Abstract—
Asparaginase is one of the most important chemotherapeutic agents against acute lymphoblastic leukemia, the most common form of blood cancer. To date, both asparaginases from E. coli and Dickeya dadantii (formerly known as Erwinia chrysanthemi), used in hematology, induce chemoresistance in cancer cells and side effects in the form of hypersensitivity of immune reactions. Leukemic cells may be resistant to asparaginase due to the increased activity of asparagine synthetase and other mechanisms associated with resistance to asparaginase. Therefore, the search for new sources of L-asparaginases with improved pharmacological properties remains a promising and prospective study. This article discusses the mechanisms of development of resistance and drug resistance to L-asparaginase, as well as possible ways to overcome them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Preparations with enzymatic activity have been used in clinical medicine for more than 50 years. Replacement therapy of pancreatic insufficiency, acceleration of wound healing or thrombolytic treatment are among the most successful applications of enzyme preparations. Enzymes that irreversibly destroy certain vital amino acids are being developed as anticancer therapeutics [1]. The first bacterial enzyme introduced into clinical practice was L-asparaginase (L-asparaginamidohydrolase (EC 3.5.1.1)) [2]. Currently, native L-asparaginase from Escherichia coli (EcA) and Dickeya dadantii (formerly known as Erwinia chrysanthemi, ErA), along with the PEGylated form of E. coli asparaginase, is being successfully used to treat patients with acute lymphoblastic leukemia [3–6]. Normal and tumor cells require L‑asparagine to meet their metabolic needs. Normal cells can synthesize L-asparagine for their growth using asparagine synthetase, while tumor cells lack the ability to synthesize asparagine due to the absence or insufficient expression of this gene, and therefore depend on the exogenous supply of this amino acid from the bloodstream [7]. The antitumor effect of L‑asparaginase is based on its ability to hydrolyze L‑asparagine to L-aspartate and ammonia. The effect of L-asparaginase on tumor cells, mainly leukemic cells, leads to disruption of protein synthesis and starvation of cancer cells, causing their death [8]. L-asparaginases have been identified in mammals, birds, plants, fungi and a wide range of bacteria [9, 10]. To date, dozens of microbial sources of L-asparginases have been identified, although not all have demonstrated cytotoxicity to leukemic cells or tumor-inhibiting effects [1, 10].
Historically, L-asparaginases have been classified into three families: plant type, Rhizobium etili type, and bacterial type. Bacterial L-asparaginases, in turn, can be divided into two types depending on inducibility, cellular localization, substrate affinity, and quaternary structure [11]. L-asparaginase type I—constitutively expressed enzymes localized in the cytoplasm; they have a relatively low affinity for L-asparagine. Among the most studied type I enzymes are L-asparaginases Bacillus subtilis [12], Pyrococcus horikoshii [13] and Acinetobacter soli [14], which showed a relatively low affinity for L-asparagine resulting in no potential therapeutic application. Type II bacterial L‑asparaginases are periplasmic enzymes with induced expression during anaerobiosis that have high affinity for L-asparagine and broad substrate specificity, resulting in potent antitumor activity [15].
The therapeutic use of L-asparaginases is limited by many side effects: hepato- and nephrotoxicity, dysfunctions of the central nervous system, pancreatitis, thromboembolism, mucositis, hyperglycemia and dyslipidemia [16–18]. In addition, the genotoxic activity of L-asparaginase produced by Streptomyces ansochromogenes [19]. These side effects are thought to be due to non-specific effects of these enzymes. In addition to the well-studied antiproliferative effects of L-asparginases, which are caused by a deficiency of L‑asparagine in the environment of tumor cells, several alternative mechanisms have also been proposed. According to the first of them, as a result of the degradation of alternative enzyme substrates such as L-glutamine, D-asparagine, succinic acid monoamide and asparaginyl-tRNA [20, 21] antiproliferative or side effects may occur. According to the second, L-asparaginase from E. coli can release carbohydrates from the α2-HS-glycoprotein fetuin, and hydrolysis of cell membrane glycoproteins and inhibition of their synthesis by the enzyme can lead to cell lysis [22]. According to the third, this enzyme can also inhibit glycoprotein biosynthesis and lead to an increase in membrane permeability due to a specific effect on the concanavalin A receptor [23]. An unexpected cytotoxic asparagine-independent mechanism has been described for the mutant L-asparaginase of Rhodospirillum rubrum (RrA). RrA has demonstrated regulatory ability and downregulated telomerase activity in several human cancer cell lines, normal activated CD4+ T lymphocytes, and human solid tumor xenografts [24–26]. These observations point to the existence of multiple mechanisms of action of L-asparaginases on tumor cells, which can also affect normal cells, thereby causing a variety of side effects and the development of resistance. In this review, we reviewed the main mechanisms for the development of drug resistance in the application of L-asparaginases and the results of work to find ways to overcome it.
1 CHARACTERIZATION OF L-ASPARAGINASES USED IN CLINICAL PRACTICE
The study of the antitumor properties of L-asparaginases began in the 1950s with the detection of a reduction in lymphoma in mice that were injected with serum from the blood of guinea pigs [27, 28]. J. Broome suggested that L-asparaginase, found in the blood serum of guinea pigs, is effective against tumor cells of lymphoid tissue [29]. Over the years, about five hundred different L-asparaginases have been isolated and described from plants, terrestrial and marine microorganisms [30], of which bacterial L-asparaginases EcA and ErA had the highest antitumor activity [31]. These two enzymes, which are 77% identical in amino acid composition, exhibit similar properties (Table 1).
Both enzymes belong to class II L-asparaginases and function as homotetramers with a molecular weight of about 140 kDa. Every 2 monomers are combined into dimers, forming a tetramer. There are 4 catalytic sites between the N- and C-terminal domains (Fig. 1). Only the tetramer has enzymatic activity [33].
Homotetramers of L-asparaginases (a) EcA and (b) ErA, showing the identity of their quaternary structures. Each monomer is highlighted with an individual color. The L-aspragine substrate molecule at the active site of each monomer is shown in yellow. Protein Data Bank data source: for EcA 5f52; for ErA 6v5f. The structures were visualized using the PyMOL program (Schrödinger Inc., USA).
The known mechanism of the catalytic reaction consists in the interaction of nucleophilic threonine located near the active site of the enzyme with the carbonyl group of asparagine to form an acyl enzyme. In this case, an ammonia molecule is split off from the substrate, and the acyl enzyme reacts with a water molecule to form aspartate and a free enzyme (Fig. 2) [33].
Mechanism of the reaction in catalytic triads based on the enzyme EcA. Triad I acylates the substrate (L-asparagine) to form a β-aspartyl enzyme intermediate. Triad II deacylates the intermediate in the presence of a water molecule, releasing L‑aspartic acid and ammonia as products. In the first reaction, the electron density migrates from Glu283 to Tyr25 oxygen and then to Thr 12 oxygen. A nucleophilic attack occurs, leading to the release of ammonia and the formation of an ether. In the second reaction, due to the presence of a charge on Asp90, an ionic bond is formed with the amino group Lys162, which leads to the removal of a proton from Thr 89, followed by a nucleophilic attack of a water molecule on the ester carbon. Thus, a deacetylation reaction occurs.
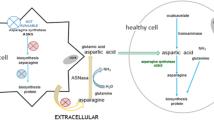
The antitumor effect of these enzymes is to reduce the concentration of L-asparagine in the blood, which is necessary for the synthesis of proteins (primarily membrane proteins) and nitrogenous bases of rapidly dividing tumor cells, and to ensure a normal cell cycle. [7]. The rapid proliferation of tumor cells leads to a deficiency of L-asparagine in them, and the introduction of L-asparaginase causes depletion of extracellular L-asparagine. In leukemia, lymphoblasts are not able to produce asparagine to compensate for the deficiency of extracellular asparagine (due to the extremely low activity of the asparagine synthetase enzyme), therefore, the cell cycle stops in the postmitotic G1 phase and subsequent death along the apoptosis pathway (Fig. 3) [34, 35].
Mechanisms of antitumor effects of L-asparaginase. Cleavage of asparagine induces the expression of the asparagine synthetase gene, which leads to a decrease in asparagine deficiency and can cause resistance to L-asparaginase. Upon hydrolysis of asparagine (and/or glutamine), cells activate GCN2 kinase, which phosphorylates the α-subunit of the eukaryotic translation initiation factor 2. This phosphorylation reduces the rate of protein synthesis and results in energy savings needed for cell survival. Overall protein synthesis is reduced, but preferential translation of mRNA of pro-apoptotic proteins occurs. One of them, transcription activating factor 4 (ATF4), can result in both the expression of the asparagine synthetase gene, which promotes cell survival, but can also induce the expression of proapoptotic factors. L-asparaginase affects the mTOR signaling pathway, which controls cell growth and division in response to environmental conditions. Under the action of L-asparaginase, it leads to inhibition of protein synthesis.
L-asparaginases EcA and ErA are the key drug in all protocols for the treatment of such oncological diseases as: lymphoid leukemia, which is divided into T- and B-, with further subdivision into acute (ALL) and chronic (CLL), chronic myeloid leukemia (CML), non-Hodgkin lymphoma, NK/T-lymphoma, glioblastoma, reticuloblastoma, and some solid tumors [10, 36]. Currently, 3 drugs are used in the clinic: a native enzyme from EcA (under the trade names Elspar, Leinase, Kidrolase, Krasnitin, asparaginase Medak and VeroAsparginase), a PEGylated form of this enzyme PEG-L-asparaginase (a conjugate of a native enzyme covalently bound to PEG in places that do not affect the enzymatic activity, Oncaspar) and the ErA enzyme (Ervinase). The efficacy of various asparaginase-based regimens has been shown in studies such as SMILE [37], GELOX [38], AspaMetDex [39] and DDGP [40].
The above drugs proved to be most effective in the treatment of ALL in children. Remission in them reaches 40−60% of cases with single drug therapy, and up to 90−95% with the additional use of the cytostatic vincristine in combination with prednisolone [41]. However, L-asparaginase preparations are of limited use because they cause severe adverse reactions: disseminated vascular coagulation syndrome (clot formation), severe hypertriglyceridemia (contributing to the development of atherosclerosis, pancreatitis and Alzheimer’s disease), acute hyperglycemia (diabetes mellitus), hypofibrinogenemia (hemorrhage), osteonecrosis (death of the bone marrow and bone structure) [42]. These reactions are due to the formation of antibodies to the enzyme due to its bacterial origin and large molecular weight. Antibodies produced by immune cells in response to the introduction of L‑asparaginases are divided into neutralizing and non-neutralizing. Neutralizing antibodies are able to bind to the active site of the therapeutic protein, inhibit the activity and reduce the effectiveness of the drug, necessitating higher and more frequent doses to achieve a clinical effect. Non-neutralizing antibodies do not bind to the active site, but are able to accelerate drug clearance by forming immune complexes with biotherapeutic drugs and removing them from circulation through the system of mononuclear phagocytes.
The study of the immunogenicity of native and PEGylated EcA and native Erwinia carotovora (EwA) asparaginase by the surface plasmon resonance method, which makes it possible to determine the kinetic, equilibrium and thermodynamic parameters of intermolecular interactions (including detection of antibody specificity), showed that 96.4% of the produced antibodies are neutralizing [43]. Frequent administration of EcA, which has the highest immunogenicity, can lead to the development of anaphylactic shock [18].
The development of resistance is also one of the factors limiting the use of this drug. The reasons for the resistance of tumor cells to chemotherapy may be the result of a number of processes: a decrease in the intracellular concentration of the antitumor drug due to active ATP-dependent excretion of the substance into the intercellular environment (such transport is carried out by the plasma membrane protein P-glycoprotein (Pgp), which is a product of the MDR1 gene); violation of apoptosis in the tumor cells themselves (mutation or deficiency of the p53 gene, overexpression of the Bcl-2 gene, making cells insensitive to proapoptotic stimuli); activation of detoxifying systems such as glutathione/glutathione-S transferases; sequestration of drugs into intracellular vesicles of the lysosome and endosome; change in the targets of topoisomerase II, which affects the topology of DNA; increased repair of drug-induced DNA damage; overexpression of multidrug resistance genes (MDR, MRP, BCRP, etc.); change in lipid metabolism incl. in the ceramide pathway (ceramides are a component of the cell membrane, and also serve as a signal molecule in the processes of proliferation, differentiation and apoptosis); inhibition of the capture of drugs when changing surface receptors and carriers; overexpression of target enzymes, such as thymidylate synthetase, which is a key enzyme that controls DNA replication (is a predictive factor in the treatment of solid tumors); chromosomal abnormalities in tumor cells leading to overexpression of antiapoptotic genes [44]. According to Rajic et al. [45], the risk of developing complications in treatment with L-asparaginase is of a genetic nature, at least in children whose entire exome sequencing revealed a polymorphism of the gene encoding the GRIA1 protein, a heteromeric protein complex of the glutamate receptor, which is an excitatory neurotransmitter in the mammalian brain.
2 METHODS FOR REDUCING THE IMMUNOGENICITY OF L-ASPARAGINASES
The immunogenicity of a protein can be reduced by altering the amino acid sequences recognized by B cell epitopes, or sequences that are associated with a major histocompatibility complex that elicits T cell dependent immune responses. The identification and removal of B cell epitopes is difficult due to their conformational nature and the lack of knowledge of the B cell antibody repertoire in people of various ethnic groups. Enzyme T cell epitopes are usually present in large numbers and their removal requires significant alteration of the sequence of the polypeptides. Developed by Cantor et al. [46] a neutral drift system using the IEDB epitope database (http:www.iedb.org.) made it possible to create an EcA mutant containing 8 amino acid substitutions, 3 of which are not found in any of the 500 bacterial-type asparaginases. This EcA mutant had low immunogenicity with preserved catalytic activity and stability. In works performed prior to the creation of the IEDB database, Moola et al. [47] determined that the main antigenic epitope of EwA is the sequence near the C-terminus—282GIV-PPDEELP287. They obtained a mutant form of the enzyme with an 8-fold reduced antigenicity, replacing Pro285 with Thr285. In M.N. Offman two EcA mutants resistant to degradation by protease B and human asparaginyl endopeptidase, an enzyme produced by leukemic blast cells, were obtained [48], and the replacement of three amino acids 195RKH197 with 195AAA197 made it possible to reduce the antigenicity of the enzyme by 5 times, which was proved by ELISA using polyclonal antibodies against wild-type asparaginase [49]. Sequence 254NLYKSVF260 identified in EcA structure causing activation of immune responses in ALL therapy [50]. In the work by Mechta and co-authors, significant amino acids in B-cell epitopes were determined by ELISA: Ser122, Tyr-176, Tyr181 and by site-directed mutagenesis, 10 times less immunogenic proteins were obtained, which were also able to reduce the transcription of asparagine synthetase [51].
The ErA enzyme has a lower ability to induce allergic and other adverse reactions and the immunogenicity of this enzyme is 5 times lower compared to EcA [52]. Also, PEGylated forms of EcA (1 day) and ErA (0.6 days) have a lower immunogenicity and a longer half-life [53]. The PEGylated form of ErA maintained complete depletion of plasma asparagine in mice for 72 hours using a 50-fold lower dose of the enzyme and did not cause the formation of specific antibodies [6].
Does not cause the production of cross-antibodies with EcA and ErA L-asparaginase Rodospyrillum rubrum (RrA) [54], consisting of 172 amino acid residues (18 kDa), having a low degree of homology with the above enzymes. RrA does not require cofactors for functioning and has low glutaminase activity (no more than 0.1% of asparaginase activity). By site-directed mutagenesis, RrA clones carrying D60K, F61L, R118H, and G120R substitutions were obtained, which showed an improvement in kinetic parameters and enzyme stability. E149R and V150P substitutions led to an increase in antitumor activity and a decrease in toxicity to normal cells [54, 55], and in vivo studies in mice with tumors have shown a doubling of life expectancy, and in 14% of mice a complete cure [56]. Asparaginase variants RrA E149R, V150P, and F151T were also found to enter breast cancer cells and normal lymphocyte cells [26, 57]. Penetration occurs with the help of the clathrin protein, which provides receptor-mediated endocytosis, that is, the selective uptake of substances by an animal cell, in which the macromolecule binds to the cell surface receptor and enters the cell in vesicles bordered by clathrin. In addition to asparaginase activity, the enzyme exhibits antibody activity inside cell nuclei, reducing the expression of the hTERT subunit of telomerase and inhibiting telomerase activity by 80%, and thereby blocking the division of the tumor cell. Therefore, RrA can potentially be used as an antitumor agent with a dual mechanism of action. The immunogenicity of RrA can also be reduced by PEGylation, as due to enlargement of the molecule, less antibodies are formed and the uptake of L-asparaginase by the monocyte-macophage cell system decreases [6, 53].
Chito-PEGylation was performed to stabilize RrA [58]. This process is based on the formation of enzyme conjugates with branched graft copolymers based on PEG-modified ionogenic chitosan. The physicochemical properties of the polymer can purposefully be varied over a wide range depending on the degree of polymerization of the chitosan polysaccharide and the degree of PEGylation. The polyelectrolyte nature of copolymers causes a multipoint electrostatic interaction with the protein surface, helping to stabilize the conformation of the enzyme. Conjugation of RrA with PEG-chitosan did not change the structure of the enzyme (the content of α- and β-helices remained the same) and increased the specific activity by almost 30%. The conjugates, due to their polycationic properties, contributed to the pH shift towards physiological values (from pH 9.2 to pH 7.5). Therefore, the chito-PEGylation method is promising for the development of highly effective drugs of L-asparaginases.
To reduce immunogenicity problems, attempts have been made, so far unsuccessful, to replace bacterial enzymes with human enzymes. Mammalian and, in particular, human asparaginases, designated hASNase 1 and hASNase 3, radically differ in structure from bacterial L-asparaginases. Thus, hASNase 1 (asparaginase-like protein, glial asparaginase) exhibits activity as L-asparaginase, as well as β-aspartyl peptidase. In addition, hASNase3 is the N-terminal domain of human lysophospholipase, which has properties similar to bacterial L-asparaginases [59]. A phospholipase with 3 enzymatic activities was isolated and characterized from the liver of rats: phospholipases, acetylhydrolases, and L-asparaginases. The N‑terminal domain of this enzyme possessed L‑asparaginase activity. The results of kinetic analysis, mutagenesis, structural modeling and fluorescent labeling indicate its homology with EcA type I. All obtained human asparaginases have very high (about 50 mM) Km values for asparagine and therefore cannot be used as therapeutic agents. Unlike human, guinea pig L‑asparaginase (gp ASNase 1) has a low Km for asparagine (57.7 μM), has no glutaminase activity, and is also 70% homologous to human hASNase 1. As a result of the humanization of gp ASNase 1 (replacement of the C-terminal domain of gpASNase 1 with the domain of hASNase 1), 2 clones were obtained, 100 and 140 times greater catalytic activity than hASNase 1. They had antiproliferative activity and had Km like gp ASNase 1, what is the basis for creating a fundamentally new drug for the clinic of leukemia [60].
3 FACTORS DETERMINING RESISTANCE AND HYPERSENSITIVITY TO L-ASPARAGINASES
Not all leukemic cells are sensitive to the action of L-asparaginase, and asparaginase resistance is an unfavorable prognostic factor. The first established mechanism for such resistance is the ability of tumor cells to express asparagine synthetase (ASNS). ASNS catalyses the synthesis of asparagine and glutamate from aspartate and glutamine in an ATP-dependent amidotransferase reaction [61]. In ALL cells, ASNS expression is absent, which is the rationale for L‑asparaginase therapy. However, in some cancers, ASNS is overexpressed, promoting cell proliferation, chemoresistance, and metastasis. Particularly high levels of expression were found in tumor cells of pancreatic, brain, thyroid, and testis cancers [62]. The exact role of ASNS in tumor growth modulation is unknown, but the expression of this gene is increased with the proliferation of solid tumors and the development of resistance to chemotherapy with L-asparaginase. A mechanism has been proposed according to which asparagine depletion increases the activity of GCN2 kinase, which phosphorylates the α-subunit of the translation initiation factor elf2α, which, in turn, reduces the rate of protein synthesis and, ultimately, promotes the survival of leukemic cells [63]. Simultaneously with the slowdown in protein synthesis, the translation of total mRNA, including the transcription factor ATF4, increases, the induction of which promotes tumor proliferation under nutrient restriction and induces the expression of ASNS as a key factor in tumor initiation and growth under conditions of a limited amount of amino acids. Normally, ASNS is required for normal brain development, and underexpression caused by 15 unique mutations in the gene leads to developmental delay in children, microcephaly, and progressive brain atrophy [63]. W. Liu et al. [64] studied the relationship between ASNS mRNA levels and response to L-asparagine in NK/T lymphoma cell lines. The authors were unable to draw a clear conclusion whether ASNS is an oncogene or an anti-oncogene. A. Aslanian et al. [65] demonstrated the resistance of MOLT-4 tumor cells to the action of L-asparaginase when ASNS was overexpressed. It was also found that increased ASNS activity was associated with a polymorphism of the main leucine-activating transcription factor ATF5 [66]. Measurement of blood ASNS levels can predict resistance to L-asparaginase therapy, but ASNS levels are important, but not the only factor in resistance of leukemic cells to the action of a therapeutic enzyme [67].
The use of genome-wide screening for ASNS knockout revealed that sensitization to L-asparaginase is mediated in mammals by WNT, a signaling system that regulates the development of malignant tumors. WNT-dependent protein stabilization depends on proteasomal degradation (catabolic source of asparagine) and glycogen synthase 3. Inhibition of this enzyme was found to increase sensitivity to L-asparaginase in resistant leukemia cells [68]. Therefore, ASNS inhibition may represent a promising strategy for the treatment of ASP-resistant leukemias, the so-called asparagine synthetase chemotherapy.
The first candidate for an inhibitor АSNS in human leukemia cell culture [69] adenylated sulfoximine, which inhibited АSNS в nanomolar concentrations. However, its ability to pass through cell membranes is limited by the presence of amine and carboxylate functional groups that recognize and bind aspartate. Study of the kinetic parameters of action ASNS suggests that β-aspartyladenylate analogs, sulfur-containing aspartate analogs, cysteine sulfonic acid, and N-acylsulfonamide may also be inhibitors of this enzyme [69].
In the development of resistance to the action of L‑asparaginase, glutamine synthetase plays a significant role, since L-asparaginase can also hydrolyze L‑glutamine to L-glutamic acid and ammonia. It was found that L-asparaginase-resistant cells produced more glutamine by increasing the activity of glutamine synthetase [70]. Indeed, the effectiveness of the therapeutic action of L-asparaginases depends on its ability to hydrolyze L-glutamine, which competes for the active center of the enzyme with L-asparagine. Glutamine is able to restore asparagine-deprived cells through a transamidation reaction, where glutamine is an amino group donor in the synthesis of asparagine with the participation of ASNS. L-glutamine deficiency inhibits tumor growth through inhibition of the mTOR pathway (in particular, protein kinase, which is part of signaling multimolecular complexes mTOR regulates cell growth and survival), which leads to inhibition of protein synthesis. But, having a sufficient amount of glutamine, ASNS increases the concentration of asparagine, which inhibits GSN2 (a protein kinase that phosphorylates the hydroxyl residues of serine and threonine) and prevents apoptosis [33].
From the above, it follows that for successful antileukemic activity of L-asparaginase, in addition to the depletion of asparagine, a decrease in glutamine levels is also necessary. Therefore, L-asparaginase ErA, which has a relatively high level of glutaminase activity, has become a therapeutic drug that gives fewer side effects compared to EcA drugs [71].
4 WAYS TO OVERCOME DRUG RESISTANCE TO ASPARAGINASES
The main factor limiting the use of asparaginases are hypersensitivity reactions that develop according to various estimates in 5–45% patients [72, 73]. To reduce immunogenicity and overcome drug resistance to asparaginases, several approaches have been proposed that are used to improve the pharmacological characteristics of L-asparaginase: the search for new natural and development of recombinant asparaginases with improved properties; enzyme immobilization on polymeric carriers; incorporation of asparaginase into artificial polymeric vesicles or erythrocytes.
The modern development of genetic engineering makes it possible to create recombinant enzymes that are encoded by the genes of some organisms in the cells of others, which makes it possible to obtain enzymes with the desired properties and simplify the technology of their production and purification. Currently in Protein Data Bank order data available 500 L-asparaginases of various origins, including genetically engineered ones. Theoretical studies and practical experience have made it possible to determine the most significant amino acids involved in the catalytic process and to obtain enzymes with improved properties. Many mutant forms of L-asparginases with improved pharmacological properties have been obtained compared to the original enzymes [74]. Recent progress in the development of programmable nucleases such as zinc finger nucleases (ZFNs), effector nucleases like transcription activators (TALENs), and clustered nucleases associated with short palindromic repeats (CRISPR)-Cas, related to Cas enabled gene editing to move from concept to clinical practice [75]. These methods have been shown to be effective in minimizing the immunogenicity of certain enzymes, improving their substrate specificity and stability. Actively developing approaches to protein engineering de novo с using deep learning neural networks such as trRosetta [76] or AlphaFold 2 [77], become attractive tools for designing proteins with desired properties. These methods can be used to create potent artificial L-asparaginases with reduced immunogenicity, low specificity for L-glutamine, and increased blood stability. An important criterion for the effectiveness of L-asparginases is the half-life of the enzyme, due to the activity of blood serine proteases such as blood coagulation factors II, VII, IX, X, XI, XII and the Fletcher factor. The resistance of the enzyme to proteases under experimental conditions is tested by its resistance to trypsin, since the selectivity of the action of these proteases is ensured by the specific structures of the N-terminal regions of the molecules and the amino acid sequence of the regions of blood coagulation factors is homologous to those of trypsin. Trypsin resistance (maintenance of 75% activity) showed a chimeric protein, EcA with protective single chain antibody [78].
In order to improve the pharmacological properties of L-asparginases, attempts have been made for the last 25 years to obtain immobilized enzymes. The term “immobilized enzymes” means that the enzyme is localized in a certain region of space with the preservation of its catalytic activity. Immobilized enzymes can be used continuously and repeatedly. However, the carrier may change such physico-chemical and pharmacological properties enzyme as: solubility, thermal and storage stability, pH and temperature optima, half-life, substrate affinity (Кm), half-life and cytotoxicity. The carrier also protects the enzyme from the action of proteases, blocks the sites that cause the production of antibodies, creates a shell that prevents the molecule from being accessible to proteins and cells of the immune system, but is permeable to the substrate [33]. The following approaches are used for immobilization: physical adsorption under the action of hydrogen, Coulomb and dispersion forces; covalent attachment, characterized by the establishment of an irreversible chemical bond between the functional groups of the enzyme and the carrier material of the nanoparticles; capture of the enzyme by the type of “lattice” i.e. capture of the enzyme by a water-insoluble polymer (most often polyacrylamide or polyvinyl alcohol); capture of the enzyme by the type of “microcapsules” i.e. the enzyme is surrounded by a semi-permeable polymeric membrane [71]. The main carriers used to modulate the functions of L-asparginase are biological and biogenic nanoparticles in the form of polyethylene glycol (PEG), polyglycolic and polylactic acids; liposomes (large, small and multilayer), dendrimers (polyamidoamine, polylysine), carbon nanotubes (fullerenes, graphene), metals (Au, Ag, Pt, Ti, Fe, SiO2) [79]. Table 2 presents the main polymer carriers of L-asparginases and their properties.
The results of numerous studies demonstrate the advantages of PEGylated L-asparaginase over the native enzyme, since PEGylation blocks potential immunogenic epitopes of the native enzyme molecule, thereby reducing the immune response and hypersensitivity reactions. PEGylation provides a delay in the excretion of the enzyme and prolongs the time of its circulation in the body up to 8 days [78]. The high efficacy of PEGylated EcA as a first-line chemotherapy drug was registered in the protocol of intensive care for ALL in children [80]. The drug also showed a reduced titer of antibodies to the enzyme, a longer half-life and remission in patients not only with ALL, but also with lymphoid tumors [81].
Encapsulation of the enzyme is used to overcome drug resistance. the inclusion of various microparticles inside, which makes it possible to protect the enzyme from active plasma proteases and from the immune system. This leads to the fact that practically no antibodies are formed against the injected foreign protein, which makes it possible to greatly lengthen the lifetime of the drug inside these particles (and, accordingly, in the bloodstream, if the particles themselves are sufficiently long-lived). In addition, the enzyme exhibits catalytic activity while inside the particles, so there are no high concentrations of free enzyme in the bloodstream, which cause many side effects. Various artificial polymeric and natural carriers are used as particles for incorporating L-asparaginase (Table 3).
Work is underway on the encapsulation of L-asparaginases in erythrocytes [82]. The advantages of erythrocytes as microvesicles for enzyme delivery are: ease of obtaining autologous cells from the blood, ideal biocompatibility, the ability to protect the enzyme from inactivation and immunological reactions of the body and, as a result, a longer (up to 3 months) duration of action [83]. Methods for incorporating L‑asparaginases into erythrocytes are: a method of reversible hypoosmotic effect on erythrocytes, when erythrocytes are incubated in a hypotonic medium in the presence of an enzyme, while the enzyme enters the cells, and then the tonicity of the medium is restored [82]; through disulfide bonding with arginine-rich low molecular weight protamine, which has a powerful penetrating ability, which allows you to maintain the morphological integrity of the erythrocyte and double the half-life [83]; immobilization of the enzyme on the surface of erythrocytes, which increases the pharmacodynamic effect by 10 times and reduces the antibody titer by 1000 times [84].
CONCLUSIONS
Analysis of the mechanisms of drug resistance to asparaginases and ways to overcome them allows us to draw the following conclusions.
(1) Mandatory monitoring of the level of asparagine in the blood of cancer patients is required to adjust the treatment.
(2) There is a cross-production of antibodies to all L-asparaginase preparations, the source of which is E. сoli.
(3) PEG-asparaginase allows to reduce the number of injections and reduce the formation of antibodies when prescribed in the induction of remission.
(4) In addition to the search for new asparaginases suitable for the treatment of cancer, methods are being developed to improve existing drugs. Such methods are: immobilization, encapsulation and incorporation of the enzyme into erythrocytes.
REFERENCES
Pokrovsky, V.S., Chepikova, O.E., Davydov, D.Z., Zamyatnin, A.A., Jr., Lukashev, A.N., and Lukashe-va, E.V., Curr. Med. Chem., 2019, vol. 26, pp. 446–464. https://doi.org/10.2174/0929867324666171006132729
Beckett, A. and Gervais, D., World J. Microbiol. Biotechnol., 2019, vol. 35, 152. https://doi.org/10.1007/s11274-019-2731-9
Dinndorf, P.A., Gootenberg, J., Cohen, M.H., Keegan, P., and Pazdur, R., Oncologist, 2007, vol. 12, pp. 991–998. https://doi.org/10.1634/theoncologist.12-8-991
Jaccard, A., Petit, B., Girault, S., Suarez, F., Gressin, R., Zini, J.-M., Coiteux, V., Larroche, C., Devidas, A., Thiéblemont, C., Gaulard, P., Marin, B., Gachard, N., Bordessoule, D., and Hermine, O., L-asparaginase-based treatment of 15 western patients with extranodal NK/T-cell lymphoma and leukemia and a review of the literature. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol., 2009, vol. 20, pp. 110–116. https://doi.org/10.1093/annonc/mdn542
Völler, S., Pichlmeier, U., Zens, A., and Hempel, G., Cancer Chemother. Pharmacol., 2018, vol. 81, pp. 305–314. https://doi.org/10.1007/s00280-017-3492-5
Chien, W.-W., Allas, S., Rachinel, N., Sahakian, P., Julien, M., Le Beux, C., Lacroix, C.-E., Abribat, T., and Salles, G., Invest. New Drugs, 2014, vol. 32, pp. 795–805. https://doi.org/10.1007/s10637-014-0102-9
Sharma, D., Singh, K., Singh, K., and Mishra, A., Curr. Protein Pept. Sci., 2018, vol. 20, pp. 452–464. https://doi.org/10.2174/1389203720666181114111035
Lubkowski, J., Vanegas, J., Chan, W.-K., Lorenzi, P.L., Weinstein, J.N., Sukharev, S., Fushman, D., Rempe, S., Anishkin, A., and Wlodawer, A., Biochemistry, 2020, vol. 59, pp. 1927−1945. https://doi.org/10.1021/acs.biochem.0c00116
Ghasemian, A., Al-Marzoqi, A.H., Al-Abodi, H.R., Alghanimi, Y.K., Kadhum, S.A., Shokouhi Mostafavi, S.K., and Fattahi, A., J. Cell. Physiol., 2019, vol. 234, pp. 19271–19279. https://doi.org/10.1002/jcp.28563
Batool, T., Makky, E.A., Jalal, M., and Yusoff, M.M., Appl. Biochem. Biotechnol., 2016, vol. 178, pp. 900–923. https://doi.org/10.1007/s12010-015-1917-3
Michalska, K. and Jaskolski, M., Acta Biochim. Pol., 2006, vol. 53, pp. 627–640.
Niu, J., Meng, F., Zhou, Y., Zhang, C., Lu, Z., Lu, F., and Chen, M., Int. J. Biol. Macromol., 2021, vol. 180, pp. 677–683. https://doi.org/10.1016/j.ijbiomac.2021.03.104
Yao, M., Yasutake, Y., Morita, H., and Tanaka, I., Acta Crystallogr. D. Biol. Crystallogr. 2005, vol. 61, pp. 294–301. https://doi.org/10.1107/S0907444904032950
Jiao, L., Chi, H., Lu, Z., Zhang, C., Chia, S.R., Show, P.L., Tao, Y., and Lu, F., J. Biosci. Bioeng., 2020, vol. 129, pp. 672–678. https://doi.org/10.1016/j.jbiosc.2020.01.007
Sharafi, Z., Barati, M., Khoshayand, M.R., and Adrangi, S., Iran. J. Pharm. Res. IJPR, 2017, vol. 16, pp. 1565–1573.
Nowak-Göttl, U., Wolff, J.E.A., Kuhn, N., Boos, J., Kehrel, B., Lilienweiss, V., Schwabe, D., and Jürgens, H., Fibrinolysis, 1994, vol. 8, pp. 63–65. https://doi.org/10.1016/0268-9499(94)90248-8
Leibundgut, K., Hirt, A., Zwicky, C., and Wuillemin, W.A., Hamostaseologie, 2003, vol. 23, pp. 109–112.
Fonseca, M.H.G., Fiúza, T.D.S., de Morais, S.B., de Souza, C.B., and Trevizani, R., Biomed. Pharmacother., 2021, vol. 139, 111616. https://doi.org/10.1016/j.biopha.2021.111616
da Silva Lacerda, G.R., Cantalice, J.C.L.L., de Souza Lima, G.M., de Albuquerque, L.E.F., da Silva, I.D.G., de Melo, M.E.B., Adam, M.L., and do Na-scimento, S.C., World J. Microbiol. Biotechnol., 2019, vol. 35, 41. https://doi.org/10.1007/s11274-019-2612-2
Aghaiypour, K., Wlodawer, A., and Lubkowski, J., Biochemistry, 2001, vol. 40, pp. 5655–5664. https://doi.org/10.1021/bi0029595
Kessel, D., BBA Sect. Nucleic Acids Protein Synth., 1971, vol. 240, pp. 554–557. https://doi.org/10.1016/0005-2787(71)90712-X
Bosmann, H.B. and Kessel, D., Nature, 1970, vol. 226, pp. 850–851. https://doi.org/10.1038/226850a0
Ankel, E.G., Zirneski, J., Ring, B.J., and Holcenberg, J.S., In Vitro, 1984, vol. 20, pp. 376–384. https://doi.org/10.1007/BF02619582
Zhdanov, D.D., Pokrovsky, V.S., Pokrovskaya, M.V., Alexandrova, S.S., Eldarov, M.A., Grishin, D.V., Basharov, M.M., Gladilina, Y.A., Podobed, O.V., and Sokolov, N.N., Biochem. Biophys. Res. Commun., 2017, vol. 492, pp. 282−288. https://doi.org/10.1016/j.bbrc.2017.08.078
Zhdanov, D.D., Pokrovsky, V.S., Pokrovskaya, M.V., Alexandrova, S.S., Eldarov, M.A., Grishin, D.V., Basharov, M.M., Gladilina, Y.A., Podobed, O.V., and Sokolov, N.N., Cancer Med., 2017, vol. 6, pp. 2697−2712. https://doi.org/10.1002/cam4.1218
Plyasova, A.A., Pokrovskaya, M.V., Lisitsyna, O.M., Pokrovsky, V.S., Alexandrova, S.S., Hilal, A., Sokolov, N.N., and Zhdanov, D.D., Pharmaceuticals, 2020, vol. 13, 286. https://doi.org/10.3390/ph13100286
Kidd, J.G. and Sobin, L.H., Cancer Res., 1966, vol. 26, pp. 208–211.
Kidd, J.G., J. Exp. Med., 1953, vol. 98, pp. 565–582. https://doi.org/10.1084/jem.98.6.565
Broome, J.D., J. Natl. Cancer Inst., 1965, vol. 35, pp. 967–974.
Ponomarenko, J., Papangelopoulos, N., Zajonc, D.M., Peters, B., Sette, A., and Bourne, P.E., Nucleic Acids Res., 2011, vol. 39, pp. D1164−D1170. https://doi.org/10.1093/nar/gkq888
Covini, D., Tardito, S., Bussolati, O., Chiarelli, L.R., Pasquetto, M.V., Digilio, R., Valentini, G., and Scotti, C., Drug Discov., 2012, vol. 7, pp. 4–13. https://doi.org/10.2174/157489212798358001
Sokolov, N.N., Eldarov, M.A., Pokrovskaya, M.V., Aleksandrova, S.S., Abakumova, O.Y., Podobed, O.V., Melik-Nubarov, N.S., Kudryashova, E.V., Gri-shin, D.V., and Archakov, A.I., Biomeditsinskaya khimiya, 2015, vol. 61, no. 3, 312–324. https://doi.org/10.18097/PBMC20156103312
Cantor, J.R., Panayiotou, V., Agnello, G., Georgiou, G., and Stone, E.M., Methods Enzymol., 2012, vol. 502, pp. 291–319. https://doi.org/10.1016/B978-0-12-416039-2.00015-X
Hermanova, I., Zaliova, M., Trka, J., and Starkova, J., Exp. Hematol., 2012, vol. 40, pp. 657–665. https://doi.org/10.1016/j.exphem.2012.04.005
Hawkins, D.S., Park, J.R., Thomson, B.G., Felgenhauer, J.L., Holcenberg, J.S., Panosyan, E.H., and Avramis, V.I., Clin. Cancer Res., 2004, vol. 10, pp. 5335–5341. https://doi.org/10.1158/1078-0432.CCR-04-0222
Krishnapura, P.R., Belur, P.D., and Subramanya, S., Crit. Rev. Microbiol., 2016, vol. 42, pp. 720–737. https://doi.org/10.3109/1040841X.2015.1022505
Yamaguchi, M., Kwong, Y.-L., Kim, W.S., Maeda, Y., Hashimoto, C., Suh, C., Izutsu, K., Ishida, F., Isobe, Y., Sueoka, E., Suzumiya, J., Kodama, T., Kimura, H., Hyo, R., Nakamura, S., Oshimi, K., and Suzuki, R., J. Clin. Oncol., 2011, vol. 29, pp. 4410–4416. https://doi.org/10.1200/JCO.2011.35.6287
Wang, L., Wang, Z., Chen, X., Li, Y., Wang, K., Xia, Y., and Xia, Z., Cancer, 2013, vol. 119, pp. 348–355. https://doi.org/10.1002/cncr.27752
Jaccard, A., Gachard, N., Marin, B., Rogez, S., Audrain, M., Suarez, F., Tilly, H., Morschhauser, F., Thieblemont, C., Ysebaert, L., Devidas, A., Petit, B., de Leval, L., Gaulard, P., Feuillard, J., Bordessou-le, D., and Hermine, O., Blood, 2011, vol. 117, pp. 1834–1839. https://doi.org/10.1182/blood-2010-09-307454
Li, X., Cui, Y., Sun, Z., Zhang, L., Li, L., Wang, X., Wu, J., Fu, X., Ma, W., Zhang, X., Chang, Y., Nan, F., Li, W., Su, L., Wang, J., Xue, H., and Zhang, M., Clin. Cancer Res., 2016, vol. 22, pp. 5223–5228. https://doi.org/10.1158/1078-0432.CCR-16-0153
Hijiya, N. and van der Sluis, I.M., Leuk. Lymphoma, 2016, vol. 57, pp. 748–757. https://doi.org/10.3109/10428194.2015.1101098
van den Berg, H., Leuk. Lymphoma, 2011, vol. 52, pp. 168–178. https://doi.org/10.3109/10428194.2010.537796
Avramis,V.I., Avramis, E.V., Hunter, W., and Long, M.C., Anticancer Res., 2009, vol. 29, pp. 299–302.
Baryshnikova, M.A., Baryshnikov, A.Y., and Afanasieva, D.A., Russ. J. Biother., 2015, vol. 14, pp. 3–10. https://doi.org/10.17650/1726-9784-2015-14-1-3-10
Rajić, V., Debeljak, M., Goričar, K., and Jazbec, J., Leuk. Lymphoma, 2015, vol. 56, pp. 3103–3108. https://doi.org/10.3109/10428194.2015.1020802
Cantor, J.R., Yoo, T.H., Dixit, A., Iverson, B.L., Forsthuber, T.G., and Georgiou, G., Proc. Natl. Acad. Sci. USA, 2011, vol. 108, pp. 1272–1277. https://doi.org/10.1073/pnas.1014739108
Moola, Z.B., Scawen, M.D., Atkinson, T., and Nicholls, D.J., Biochem. J., 1994, vol. 302, Pt 3, pp. 921–927. https://doi.org/10.1042/bj3020921
Offman, M.N., Krol, M., Patel, N., Krishnan, S., Liu, J., Saha, V., and Bates, P.A., Blood, 2011, vol. 117, pp. 1614–1621. https://doi.org/10.1182/blood-2010-07-298422
Jianhua, C., Yujun, W., Ruibo, J., Min, W., and Wutong, W., Mol. Biotechnol., 2006, vol. 33, pp. 57–65. https://doi.org/10.1385/MB:33:1:57
Werner, A., Röhm, K.-H., and Müller, H.-J., Biol. Chem. 2005, vol. 386, pp. 535–540. https://doi.org/10.1515/BC.2005.063
Mehta, R.K., Verma, S., Pati, R., Sengupta, M., Khatua, B., Jena, R.K., Sethy, S., Kar, S.K., Mandal, C., Roehm, K.H., and Sonawane, A., J. Biol. Chem., 2014, vol. 289, pp. 3555–3570. https://doi.org/10.1074/jbc.M113.486530
Zeidan, A., Wang, E.S., and Wetzler, M., Expert Opin. Biol. Ther., 2009, vol. 9, pp. 111–119. https://doi.org/10.1517/14712590802586058
Medawar, C.V., Mosegui, G.B.G., de M. Vian-na, C.M., and da Costa T.M.A., Hematol. Transfus. Cell Ther., 2019, vol. 42, pp. 54–61. https://doi.org/10.1016/j.htct.01.013
Pokrovsky, V.S., Kazanov, M.D., Dyakov, I.N., Pokrovskaya, M.V., and Aleksandrova, S.S., BMC Cancer, 2016, vol. 16, 89. https://doi.org/10.1186/s12885-016-2125-4
Pokrovskaya, M.V., Aleksandrova, S.S., Pokrovsky, V.S., Veselovsky, A.V., Grishin, D.V., Abakumova, O.Y., Podobed, O.V., Mishin, A.A., Zhdanov, D.D., and Sokolov, N.N., Mol. Biotechnol., 2015, vol. 57, pp. 251–264. https://doi.org/10.1007/s12033-014-9819-0
Pokrovskaya, M.V., Pokrovskiy, V.S., Aleksandro-va, S.S., Anisimova, N.Y., Andrianov, R.M., Treschalina, E.M., Ponomarev, G.V., and Sokolov, N.N., Biomeditsinskaya Khimiya, 2012, vol. 59, no. 2, pp. 192–208. https://doi.org/10.18097/pbmc20135902192
Pokrovskaya, M.V., Zhdanov, D.D., Eldarov, M.A., Aleksandrova, S.S., Veselovsky, A.V., Pokrovskiy, V.S., Grishin, D.V., Gladilina, J.A., and Sokolov, N.N., Biomeditsinskaya Khimiya, 2017, vol. 63, no. 1, pp. 62–74. https://doi.org/10.18097/PBMC20176301062
Malakhova, M.A., Pokrovskaya, M.V., Alexandro-va, S.S., Sokolov, N.N., and Kudryashova, E.V., Moscow Univ. Chem. Bull., 2018, vol. 73, pp. 185–191. https://doi.org/10.3103/S0027131418040065
Karamitros, C.S. and Konrad, M., J. Biol. Chem., 2014, vol. 289, pp. 12962–12975. https://doi.org/10.1074/jbc.M113.545038
Rigouin, C., Nguyen, H.A., Schalk, A.M., and Lavie, A., Sci. Rep., 2017, vol. 7, 10224. https://doi.org/10.1038/s41598-017-10758-4
Haskell, C.M. and Canellos, G.P., Biochem. Pharmacol., 1969, vol. 18, pp. 2578–2580. https://doi.org/10.1016/0006-2952(69)90375-x
Chiu, M., Taurino, G., Bianchi, M.G., Kilberg, M.S., and Bussolati, O., Front. Oncol., 2019, vol. 9, 1480. https://doi.org/10.3389/fonc.2019.01480
Lomelino, C.L., Andring, J.T., McKenna, R., and Kilberg, M.S., J. Biol. Chem., 2017, vol. 292, pp. 19952–19958. https://doi.org/10.1074/jbc.R117.819060
Liu, W.-J., Wang, H., Peng, X.-W., Wang, W., Liu, N.-W., Wang, Y., and Lu, Y., Onco. Targets. Ther., 2018, vol. 11, pp. 6605–6615. https://doi.org/10.2147/OTT.S155930
Aslanian, A.M., Fletcher, B.S., and Kilberg, M.S., Biochem. J., 2001, vol. 357, pp. 321–328. https://doi.org/10.1042/0264-6021:3570321
Mei, L., Ontiveros, E.P., Griffiths, E.A., Thomp-son, J.E., Wang, E.S., and Wetzler, M., Blood Rev., 2015, vol. 29, pp. 243–349. https://doi.org/10.1016/j.blre.2015.01.001
Aslanian, A.M. and Kilberg, M.S., Biochem. J., 2001, vol. 358, pp. 59–67. https://doi.org/10.1042/0264-6021:3580059
Hinze, L., Pfirrmann, M., Karim, S., De-gar, J., Mc-Guckin, C., Vinjamur, D., Sacher, J., Stevenson, K.E., Neuberg, D.S., Orellana, E., Stanul-la, M., Gregory, R.I., Bauer, D.E., Wagner, F.F., Stegmaier, K., and Gutierrez, A., Cancer Cell, 2019, vol. 35, pp. 664−676. e7. https://doi.org/10.1016/j.ccell.2019.03.004
Richards, N.G.J. and Kilberg, M.S., Annu. Rev. Biochem., 2006, vol. 75, pp. 629–654. https://doi.org/10.1146/annurev.biochem.75.103004.142520
Chan, W.-K., Horvath, T.D., Tan, L., Link, T., Harutyunyan, K.G., Pontikos, M.A., Anishkin, A., Du, D., Martin, L.A., Yin, E., Rempe, S.B., Sukharev, S., Konopleva, M., Weinstein, J.N., and Lorenzi, P.L., Mol. Cancer Ther., 2019, vol. 18, pp. 1587–1592. https://doi.org/10.1158/1535-7163.MCT-18-1329
Nunes, J.C.F., Cristóvão, R.O., Freire, M.G., Santos-Ebinuma, V.C., Faria, J.L., Silva, C.G., and Tavares, A.P.M., Molecules, 2020, vol. 25, 5827. https://doi.org/10.3390/molecules25245827
Ohnuma, T., Holland, J.F., Freeman, A., and Sinks, L.F., Cancer Res., 1970, vol. 30, pp. 2297–22305.
Akbayram, S., Doğan, M., Akgün, C., Caksen, H., and Oner, A.F., J. Pediatr. Hematol. Oncol., 2010, vol. 32, e187−e191. https://doi.org/10.1097/MPH.0b013e3181e003c7
Pokrovskaya, M.V., Pokrovsky, V.S., Aleksandro-va, S.S., Sokolov, N.N., and Zhdanov, D.D., Pharmaceutics, 2022, vol. 14, 599. https://doi.org/10.3390/pharmaceutics14030599
Li, H., Yang, Y., Hong, W., Huang, M., Wu, M., and Zhao, X., Signal Transduct. Target. Ther., 2020, vol. 5, 1. https://doi.org/10.1038/s41392-019-0089-y
Anishchenko, I., Pellock, S.J., Chidyausiku, T.M., Ramelot, T.A., Ovchinnikov, S., Hao, J., Bafna, K., Norn, C., Kang, A., Bera, A.K., DiMaio, F., Carter, L., Chow, C.M., Montelione, G.T., and Baker D., Nature, 2021, vol. 600, pp. 547–552. https://doi.org/10.1038/s41586-021-04184-w
Evans, R., Neill, M.O., Pritzel, A., Antropova, N., Senior, A., Green, T., Židek, A., Bates, R., Blackwell, S., Yim, J., Ronneberger, O., Bodenstein, S., Zielin-ski, M., Bridgland, A., Potapenko, A., Cowie, A., Tunyasuvunakool, K., Jain R., Clancy, E., Kohli, P., Jumper, J., and Hassabis, D., BioRxiv, 2021, 10.04.463034.https://doi.org/10.1101/2021.10.04.463034
Fu, C.H. and Sakamoto, K.M., Expert Opin. Pharmacother., 2007, vol. 8, pp. 1977–1984. https://doi.org/10.1517/14656566.8.12.1977
Postnov, V.N., Naumysheva, E.B., Korolev, D.V., and Galagudza, M.M., Biotekhnosfera, 2013, vol. 30, pp. 16–29.
Rizzari, C., Citterio, M., Zucchetti, M., Conter, V., Chiesa, R., Colombini, A., Malguzzi, S., Silvestri, D., and D’Incalci, M., Haematologica, 2006, vol. 91, pp. 24–31.
Graham, M.L., Adv. Drug Deliv. Rev., 2003, vol. 55, pp. 1293–1302. https://doi.org/10.1016/s0169-409x(03)00110-8
Alpar, H.O. and Lewis, D.A., Biochem. Pharmacol., 1985, vol. 34, pp. 257–261. https://doi.org/10.1016/0006-2952(85)90133-9
He, H., Ye, J., Wang, Y., Liu, Q., Chung, H.S., Kwon, Y.M., Shin, M.C., Lee, K., and Yang, V.C., J. Control. Release, 2014, vol. 176, pp. 123–132. https://doi.org/10.1016/j.jconrel.2013.12.019
Lorentz, K.M., Kontos, S., Diaceri, G., Henry, H., and Hubbell, J.A., Sci. Adv., 2015, vol. 1, e1500112. https://doi.org/10.1126/sciadv.1500112
Funding
The work was carried out within the framework of the Program of Fundamental Scientific Research in the Russian Federation for a long-term period (2021−2030) (no. 122022800499-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This work was not related to studies on humans or animals as research objects.
Additional information
The article was translated by the author (D.D. Zhdanov).
Rights and permissions
About this article
Cite this article
Alexandrova, S.S., Gladilina, Y.A., Pokrovskaya, M.V. et al. Mechanisms of Development of Side Effects and Drug Resistance to Asparaginase and Ways to Overcome Them. Biochem. Moscow Suppl. Ser. B 16, 175–186 (2022). https://doi.org/10.1134/S1990750822030027
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990750822030027