting asparaginase to the required levels (≥ 0.1 IU/ml) in most patients [38].
Phase II trials have been conducted in patients with relapsed ALL, using doses of 2000 IU/m2 of PEGylated asparaginase once per week for 14 days, then therapy in conjunction with vincristine, prednisone, doxorubicin (40 mg/m2) and intrathecal dosing after 2 weeks. After this, 22% of patients achieved high remissions: at the end of the induction period 78% of patients were in complete or partial remission, with no anaphylactic reactions, mild allergic reactions, or pancreatitis and a low incidence of hyperglycemia [39].
Using PEGylated asparaginase in phase II every 15 days × 3 doses, achieving 80% favorable response to treatment in 7 patients with refractory ALL, of which 5/7 showed complete response and only 2 showed partial response. 2000 IU/m2 treatment was also used in 22 patients with recurrent ALL and in conjunction with methotrexate, vincristine, prednisone at day 1 and 14 of therapy, achieving complete responses of up to 93% in 14/22 of the patients [14].
Published studies on bio-improved asparaginase activity show that conjugation with oxidized inulin improves the thermal stability of the enzyme, resistance to trypsin digestion, longer half-life, and a wide range of optimum pH compared to native asparaginase. The decrease of antibodies (IgG) and immunogenicity is also reported (study in rabbits) in repeated doses [40].
In 33 previously treated patients (2009 and 2011) in an ALR3 trial (UKALL2003), PEGylated asparaginase was administered, finding that 21 responded favorably showing asparaginase activity (IM 1000 IU/m2; 200 IU/l), 1 showed antibodies against the PEGylated and native form, with no detectable activity to the PEGylated form, resulting in an adequate dose in the treatment of relapsed patients during the first-line protocol [41].
Comparative clinical trials between SC-PEG and SS-PEG (succinimidyl carbamate and succinate, respectively) in 167 pediatric patients with high-risk B-cell ALL (Pediatric Oncology Group), using SC 2500 IU/m2 and SS 2100 IU/m2 in a randomized fashion in identical therapy regimen, demonstrated longer activity for SC-PEG versus SS-PEG and similar toxicity profiles [42].
In adult patients treated for relapse of ALL, in combined treatment of PEGylated asparaginase with dexamethasone, vincristine and methotrexate, side effects including nausea, increased bilirubin and transaminases, hyperglycemia and peripheral neuropathy were observed and readjusted. The overall and complete remission rates were 28% and 39%, respectively [43].
There are reports of 615 patients evaluated between 2008 and 2011 with PEGylated asparaginase, to which they presented an allergic reaction when randomly receiving 8–15 doses of the enzyme (IM 1000 IU/m2) for 7.5 months and every 2 or 6 weeks (NOPHO ALL2008 protocol).7,9 discontinued treatment after the second dose; in 58% of patients the allergic reaction occurred 2 h after the dose with symptoms of mild systemic anaphylaxis; 9 had an anaphylactic reaction after 1 h of enzyme administration; 6% of patients subsequently treated with Erwinia also presented allergic reactions [44].
Monitoring asparaginase activity is very advantageous during the application of treatment protocols, so that dosage schedules can be optimized [45], as well as patients with silent inactivation [46] or pseudo-allergic reaction can be identified, correlating the levels of anti-asparaginase antibodies with the activity to adapt the treatment protocols to the patient and achieve better results [47].
In a study of patients treated with native asparaginase, randomized into 2 groups, customized doses, and fixed doses, respectively, of E. coli asparaginase were used, observing that the fixed dose group presented clinical hypersensitivity in some cases and the treatment was changed to another asparaginase. However, in the personalized dose group, silent inactivation were reported (it being necessary to change the enzyme), but they showed a superior event-free survival in the following 5 years (90%) compared to the fixed dose group (82%), which shows the advantage of having been able to determine the activity of the enzyme against silent inactivation [48].
The development of a longer-acting asparaginase (Irish pharmaceutical company license 2017) using PASylation® technology, based on incorporating proline, alanine and serine (PAS) polypeptides into the enzyme as these possess similar biophysical properties to PEGylated asparaginase under physiological conditions and chemically inert side chains [49].
Phase II/III clinical trials have with PEG crisantaspase to be used as a second-line drug (Asparec®) if hypersensitivity to the enzyme E. coli occurs, these studies were completed in 2015, using Intravenous (IV) Infusion in Patients with Relapsed or Refractory Hematological Malignancies, in addition, a pegylated biopharmaceutical, already approved by the FDA, was developed using succinimidyl carbamate (Calaspargase Pegol) [33, 50].
Limitations of l-asparaginase
The use of ASNase in the treatment of acute lymphoblastic leukemia causes asparagine depletion, which is also associated with a lower synthesis of proteins such as albumin, insulin and others involved in the process of coagulation and fibrinolysis, leading to thrombosis, pancreatitis, or hyperglycemia. In addition, due to its bacterial origin, it has the disadvantage of provoking immune reactions (hypersensitivity and formation of antibodies), as well as hyperammonemia, leukopenia, hepatic insufficiency, hemorrhages; these effects are also associated with the use of antitumor drugs used and with factors such as gender, age, body mass index and, in the case of adolescents the high-risk of neurotoxicity causing depression, fatigue, lethargy, dizziness [51].
Another limitation is that the patient's immune system may react against the drug in different ways, including suppression of the asparagine synthetase gene, production of specific antibodies against the drug, inactivation of caspase 3 or PARP (poly ADP-ribose polymerase), and production of glutamine in large amounts by adipocytes. In addition, the drug in its native form is rapidly eliminated from the blood serum (short half-life), so the patient needs 3 or more treatments every week, which requires frequent visits to the physician and therefore makes the overall treatment expensive [52].
Adverse effects
Pancreatitis is a common consequence among patients receiving asparaginase treatment, affecting about 17% of those involved and presenting with symptoms such as abdominal pain, anorexia, vomiting and vomiting [53]; this effect has a high incidence in patients over 4 years of age and with a median of 12 days after administration of asparaginase from E. coli [54]. The course of pancreatitis is also associated with the administration of prednisone, dexamethasone, daunomycin, and the relationship between the dose and the duration or type of asparaginase used for the occurrence of pancreatitis is not clearly known. Although most cases are acute, a large proportion of patients return to receive the enzyme at least 72 h after the onset of symptoms [55]. The evolution of pancreatitis in patients with leukemia and lymphoma treated with l-asparaginase could be influenced by their immunosuppression, frequent microbial translocation from the intestine, coagulation alterations and hyperlipidemia associated with chemotherapy combined with asparaginase [56]. It is recommended that asparaginase be reintroduced in patients who, within 48 h, have (1) no symptoms of acute pancreatitis, (2) normal amylase and lipase levels below three times the LNU, and (3) no pseudocysts or necrosis on imaging. If these patients experience a new episode of pancreatitis, treatment with l-asparaginase should be permanently discontinued [53].
Hypersensitivity is an adverse effect mainly associated with the use of ASNase from E. coli compared to PEGylated asparaginase and that from E. coli and Erwinia [57]. It occurs in about 50% of children and 15% of adults [58]. This leads to the substitution of the asparaginase formulation used, taking into account the half-life time as well as a better control of the drug exposure, since this side effect produces immune reactions that induce the formation of antibodies that inactivate the ASNase [59].
The manifestations are allergies that can be mild or severe, such as erythema at the injection site, urticaria, bronchospasm and even anaphylactic reactions in 20 to 40% of the cases, which poses a great risk to the patient's life since they usually occur silently with no manifestations of symptoms to warn about the ongoing reaction [60]. The option to continue with the therapy scheme is to change the ASNase to the PEGylated form, which is designed to mask the immunogenicity of asparaginase and act with a longer half-life in the bloodstream [61].
Hypersensitivity reactions have been associated with the generation of antibodies against bacterial proteins. Most of the episodes occur during the reinduction phases, being more frequent in consolidation and maintenance. The administration of the enzyme by IV route is also associated with a higher risk of hypersensitivity reactions [62].
Immune reactions to asparaginase classified as clinical or subclinical hypersensitivity (silent inactivation) have variable incidence rates. Clinical hypersensitivity to native E. coli asparaginase has been reported in up to 75% of patients with ALL [63] although rates generally range from 10 to 30% [63, 64] and the Clinical hypersensitivity reactions appear to be less frequent with PEGylated asparaginase, with rates of 3 to 24% reported in clinical trials [65]. Hypersensitivity reactions to the PEGylated form are more frequent when patients have been previously exposed to native E coli asparaginase due to their common bacterial origin [58].
Clinical hypersensitivity rates in patients receiving Erwinia asparaginase have been reported in 3–37% of patients in clinical trials [65, 66]. Patients developing hypersensitivity showed increased antibody formation and decreased levels of asparaginase activity compared to patients not developing hypersensitivity in the induction phase [64, 67, 68]. Clinical hypersensitivity (grades 1–4) has been reported in 20 of 89 patients (22%) administered PEG-asparaginase during the intensification phase after receiving native E. coli asparaginase during the induction phase of the Dutch Children's Oncology Group (DCOG) ALL-10 protocol [68].
The likelihood that asparaginase will elicit an immune response in patients may be influenced by several factors, including the asparaginase preparation, the intensity of treatment and the use of concomitant medications [69, 70]. The risk of antibody formation in patients increases with repeated exposure to asparaginase; the consolidation and reinduction phases show the highest incidence of hypersensitivity reactions and antibody formation [53, 71]. Prolonged exposure to asparaginase without treatment interruption, however, is associated with a decrease in antibody levels [70, 72] and consequently, hypersensitivity reactions are more frequent in the first doses of asparaginase after a treatment interruption [68]. Asparaginase frequently gives rise to antibodies that can inactivate it and these antibodies are associated with a decrease in its efficiency. Inactivation of the enzyme is reliably detected by measuring the enzyme activity of the formulation used and correlating it with the level of asparagine depletion; serum asparagine activity levels above 100 IU/l are considered to achieve asparagine depletion below quantification levels [34, 73].
Prospects for overcoming the disadvantages of enzymes
The use of genetic engineering techniques is an opportunity for the development of alternative strategies in the search for new modified asparaginases. These techniques have resulted in enzymes with improved kinetic properties, a wider range of pH and temperature for their activity, greater thermostability and specific activity, and greater resistance to proteolytic digestion [74], aiming to reduce the immunogenicity of the enzyme. This would avoid variation in its bioactivity and trying to prolong its half-life in the blood plasma to offer the patient less frequent applications in a treatment, fewer adverse effects, as well as the safety and effectiveness of the enzyme [8].
In this sense, strategies have been developed in the search for these characteristics in the enzyme; and to this end, chemical modification has been proposed by coating E. coli asparaginase with polyethylene glycol (PEG) chains, a water-soluble polymer approved for oral, topical and intravenous administration [75]. The use of soluble polymeric supports such as albumin, dextran, polyvinyl alcohol, and insoluble support matrices such as collagen, carboxymethylcellulose and polyacrylamide gels has also been suggested, with covalent coupling of l-asparaginase with PEG being the most common technique for enzyme modification. Although PEG-asparaginase is an effective alternative to E. coli and Erwinia [7].
It has limitations in terms of loss of activity and toxicity [74]. This form of asparaginase increases its stability under physiological conditions, so the strategy has improved the therapeutic efficiency of the enzyme, whether at multiple sites of the protein or at a selected amino acid residue; however, these aspects and the conformational restriction imposed by the PEG chains, as well as the production by the immune system of antibodies against the PEG formulations must be resolved [33].
Other more recent modification protocols have been applied by conjugating the enzyme with succinimidyl succinate derived from polyethylene glycol to avoid denaturation of asparaginase upon exposure to organic solvents and sonication [76]. On the other hand, the stability and activity of Cladosporium sp. asparaginase have been improved after chemical modification with ovalbumin and bovine serum albumin [77].
Other strategies adopted are those related to the induction of glycosylations (addition of sugars), as is the case of the study on the structure of Erwinia chrysanthemi asparaginase, where yeast strains called GlycoSwitch® have been used, which have been genetically modified for the production of recombinant proteins with a homogeneous pattern of N-glycosylation, representing an alternative for the recombinant production of l-asparaginase [78]. Glycosylation can improve pharmacokinetics, solubility, distribution, serum half-life, effector function and enzyme receptor binding; therefore, it can be used to reduce many of the side effects of treatment [79].
Has been investigated the pharmacological activity, immunogenicity, and anti-leukemic activity of a recombinant of Erwinia, (PEG-r-crisantaspase). It has been demonstrated that it maintains a complete depletion of asparaginase in the blood for 72 h, without detection of antibodies and inhibiting the proliferation of leukemic cells, so it could be a candidate in the treatment of ALL. However, its use in humans is currently under evaluation in phase I (NCT01251809), showing as first results that it is less immunogenic than Erwinase® and markedly increases half-life in plasma [33, 80].
Site-directed mutagenesis has taken advantage of the fact that charged amino acid residues on both the interior and surface of the enzymes contribute to biological activities and stability. Neutralization and charge reversal at critical positions of the enzyme optimizes the electrostatic surface by eliminating that unfavorable electrostatic interaction, thus conferring stability. Stability improvements have been made by altering the surface charges of E. coli l-asparaginase using this technique to replace destabilizing amino acids with stabilizing residues [81].
The most feasible engineering strategy can be predicted by bioinformatics tools for modeling and modifying asparaginase properties, such as a genetic algorithm, structure-based multiple sequence alignment, crystallographic structure analysis and molecular dynamics simulations, density functional theory (DFT), molecular docking, tetramer solvent accessibility and internal dynamics of the protein, kinetics of Asn and Gln catabolism, prediction of conformational stability, among others useful for an adequate and thorough study of the biopharmaceutical [82].In this sense, immunoinformatic analyses have been performed as a tool in the development and improvement of therapeutic proteins to clarify structural aspects and determine the immunogenicity of asparaginase from Escherichia coli and Erwinia carotovora. Regarding that, there are no significant differences in the level of immunogenicity between the two enzymes, while the asparaginase of E. coli asparaginase proves to be a major allergenic determinant. These results can be the basis for the design of asparaginase using bioengineering through the modification of immunogenic or allergenic epitopes in specific amino acids located in the enzyme structure [83].
In addition, immobilization (adsorption, covalent bonds and encapsulation) of asparaginase in nanostructured materials on different types of substrates and supports has been proposed as an alternative to protect it from the action of proteases and provide it with a longer catalytic half-life in vivo [84, 85].
The encapsulation of the enzyme in nanoparticles and liposomes has been described as an alternative; however, the development of nanotechnology-based ASNases is complex since the starting materials for the nanocarriers, such as polymers, lipids and surfactants, must be chosen considering their biodegradability and guaranteeing their subsequent elimination from the body as well as their sterilization and stability at low temperatures [86] in the case of the enzyme. In this sense, permeable polymersomes have been developed as ASNase nanobioreactors, allowing the depletion of L-asparagine without the enzyme being released from the nanostructures into the bloodstream, reducing its proteolytic degradation and the recognition of antibodies compared to free protein or PEG-conjugates [87].
In this regard, erythrocyte-encapsulated asparaginase has been developed (GRASPA® Erytech Pharma Lyon, France) as a circulating cell microbioreactor (MBC), which allows intracellular depletion of L-asparagine for a longer period than the native E. coli formulation using lower doses. This is a formula that contains ASNase encapsulated in red blood cells, with 150 IU/kg in each chemotherapy cycle, and it can be used without generating toxic products for a long period of 100–120 days, compared with PEG ASNase of 5 days or native ASNase for 26 h. This makes it possible to reduce the dosage of the enzyme and maintain its levels in the blood. Its use does not produce allergies, but it causes adverse effects such as anemia and thrombocytopenia. It is currently in phase II trials combined in low doses with cytarabine, and in patients older than 65 years (NCT01810705) with newly diagnosed acute myeloid leukemia AML not suitable for intensive chemotherapy and has only been compared with E. coli native asparaginase [33, 50].
In a GRASPALL 2005–01 study, applying three randomized controlled doses of GRASPA to evaluate the duration of phase I/II asparagine depletion in adults and children with first relapse acute lymphoblastic leukemia (ALL) 2006/2008, 18 patients received GRASPA (50 IU/kg: n = 6, 100 IU/kg: n = 6, 150 IU/kg: n = 6) after randomization, and six patients were assigned to treatment with native Escherichia coli. GRASPA was found to be effective in eliminating L-asparagine and a single dose of 150 IU/kg (NCT01518517) of GRASPA was observed to achieve [33] results similar to those obtained by IV administration of 8—10 000 IU/m2 of E. coli. The safety profile of GRASPA showed a reduction in the number and severity of allergic reactions and a tendency to reduce coagulation disorders. Other adverse effects were comparable to those observed with the native formulation and with no differences among the three doses of GRASPA used [50].
However, in June 2018, Erytech Pharma S.A. officially notified the Committee for Medicinal Products for Human Use (CHMP) of its desire to withdraw the marketing authorization application for GRASPA for the treatment of acute lymphoblastic leukemia (ALL). This formulation would be used to treat patients with a negative Philadelphia chromosome and whose response to initial treatment or if relapse occurred after treatment. The CHMP (Committee for Medicinal Products for Human Use) had considered with reservations that this formulation would not be approved due to the way in which the efficacy of the drug had been determined in the main study. Moreover, the company modified the way of producing the drug without demonstrating the effect on the efficiency of GRASPA, stating the data were not available to present to the CHMP, while Erytech Pharma reported at the time that the patients participating in clinical trials would have no consequences [88].
In the search for ASNase alternatives, it is possible to find versions of the enzyme with different pharmacological characteristics, potentially useful for the treatment of ALL and other lymphomas. One option is to include others microbial ASNases than E.coli and E. chrysanthemi and focus on the strategy with the best advantage such as PEGylation to generate improved forms with high affinity for asparagine (Asn) and low affinity for l-glutamine; with thermal and mechanical stability, better water solubility; considering the actions by the antigenic sites present on the enzyme surface, prevention of in vivo degradation by proteolytic enzymes, increase of the apparent size of the enzyme and its hydrodynamic volume (less renal filtration and longer half-life of the drug), improvement of the enzymatic activity affected by steric and conformational hindrance would be good strategies in search of decreasing the incidence of adverse side effects [74, 89].
Concluding Remarks
Asparaginase is a critical component of all pediatric ALL protocols and is increasingly used to treat patients with ALL despite the disadvantages of its use. This indicates the importance of its study and the search for improvements in its formulations, some of which have been sought to be developed over time; however, alternatives should continue to be investigated in pursuit of more favorable pharmacological and pharmacokinetic properties. Yet as with many protocols incorporating prolonged, high intensity asparaginase therapy, it is important for practitioners to be aware of all potential toxicities associated with treatment. Effective management of asparaginase toxicity will help ensure that patients receive the full course of asparaginase treatment and achieve optimal outcomes.
With the increasing demand for enzymes, methods such as genetic engineering and recombinant technologies should be exploited to produce l-asparaginase from microbes at a lower cost, with a high expression rate and alternative microbial sources to Escherichia coli and Erwinia chrysanthemi should be explored, which to date are the only sources used for medical purposes but cause side effects in their administration.
In conclusion, it is necessary to introduce new formulations resulting from studies and explorations in new sources as part of the search for better results. It can be perceived that is an effective candidate, with great potential in the treatment of malignant diseases of the lymphatic system, but it has parallel side effects that still need to be worked on and could be resolved, in our opinion, through a better characterization of the pharmacodynamics and pharmacokinetics, which would increase the efficacy of the drug. Thus, there is still much to be explored about this useful enzyme.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Lins MM, Santos MO, de Albuquerque MFPM, de Castro CCL, Mello MJG, de Camargo B. Incidence and survival of childhood leukemia in Recife, Brazil: A population-based analysis. Pediatr Blood Cancer. 2017;64:1–6. https://doi.org/10.1002/pbc.26391.
Katz AJ, Chia VM, Schoonen WM, Kelsh MA. Acute lymphoblastic leukemia: an assessment of international incidence, survival, and disease burden. Cancer Causes Control. 2015;26:1627–42. https://doi.org/10.1007/s10552-015-0657-6.
Onciu M. Acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23:655–74. https://doi.org/10.1016/j.hoc.2009.04.009.
McGraw-Hill Interamericana Editores SA de C V, editor. Vademecum Academico de Medicamentos. sexta edic. Mexico: McGraw-Hill Interamericana Editores SA; 2013.
Beckett A, Gervais D. What makes a good new therapeutic l-asparaginase? World J Microbiol Biotechnol. 2019;35:1–13. https://doi.org/10.1007/s11274-019-2731-9.
Müller HJ, Boos J. Use of l-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998;28:97–113. https://doi.org/10.1016/S1040-8428(98)00015-8.
Avramis VI, Tiwari PN. Asparaginase (native ASNase or pegylated ASNase) in the treatment of acute lymphoblastic leukemia. Int J Nanomed. 2006;1:241–54.
Batool T, Makky EA, Jalal M, Yusoff MM. A comprehensive review on l-asparaginase and its applications. Appl Biochem Biotechnol. 2016;178:900–23. https://doi.org/10.1007/s12010-015-1917-3.
Lubkowski J. Atomic resolution structure of Erwinia chrysanthemi l-asparaginase. Acta Crystallogr Sect D. 2003;59(1):84–92.
Ramya LN, Doble M, Rekha VPB, Pulicherla KK. l-Asparaginase as potent anti-leukemic agent and its significance of having reduced glutaminase side activity for better treatment of acute lymphoblastic leukaemia. Appl Biochem Biotechnol. 2012;167:2144–59. https://doi.org/10.1007/s12010-012-9755-z.
Swain AL, Jaskolski M, Housset D, Rao JKM, Wlodawer A. Crystal structure of Escherichia coli l-asparaginase, an enzyme used in cancer therapy. Proc Natl Acad Sci U S A. 1993;90:1474–8. https://doi.org/10.1073/pnas.90.4.1474.
Lopes AM, de Oliveira-Nascimento L, Ribeiro A, Tairum CA, Breyer CA, de Oliveira MA, et al. Therapeutic l-asparaginase: upstream, downstream and beyond. Crit Rev Biotechnol. 2017;37:82–99. https://doi.org/10.3109/07388551.2015.1120705.
Labrou NE, Papageorgiou AC, Avramis VI. Structure–function relationships asparaginases and clinical applications of l-asparaginases. Curr Med Chem. 2010;17(20):2183–95.
Narta UK, Kanwar SS, Azmi W. Pharmacological and clinical evaluation of l-asparaginase in the treatment of leukemia. Crit Rev Oncol Hematol. 2007;61:208–21. https://doi.org/10.1016/j.critrevonc.2006.07.009.
Vrooman LM, Kirov II, Dreyer ZE, Kelly M, Hijiya N, Brown P, Drachtman RA, Messinger YH, Ritchey AK, Hale GA, Maloney K, Lu Y, Plourde PV, Silverman LB. Activity and toxicity of intravenous Erwinia asparaginase following allergy to E. coli-derived asparaginase in children and adolescents with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2016;63:228–33. https://doi.org/10.1002/pbc.2757.
Ogawa C, Taguchi F, Goto H, Koh K, Tomizawa D, Ohara A, et al. Plasma asparaginase activity, asparagine concentration, and toxicity after administration of Erwinia asparaginase in children and young adults with acute lymphoblastic leukemia: Phase I/II clinical trial in Japan. Pediatr Blood Cancer. 2017;64:1–8. https://doi.org/10.1002/pbc.26475.
Thakur M, Lincoln L, Niyonzima FN, Sunil SM. Biotransformation isolation, purification and characterization of fungal. J Biocatal Biotransformation. 2014;2:1–9.
Peng H, Shen N, Qian L, Sun XL, Koduru P, Goodwin LO, et al. Hypermethylation of CpG islands in the mouse asparagine synthetase gene: relationship to asparaginase sensitivity in lymphoma cells. Partial methylation in normal cells. Br J Cancer. 2001;85:930–5. https://doi.org/10.1054/bjoc.2001.2000.
Shrivastava A, Khan AA, Khurshid M, Kalam MA, Jain SK, Singhal PK. Recent developments in l-asparaginase discovery and its potential as anticancer agent. Crit Rev Oncol Hematol. 2016;100:1–10. https://doi.org/10.1016/j.critrevonc.2015.01.002.
Lanvers-Kaminsky C. Asparaginase pharmacology: Challenges still to be faced. Cancer Chemother Pharmacol. 2017;79:439–50. https://doi.org/10.1007/s00280-016-3236-y.
Zeidan A, Wang ES, Wetzler M. Pegasparaginase: where do we stand? Drug Eval. 2009;91(9):111–9.
Kumar K, Kaur J, Walia S, Pathak T, Aggarwal D. L-asparaginase: an effective agent in the treatment of acute lymphoblastic leukemia. Leuk Lymphoma. 2013;55(2):256–62. https://doi.org/10.3109/10428194.2013.796047.
Costa-Silva TA, Costa IM, Biasoto HP, Lima GM, Silva C, Pessoa A, et al. Critical overview of the main features and techniques used for the evaluation of the clinical applicability of L-asparaginase as a biopharmaceutical to treat blood cancer. Blood Rev. 2020;43:100651. https://doi.org/10.1016/j.blre.2020.100651.
Atienza AL. Leucemias Leucemia linfoblástica. Pediatr Integr. 2016;6:380–9. https://doi.org/10.1017/cbo9781139107563.069.
Matloub Y, Stork L, Asselin B, Hunger SP, Borowitz M, Jones T, Bostrom B, Gastier-Foster JM, Heerema NA, Carroll A, Winick N, Carroll WL, Camitta B, Gaynon PS. Outcome of children with standard-risk T-lineage acute lymphoblastic leukemia—comparison. Pediatr Blood Cancer. 2016;63:255–61. https://doi.org/10.1002/pbc.
Benedí J, Ángeles Gómez del Río M. Fármacos antineoplásicos (I). Farm Salud 2006;20:60–4.
Tamayo-Chuc DU, Garza-González AG. Papel de CYP2B6 y ALDH1A1 en la resistencia farmacológica del meduloblastoma a ciclofosfamida. Gac Mex Oncol. 2015;14:46–52. https://doi.org/10.1016/j.gamo.2015.06.007.
Cuca L, Muñoz D. Compuestos citotóxicos de origen vegetal y su relación con proteínas inhibidoras de apoptosis (IAP). Rev Colomb Cancerol. 2016;20:124–34.
Guilleme CM, Delgado RF, Navarro JS. Actualización del tratamiento con L-asparraginasa en Pediatría. An Peditria 2013;79:329e1–11.
Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, et al. l-asparaginase treatment in acute lymphoblastic leukemia. Cancer. 2011;117:238–49. https://doi.org/10.1002/cncr.25489.
Ettinger AR. Pharmacology. J Pediatr Oncol 1995:46–8. https://doi.org/10.1177/104345429501200110.
Völler S, Pichlmeier U, Zens A, Hempel G. Pharmacokinetics of recombinant asparaginase in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2018;81:305–14. https://doi.org/10.1007/s00280-017-3492-5.
Thomas X, Le Jeune C. Erythrocyte encapsulated l -asparaginase (GRASPA) in acute leukemia. Int J Hematol Oncol. 2016;5:11–25. https://doi.org/10.2217/ijh-2016-0002.
Rizzari C, Conter V, Starý J, Colombini A, Moericke A, Schrappe M. Optimizing asparaginase therapy for acute lymphoblastic leukemia. Curr Opin Oncol. 2013;25:1–9. https://doi.org/10.1097/CCO.0b013e32835d7d85.
Garay RP, El-Gewely R, Armstrong JK, Garratty G, Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv. 2012;9:1319–23. https://doi.org/10.1517/17425247.2012.720969.
Kurtzberg J, Asselin B, Bernstein M, Buchanan GR, Pollock BH, Camitta BM. Polyethylene glycol-conjugated l-asparaginase versus native l-asparaginase in combination with standard agents for children with acute lymphoblastic leukemia in second bone marrow relapse: a children’s Oncology Group Study (POG 8866). J Pediatr Hematol Oncol. 2011;33:610–6. https://doi.org/10.1097/MPH.0b013e31822d4d4e.
Keating MJ, Holmes R, Lerner S, Ho DH. l-asparaginase and PEG asparaginase—past, present, and future. Leuk Lymphoma. 1993;10:153–7.
Vieira Pinheiro JP, Müller HJ, Schwabe D, Gunkel M, Casimiro Da Palma J, Henze G, et al. Drug monitoring of low-dose PEG-asparaginase (OncasparTM) in children with relapsed acute lymphoblastic leukaemia. Br J Haematol 2001;113:115–9. https://doi.org/10.1046/j.1365-2141.2001.02680.x.
Ettinger LJ, Kurtzberg J, Voǔte PA, Jürgens H, Halpern SL. An open-label, multicenter study of polyethylene glycol-l-asparaginase for the treatment of acute lymphoblastic leukemia. Cancer. 1995;75:1176–81. https://doi.org/10.1002/1097-0142(19950301)75:5%3c1176::AID-CNCR2820750519%3e3.0.CO;2-Y.
Brumano LP, Silva FVS, Costa-Silva TA, Apolinario AC, Santos JHP, Kleingesinds EK, Monteiro G, Rangel-Yagui CO, Benyahia B, Pessoa A Jr. Development of l-asparaginase biobetters: current research status and review of the desirable quality profiles. Bioeng Biotechnol. 2019;6:1–9. https://doi.org/10.3389/fbioe.2018.00212.
Masurekar A, Fong C, Hussein A, Revesz T, Hoogerbrugge PM, Love S, et al. The optimal use of PEG-asparaginase in relapsed ALL-Lessons from the ALLR3 clinical trial. Blood Cancer J. 2014;4:4–6. https://doi.org/10.1038/bcj.2014.26.
Angiolillo AL, Schore RJ, Devidas M, Borowitz MJ, Carroll AJ, Gastier-Foster JM, et al. Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: results from children’s oncology group study AALL07P4. J Clin Oncol. 2014;32:3874–82. https://doi.org/10.1200/JCO.2014.55.5763.
Lehmann-horn K, Sagan SA, Bernard CCA, Sobel A, Zamvil SS, Wanna AGB, et al. This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please c. Laryngoscope 2014:2–31.
Henriksen LT, Harila-Saari A, et al. PEG-asparaginase allergy in children with acute lymphoblastic leukemia in the NOPHO ALL2008 protocol. Pediatr Blood Cancer. 2015;1:1–7. https://doi.org/10.1002/pbc.25319.
Pession A, Valsecchi MG, Masera G, Kamps WA, Magyarosy E, Rizzari C, et al. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:7161–7. https://doi.org/10.1200/JCO.2005.11.411.
Yen HJ, Chang WH, Liu HC, Yeh TC, Hung GY, Wu KH, et al. Outcomes following discontinuation of E. coli L-asparaginase upon severe allergic reactions in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2016;63:665–70. https://doi.org/10.1002/pbc.25869.
Willer A, Gerß J, König T, Franke D, Kühnel HJ, Henze G, et al. Anti-Escherichia coli asparaginase antibody levels determine the activity of second-line treatment with pegylated E. coli asparaginase: a retrospective analysis within the ALL-BFM trials. Blood. 2011;118:5774–82. https://doi.org/10.1182/blood-2011-07-367904.
Vrooman LM, Stevenson KE, Supko JG, O’Brien J, Dahlberg SE, Asselin BL, et al. Postinduction dexamethasone and individualized dosing of Escherichia coli l-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study—Dana-Farber Cancer Institute ALL. J Clin Oncol. 2013;31:1202–10. https://doi.org/10.1200/JCO.2012.43.2070.
Gebauer M, Skerra A. Prospects of PASylation® for the design of protein and peptide therapeutics with extended half-life and enhanced action. Bioorg Med Chem. 2018;26:2882–7. https://doi.org/10.1016/j.bmc.2017.09.016.
Halfon-Domenech C, Thomas X, Chabaud S, Baruchel A, Gueyffier F, Mazingue F, et al. L-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005–01 randomized trial. Br J Haematol. 2011;153:58–65. https://doi.org/10.1111/j.1365-2141.2011.08588.x.
El-Naggar NEA, El-Ewasy SM, El-Shweihy NM. Microbial L-asparaginase as a potential therapeutic agent for the treatment of acute lymphoblastic leukemia: the pros and cons. Int J Pharmacol. 2014;10:182–99. https://doi.org/10.3923/ijp.2014.182.199.
Ali U, Naveed M, Ullah A, Ali K, Shah SA, Fahad S, et al. L-asparaginase as a critical component to combat Acute Lymphoblastic Leukaemia (ALL): a novel approach to target ALL. Eur J Pharmacol. 2016;771:199–210. https://doi.org/10.1016/j.ejphar.2015.12.023.
Raja RA, Schmiegelow K, Frandsen TL. Asparaginase-associated pancreatitis in children. Br J Haematol. 2012;159:18–27. https://doi.org/10.1111/bjh.12016.
Knoderer HM, Robarge J. Predicting asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2007;49:634–9.
Flores-Calderón J, Exiga-Gonzaléz E, Morán-Villota S, Martín-Trejo J, Yamamoto-Nagano A. Acute pancreatitis in children with acute lymphoblastic leukemia treated with L-asparaginase. J Pediatr Hematol Oncol. 2009;31:790–3. https://doi.org/10.1097/MPH.0b013e3181b794e8.
Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262:751–64. https://doi.org/10.1148/radiol.11110947.
Avramis VI, Sencer S, Periclou AP, Sather H, Bostrom BC, Cohen LJ, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2002;99:1986–94. https://doi.org/10.1182/blood.V99.6.1986.
Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the children’s oncology group. Blood. 2008;111:2548–55. https://doi.org/10.1182/blood-2007-02-070342.
Wang B, Relling MV, Storm MC, Woo MH, Ribeiro R, Pui CH, et al. Evaluation of immunologic crossreaction of antiasparaginase antibodies in acute lymphoblastic leukemia (ALL) and lymphoma patients. Leukemia. 2003;17:1583–8. https://doi.org/10.1038/sj.leu.2403011.
Burke MJ. How to manage asparaginase hypersensitivity in acute lymphoblastic leukemia. Futur Oncol. 2014;10:2615–27. https://doi.org/10.2217/fon.14.138.
Pui CH, Liu Y, Relling MV. How to solve the problem of hypersensitivity to asparaginase? Pediatr Blood Cancer. 2018;65:19–20. https://doi.org/10.1002/pbc.26884.
Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol. 1993;11:1780–6. https://doi.org/10.1200/JCO.1993.11.9.1780.
Leukemia L, Wacker P, Land VJ, Camitta BM, Kurtzberg J, Pullen J, et al. Allergic reactions to E. coli l-asparaginase do not affect outcome in childhood B-precursor acute. J Pediatr Hematol Oncol. 2007;29:627–32.
Vrooman LMGS. Erwinia asparaginase after allergy to E. coli asparaginase in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2010;54:199–205. https://doi.org/10.1002/pbc.22225.
Raetz EA, Salzer WL. Tolerability and efficacy of l-asparaginase therapy in pediatric patients with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010;32:554–63.
Jazz Pharmaceuticals I. ERWINAZE [package insert]. Palo Alto, 2014.
Paul V. Plourde, Sima Jeha, Nobuko Hijiya, Frank G. Keller, Lewis B. Silverman, Susan R. Rheingold, ZoAnn E. Dreyer, Gary V. Dahl, Taheri Mercedes, Chinglin Lai, and Tim Corn M. Safety profile of asparaginase erwinia chrysanthemi in a large compassionate-use trial. Pediatr Blood Cancer 2014;61:1232–8. https://doi.org/10.1002/pbc.
Tong WH, Pieters R, Kaspers GJL, Te Loo DMWM, Bierings MB, Van Den Bos C, et al. A prospective study on drug monitoring of PEG asparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123:2026–33. https://doi.org/10.1182/blood-2013-10-534347.
Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95–01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. https://doi.org/10.1182/blood-2006-06-027714.
Avramis VI. Asparaginases: biochemical pharmacology and modes of drug resistance. Anticancer Res. 2012;32:2423–37.
Zalewska-Szewczyk B, et al. The anti-asparagines antibodies correlate with L-asparagines activity and may affect clinical outcome of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2007;48(5):931–6.
Kawahara Y, Morimoto A, Hayase T, Kashii Y, Fukuda T, Momoi MY. Monitoring of anti-l-asparaginase antibody and l-asparaginase activity levels in a pediatric patient with acute lymphoblastic leukemia and hypersensitivity to native Escherichia coli l-asparaginase during desensitization courses. J Pediatr Hematol Oncol. 2014;36:2013–5. https://doi.org/10.1097/MPH.0b013e3182986559.
Pieters R, Appel I, Kuehnel HJ, Tetzlaff-Fohr I, Pichlmeier U, Van Der Vaart I, et al. Pharmacokinetics, pharmacodynamics, efficacy, and safety of a new recombinant asparaginase preparation in children with previously untreated acute lymphoblastic leukemia: a randomized phase 2 clinical trial. Blood. 2008;112:4832–8. https://doi.org/10.1182/blood-2008-04-149443.
Krishnapura PR, Belur PD, Subramanya S. A critical review on properties and applications of microbial l-asparaginases. Crit Rev Microbiol. 2016;42:720–37. https://doi.org/10.3109/1040841X.2015.1022505.
D’souza AA, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13:1257–75. https://doi.org/10.1080/17425247.2016.1182485.
Suri Vasudev S, Ahmad S, Parveen R, Ahmad FJ, Anish CK, Ali M, et al. Formulation of PEG-ylated l-asparaginase loaded poly (lactide-co-glycolide) nanoparticles: Influence of PEGylation on enzyme loading, activity and in vitro release. Pharmazie. 2011;66:956–60. https://doi.org/10.1691/ph.2011.1058.
Kumar S, Venkata Dasu V, Pakshirajan K. Purification and characterization of glutaminase-free l-asparaginase from Pectobacterium carotovorum MTCC 1428. Bioresour Technol. 2011;102:2077–82. https://doi.org/10.1016/j.biortech.2010.07.114.
Effer B, Lima GM, Cabarca S, Pessoa A, Farías JG, Monteiro G. L-Asparaginase from E. chrysanthemi expressed in glycoswitch®: effect of His-Tag fusion on the extracellular expression. Prep Biochem Biotechnol 2019;49:679–85. https://doi.org/10.1080/10826068.2019.1599396.
Nadeem T, Khan MA, Ijaz B, Ahmed N, Rahman Zur, Latif MS, et al. Glycosylation of recombinant anticancer therapeutics in different expression systems with emerging technologies. Cancer Res 2018;78:2787–98. https://doi.org/10.1158/0008-5472.CAN-18-0032.
Chien WW, Allas S, Rachinel N, Sahakian P, Julien M, Le Beux C, et al. Pharmacology, immunogenicity, and efficacy of a novel pegylated recombinant Erwinia chrysanthemi-derived L-asparaginase. Invest New Drugs. 2014;32:795–805. https://doi.org/10.1007/s10637-014-0102-9.
Vidya J, Ushasree MV, Pandey A. Effect of surface charge alteration on stability of l-asparaginase II from Escherichia sp. Enzyme Microb Technol. 2014;56:15–9. https://doi.org/10.1016/j.enzmictec.2013.12.012.
Brumano LP, da Silva FVS, Costa-Silva TA, Apolinário AC, Santos JHPM, Kleingesinds EK, et al. Development of l-asparaginase biobetters: current research status and review of the desirable quality profiles. Front Bioeng Biotechnol. 2019;6:1–22. https://doi.org/10.3389/fbioe.2018.00212.
Belén LH, Lissabet JB, de Oliveira R-Y, Effer B, Monteiro G, Pessoa A, et al. A structural in silico analysis of the immunogenicity of l-asparaginase from Escherichia coli and Erwinia carotovora. Biologicals. 2019;59:47–55. https://doi.org/10.1016/j.biologicals.2019.03.003.
Datta S, Christena LR, Rajaram YRS. Enzyme immobilization: an overview on techniques and support materials. Biotechnology. 2013;3:1–9. https://doi.org/10.1007/s13205-012-0071-7.
Bosio VE, Islan GA, Martínez YN, Durán N, Castro GR. Nanodevices for the immobilization of therapeutic enzymes. Crit Rev Biotechnol. 2016;36:447–64. https://doi.org/10.3109/07388551.2014.990414.
Keck CM, Müller RH. Nanotoxicological classification system (NCS)—a guide for the risk-benefit assessment of nanoparticulate drug delivery systems. Eur J Pharm Biopharm. 2013;84:445–8. https://doi.org/10.1016/j.ejpb.2013.01.001.
Blackman LD, Varlas S, Arno MC, Houston ZH, Fletcher NL, Thurecht KJ, et al. Confinement of therapeutic enzymes in selectively permeable polymer vesicles by polymerization-induced self-assembly (PISA) reduces antibody binding and proteolytic susceptibility. ACS Cent Sci. 2018;4:718–23. https://doi.org/10.1021/acscentsci.8b00168.
European Medicines Agency. Graspa: Withdrawal of the marketing authorisation application. EMA/431413/2018; 2018.
Torres-Obreque K, Meneguetti GP, Custódio D, Monteiro G, Pessoa-Junior A, de Oliveira R-Y. Production of a novel N-terminal PEGylated crisantaspase. Biotechnol Appl Biochem. 2019;66:281–9. https://doi.org/10.1002/bab.1723.
Funding
This work was supported by the Agencia Nacional de Investigación y Desarrollo (ANID) Fellowship No. 21210710 and the National Council for Scientifc and Technological Development (CNPq/Brazil, Fellowship # 301832/2017–0).
Author information
Authors and Affiliations
Contributions
María Tosta and Lisandra Herrera had the idea for the article; María Tosta, Pablo Letelier and Yolana Calle performed the literature search; Lisandra Herrera drafted the article, and Adalberto Pessoa and Jorge Farías critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tosta Pérez, M., Herrera Belén, L., Letelier, P. et al. l-Asparaginase as the gold standard in the treatment of acute lymphoblastic leukemia: a comprehensive review. Med Oncol 40, 150 (2023). https://doi.org/10.1007/s12032-023-02014-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02014-9