Abstract
To evaluate outcome in patients treated with stereotactic body radiotherapy (SBRT) on bone oligometastases from castration-sensitive prostate cancer after primary treatment. We retrospectively collected data of patients with less than five lesions at time of SBRT and hormone-naïve disease at the first extra-regional localization, treated between 03/2012 and 11/2016. Prostate-specific antigen (PSA) was measured every 3 months after SBRT. Imaging was performed in case of progression. Survival analysis was performed with Kaplan–Meier (log-rank test) approach. Fifty-five patients were treated on 77 bone oligometastases. Median age, initial PSA and pre-SBRT PSA were 72 years, 9.12 and 3.5 ng/mL, respectively. Twenty-five patients (45%) received SBRT alone while the remaining 30 patients (55%) received concomitant ADT. Median follow-up was 24.6 months (range 3.0–67.2 months). No acute or late toxicity of grade > 1 was reported. Clinical progression was observed in 38 (69%) patients. 1-year biochemical progression-free survival (b-PFS), clinical progression-free survival (c-PFS), prostate-specific survival (PCSS) and local control (LC) rates were 51, 56, 100 and 83%, respectively. Comparing patients treated with SBRT alone and with concomitant ADT, no significant differences were found for those outcomes. SBRT is safe and allows high 1-year LC rate (83%) with low toxicity profile. No significant improvement in outcomes was registered with the addition of ADT to SBRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second most common cancer among men globally, with an estimated 1.1 million new cases and over 300,000 deaths annually [1]. In the last decade, the debate principally involves the management of the oligometastatic disease, defined as an intermediate state of tumor spread with limited metastatic capacity [2, 3]. This concept has changed the clinical practice allowing for a local treatment, such as surgery or radiation therapy, rather than a systemic approach, given the limited number and site of metastatic lesions.
PCa is a radiosensitive disease, and stereotactic body radiation therapy (SBRT) is emerging as a promising treatment option with low toxicity for the management of the oligometastatic patient both at diagnosis and at recurrence (local consolidative therapy and metastasis-directed therapy) [4]. SBRT, similar to surgery, is a spatially targeted therapy with the advantage over other local therapies to add margins for subclinical disease extent. However, despite the advances in technology, the use of SBRT remains limited and androgen deprivation therapy (ADT) is still the most used therapeutic modality even in the oligometastatic setting. Other emerging approaches to metastatic PCa include chemotherapy and new agents, but their role in oligometastatic disease is not yet clear [5,6,7].
In metastatic PCa setting, delaying the castration resistance is a key point for the long-term management of these patients since this condition is widely recognized as a signal of forthcoming disease progression. In the low-volume metastatic scenario, metastasis-directed therapy is a valid option for the procrastination of ADT beginning and consequently of castration resistance [8]. For these reasons, some authors introduced the concept of ADT-free survival (ADT-FS), intended as the time to the delayed start of systemic therapy, which is now emerging as a way to spare the negative side effects of this treatment, such as the increased occurrence of cardiovascular events and metabolic syndrome that significantly affect the quality of life [9].
Currently, limited studies focus on metastasis-directed intervention and the vast majority includes lymph node oligometastases. The knowledge is mainly based on retrospective and non-randomized studies, thus suffering from heterogeneous population (oligo/polymetastatic disease, different sites of metastatic disease like lymph nodes, bone, visceral lesions and biases in patient selection) and inappropriate sample power. A large consensus on oligorecurrent PCa management has not been obtained so far.
In this study, we reviewed 55 oligometastatic PCa patients for a total of 77 bone metastases treated with SBRT, with or without concomitant ADT, analyzing biochemical progression-free survival (b-PFS), clinical progression-free survival (c-PFS), PCa-specific survival (PCSS), local control (LC) and the ADT-FS.
Patients and methods
The study was a part of general SBRT and image-guided radiation therapy (IGRT) research notified to the Ethical Committee of the European Institute of Oncology, Milan, Italy (notifications No. 79/10, 86/11, 87/11, 93/11).
Patient selection
The inclusion criteria for this retrospective study were as follows:
-
1.
Histologic diagnosis of PCa on primary tumor biopsy;
-
2.
Documented radiological stage M1b;
-
3.
Number of bone metastases less or equal to 5 (based on the current definition of oligometastatic disease);
-
4.
Hormone-naïve disease at the first extra-regional localization;
-
5.
No previous radiation therapy on the same lesion;
-
6.
SBRT delivered on all detected lesions;
-
7.
Written informed consent for SBRT;
-
8.
Written informed consent for the use of the anonymized data for research or educational purpose; and
-
9.
Specific approval by the multidisciplinary uro-oncology board.
Any kind of previous therapy on the primary tumor was permitted (radical prostatectomy or radiation therapy with or without ADT). The diagnosis of a clinically evident bone recurrent PCa was based on an evidence of biochemical progression and imaging studies. Biochemical recurrence after the primary therapy was verified according to Phoenix criteria, based on a prostate-specific antigen (PSA) value corresponding to PSA nadir + 2 ng/mL for patients treated with radiation therapy with or without ADT and according to the ASTRO criteria [10], based on three consecutive rises of PSA level above the nadir; for patients treated with radical prostatectomy, PSA value ≥ 0.2 ng/mL was considered suggestive for biochemical progression as established in EAU guidelines [11, 12].
At biochemical progression, patients were staged according to the EAU guidelines [11, 12], with 11C-choline positron emission tomography with co-registered computer tomography (11C-choline-PET/CT), magnetic resonance (MR), total body CT or 68Ga-prostate-specific membrane antigen ligand PET/CT (68Ga-PSMA-PET/CT).
Treatment protocol
The SBRT dose prescription depended on the volume and localization of the recurrence. In case of unfavorable localization, lower dose/fraction or lower total dose was administered.
Spinal lesions were principally treated with CyberKnife® system (Accuray, Inc., Sunnyvale, CA), while for non-spinal metastases VERO system (Mitsubishi Heavy Industries, Ltd., Japan and BrainLab AG, Feldkirchen, Germany) was preferred.
For CyberKnife SBRT, treatment was planned with MultiPlan® treatment planning system (v.5.2 Accuray, USA) and delivered with photon energy 6 MV, using Xsight spine-tracking mode with no implanted fiducials. All patients were immobilized during CT simulation and treatment, using a customized external vacuum-type cast. Dose was prescribed to the mean 75% isodose using a non-isocentric and non-coplanar CyberKnife treatment technique.
For VERO System SBRT, iPlanRT (v. 4.5.3 BrainLab, Germany) was employed. All patients were immobilized during CT simulation and treatment using Combifix™ device (CIVCO Medical solutions, lowa, USA). Seven infrared markers were put on the chest wall during CT simulation and treatment in order to correct the setup errors and the shifts during the treatment. Coplanar and non-coplanar dynamic conformal arcs were employed for treatment planning. During beam delivery, the ExacTrac® system (BrainLAB, Feldkirchen, Germany) monitored the position of the target using the infrared markers. Cone-beam CT or orthogonal kilovoltage X-rays were evaluated before dose delivery.
The definitions of the organs at risk (OARs) depended on the localization of the bone recurrence. For cervical spine lesions, spinal cord was considered OAR, for thoracic spine lesions spinal cord, lungs and esophagus, for lumbar spine lesions spinal cord or cauda equina and small bowel. For hip bones, pelvic organs were considered OARs, namely urinary bladder, rectum and small bowel. The dose volume constraints proposed by Timmerman were applied [13].
Follow-up procedure
After SBRT, a PSA-level dosage and clinical assessment of toxicities were recorded at 3 months. Then, PSA test was performed every 3 months and clinical examination every 6 months.
LC was measured from the beginning of SBRT and the diagnosis of in-field relapse, intended as a morphologic or metabolic increase in the planning target volume. b-PFS was measured as the time from the beginning of SBRT to the PSA increase after SBRT, according to the criteria defined in Jereczek-Fossa et al. [14]. In case of progression, routine radiological or 11C-choline-PET/CT re-evaluation was requested to evaluate in- or out-field relapse. c-PFS was measured as the time between the beginning of SBRT and the clinical progression, defined as the radiological detection of local progression or distant disease. ADT-FS was defined as the time between SBRT and the start of palliative ADT. At progression metastasis, directed treatment and/or ADT was proposed according to clinical presentation, current guidelines and evidences. PCSS was defined as the time from the beginning of SBRT to the time of mortality from PCa.
Although a systematic evaluation of pain response with a validated pain scale was not performed, patient subjective perception of pain changing after SBRT was collected retrospectively. Treatment toxicity was evaluated according to the Common Terminology Criteria for Adverse Event, version 3 (CTCAE.v3) [15].
Follow-up data were reported until January 31, 2018.
Statistical analysis
Patient and tumor characteristics were represented as frequencies and percentages when classified with categorical variables and with median values and range for continuous variables [16]. The correlation between patient- and treatment-related characteristics and clinical outcome was investigated with Cox regression analysis. Survival analysis was performed with Kaplan–Meier approach [17], and differences between groups were evaluated with log-rank test. A p value < 0.05 was considered significant.
Results
We selected 55 oligorecurrent PCa patients for a total of 77 bone metastases, treated at the European Institute of Oncology (Milan, Italy) between May 2012 and November 2016. Forty-five patients were staged with 11C-choline-PET/CT, eight patients with MR, one patient with total body CT and one with 68Ga-PSMA-PET/CT.
Twenty-five patients (45%) received SBRT alone while the remaining 30 patients (55%) received concomitant SBRT and ADT. Thirty-four out of 77 (44%) were spinal lesions. Patient and tumor characteristics are listed in Table 1. Treatment-related characteristics are shown in Table 2.
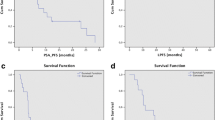
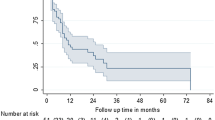
With a median follow-up of 24.6 months (range 3–67.2), in-field progression was observed in ten out of 77 (13%) irradiated bone metastases corresponding to 7 out 55 patients. 1- and 2-year LC rates were 83 and 76%, respectively. Forty-four (80%) patients experienced biochemical recurrence. 1- and 2-year b-PFS rates were 51 and 13%, respectively. Clinical progression was always preceded by biochemical recurrence and was recorded in 38 (69%) patients. 1- and 2-year c-PFS rates were 55 and 27%, respectively. At last follow-up, six patients died for PCa. 1- and 2-year PCSS rates were 100 and 96%, respectively (Fig. 1).
No significant differences between patients treated with SBRT alone or in combination with ADT were found for b-PFS (p = 0.57), c-PFS (p = 0.93), PCSS (p = 0.09) and LC (p = 0.25), as shown in the Kaplan–Meier curves (Fig. 2).
a Biochemical progression-free survival (b-PFS), b clinical progression-free survival (c-PFS), c local control (LC) and d prostate cancer-specific survival (PCSS) in the two subgroups of patients treated with stereotactic body radiotherapy (SBRT) alone (25 patients) and SBRT with concomitant androgen deprivation therapy (ADT) (30 patients)
The analysis of correlation of the T stage, number of lesions, type of treatment on primary tumor, equivalent dose in 2 Gy per fraction (EQD2) calculated with α/β = 3 [18, 19] and the use of ADT with the local recurrence showed no statistically significant correlations.
Of the 13 patients complaining pain at the time of SBRT, no patient experienced pain progression at the first examination after SBRT and seven patients (54%) of them reported a complete pain regression.
One patient showed acute toxicity, with an absolute rate of acute grade 1 gastrointestinal toxicity of 1.8%, without any 2 and 3 grade toxicity. No late gastrointestinal, genitourinary and other organ toxicity was observed.
Discussion
Our study, including data of 55 patients treated with SBRT for 77 bone oligometastases from castration naïve PCa, showed high LC and excellent toxicity profile. At 1 year after SBRT, one out of two patients was free of progression.
Oligorecurrent PCa has become more frequently diagnosed as a result of the improvement in cancer imaging due to both better MR sequencing and to novel nuclear tracer. Recent works confirmed the efficacy and tolerability of metastasis-directed therapy [14, 20]. No consensus exists about the optimal treatment schedule. Otherwise, our SBRT doses are in line with a study by Muldermans et al. [21] that tried to investigate the better dose with the lower toxicity to use in the treatment of oligometastatic PCa, identifying a ≥ 18 Gy total dose as the probably good compromise.
Actually, no consensus exists for response criteria in bone metastases. Therefore, in-field progression was defined as an increased uptake of radiopharmaceutical tracer in six out of seven patients (nine out of ten in-field progressive lesions). For the only patient revaluated with CT scan, the images reviewed by the multidisciplinary tumor board confirmed the disease progression and finally the patient underwent a second irradiation. Similar clinical results were obtained in a recent study by Habl et al. [19] that reported a median b-PFS of 6.9 months and a LC after 2 years of 100% in patients treated with SBRT for oligometastases from PCa. Furthermore, in patients with up to three metastases treated with SBRT, Decaestecker et al. [22] showed that 18 out of 50 patients remained free of disease, while 32 patients developed distant metastasis at 2 years. In our analysis, 21 out of 55 patients developed out-field distant progression, arising the question of a downstaging phase in patient selection before the SBRT treatment. This confirms the assumption that a distinction of polymetastatic from oligometastatic disease becomes a crucial step in the management of these patients.
Based on the fact that our LC rate at 1 year after SBRT was high (83%) we might assume that both biochemical and clinical progression can be slowed down, at least temporarily, by the SBRT treatment. Furthermore, the pattern of recurrence appeared to be again oligometastatic in 30% of patients, allowing a new treatment with SBRT. Moreover, our study investigated the role of the two possible approaches in the management of oligometastic patients: local versus local and systemic therapy. Here, we hypothesize that SBRT alone is a feasible and effective treatment, with low toxicity and low detrimental effect on patient quality of life, allowing for the deferral of ADT. Oligometastatic patients fulfilling the inclusion criteria mentioned above often have a long survival time; therefore, a noninvasive low toxicity approach could be of a great value for this population. Consequently, therapeutic approaches meant to defer ADT-induced morbidity should be considered in well-selected patients with low-volume metastatic disease.
When we compared the b-PFS, c-PFS and PCSS in the two different groups (SBRT alone vs SBRT and ADT), no statistically significant differences were found and the two subgroups seem to have similar outcomes. We are aware that these findings should be considered extremely carefully, first of all for the retrospective nature of the study in addition to the exiguous number of our cohort. Our results, however, could help clinicians in the decision-making process especially for those patients with worse performance status or higher number of comorbidities that contraindicate systemic approaches.
The role of metastasis-directed therapy with SBRT in this setting is controversial and subject of active investigation, such as in the STOMP study, a randomized phase II trial that demonstrates the deferral of palliative ADT in oligometastatic PCa of 21 months [23, 24].
Although we acknowledge the abovementioned limitations, this study showed also some strength. First of all, a homogeneous population has been analyzed: All patients were evaluated for progression after primary treatment and studied for bone localizations. Furthermore, the promising initial results for SBRT are based on the good c-PFS in the two subgroups of patients, the feasibility and the very low toxicity profile. We are aware that larger population with more mature data would lead to a widely accepted consensus and finally to definitive guidelines. In the meantime, these results could drive clinicians to offer different therapeutic choices to adapt on the single patient conditions.
In conclusion, SBRT is effective in the management of bone oligometastatic PCa in terms of LC as well as systemic control of disease. Potential positive effects can be found in the deferral of ADT even if no consensus has been found in the optimal combination of SBRT and ADT. Finally, this approach should be encouraged not only for the very low toxicity profile but also for preventing future skeletal-related events.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. https://doi.org/10.1002/ijc.29210 (Epub 2014 Oct 9).
Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R, Carver B, Castellano D, Chung BH, Clarke N, Daugaard G, Davis ID, de Bono J, Borges A, DosReis R, Drake CG, Eeles R, Efstathiou E, Evans CP, Fanti S, Feng F, Fizazi K, Frydenberg M, Gleave M, Halabi S, Heidenreich A, Higano CS, James N, Kantoff P, Kellokumpu-Lehtinen PL, Khauli RB, Kramer G, Logothetis C, Maluf F, Morgans AK, Morris MJ, Mottet N, Murthy V, Oh W, Ost P, Padhani AR, Parker C, Pritchard CC, Roach M, Rubin MA, Ryan C, Saad F, Sartor O, Scher H, Sella A, Shore N, Smith M, Soule H, Sternberg CN, Suzuki H, Sweeney C, Sydes MR, Tannock I, Tombal B, Valdagni R, Wiegel T, Omlin A. Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference APCCC 2017. Eur Urol. 2018;73(2):178–211. https://doi.org/10.1016/j.eururo.2017.06.002.
Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–82. https://doi.org/10.1038/nrclinonc.2011.44.
Tree AC, Khoo VS, Eeles RA, Ahmed M, Dearnaley DP, Hawkins MA, Huddart RA, Nutting CM, Ostler PJ, van As NJ. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:e28–37. https://doi.org/10.1016/S1470-2045(12)70510-7.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, De Porre P, Kheoh T, Park YC, Todd MB, Chi KN, LATITUDE Investigators. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017. https://doi.org/10.1056/NEJMoa1704174.
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, Ritchie AWS, Amos CL, Gilson C, Jones RJ, Matheson D, Millman R, Attard G, Chowdhury S, Cross WR, Gillessen S, Parker CC, Russell JM, Berthold DR, Brawley C, Adab F, Aung S, Birtle AJ, Bowen J, Brock S, Chakraborti P, Ferguson C, Gale J, Gray E, Hingorani M, Hoskin PJ, Lester JF, Malik ZI, McKinna F, McPhail N, Money-Kyrle J, O’Sullivan J, Parikh O, Protheroe A, Robinson A, Srihari NN, Thomas C, Wagstaff J, Wylie J, Zarkar A, Parmar MKB, Sydes MR, STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017. https://doi.org/10.1056/nejmoa1702900.
Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, Dreicer R, Vogelzang NJ, Picus J, Shevrin D, Hussain M, Garcia JA, DiPaola RS. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–46. https://doi.org/10.1056/NEJMoa1503747.
Martínez-Fernández MI, Pérez Gracia JL, Gil-Bazo I, Martínez-Monge R. Stereotactic body radiation therapy (SBRT) delays the emergence of castration resistance in patients with oligometastatic prostate cancer. Clin Transl Oncol. 2016;18(7):743–7. https://doi.org/10.1007/s12094-015-1414-8.
Berkovic P, De Meerleer G, Delrue L, Lambert B, Fonteyne V, Lumen N, Decaestecker K, Villeirs G, Vuye P, Ost P. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin Genitourin Cancer. 2013;11(1):27–32. https://doi.org/10.1016/j.clgc.2012.08.003.
Horwitz EM, Uzzo RG, Hanlon AL, Greenberg RE, Hanks GE, Pollack A. Modifying the ASTRO definition of biochemical failure to minimize the influence of backdating in patients with prostate cancer treated with 3D conformal radiation therapy alone. J Urol. 2003;169:2153–9.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N, European Association of Urology. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65(1):124–37. https://doi.org/10.1016/j.eururo.2013.09.046.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N, European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–79. https://doi.org/10.1016/j.eururo.2013.11.002.
Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18:215–22. https://doi.org/10.1016/j.semradonc.2008.04.001.
Jereczek-Fossa BA, Fanetti G, Fodor C, Ciardo D, Santoro L, Francia CM, Muto M, Surgo A, Zerini D, Marvaso G, Timon G, Romanelli P, Rondi E, Comi S, Cattani F, Golino F, Mazza S, Matei DV, Ferro M, Musi G, Nolè F, de Cobelli O, Ost P, Orecchia R. Salvage stereotactic body radiotherapy for isolated lymph node recurrent prostate cancer: single institution series of 94 consecutive patients and 124 lymph nodes. Clin Genitourin Cancer. 2017. https://doi.org/10.1016/j.clgc.2017.01.004.
https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
Marubini E, Valsecchi MG. Analysing survival data from clinical trials and observational studies. Chichester: Wiley; 1995.
Kaplan E, Meier P. Non–parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Bhattacharya IS, Woolf DK, Hughes RJ, Shah N, Harrison M, Ostler PJ, Hoskin PJ. Stereotactic body radiotherapy (SBRT) in the management of extracranial oligometastatic (OM) disease. Br J Radiol. 2015;88(1048):20140712. https://doi.org/10.1259/bjr.20140712.
Habl G, Straube C, Schiller K, Duma MN, Oechsner M, Kessel KA, Eiber M, Schwaiger M, Kübler H, Gschwend JE, Combs SE. Oligometastases from prostate cancer: local treatment with stereotactic body radiotherapy (SBRT). BMC Cancer. 2017;17(1):361. https://doi.org/10.1186/s12885-017-3341-2.
Ost P, Bossi A, Decaestecker K, Meerleer G, Giannarini G, Karnes RJ, Roach M, Briganti A. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015;67(5):852–63. https://doi.org/10.1016/j.eururo.2014.09.004.
Muldermans JL, Romak LB, Kwon ED, Park SS, Olivier KR. Stereotactic body radiation therapy for oligometastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2016;95(2):696–702. https://doi.org/10.1016/j.ijrobp.2016.01.032 (Epub 2016 Jan 29).
Decaestecker K, De Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T, De Vos F, Huysse W, Hautekiet A, Maes G, Ost P. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol. 2014;12(9):135. https://doi.org/10.1186/1748-717X-9-135.
Decaestecker K, De Meerleer G, Ameye F, Fonteyne V, Lambert B, Joniau S, Delrue L, Billiet I, Duthoy W, Junius S, Huysse W, Lumen N, Ost P. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): study protocol for a randomized phase II trial. BMC Cancer. 2014;15(14):671. https://doi.org/10.1186/1471-2407-14-671.
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, Lambert B, Delrue L, Bultijnck R, Claeys T, Goetghebeur E, Villeirs G, De Man K, Ameye F, Billiet I, Joniau S, Vanhaverbeke F, De Meerleer G. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446–53. https://doi.org/10.1200/JCO.2017.75.4853.
Acknowledgements
This work was partially supported by the research Grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC): IG-13218 “Short-term high precision RT for early prostate cancer with concomitant boost to the dominant lesion,” registered at ClinicalTrials.gov NCT01913717, approved by IEO S768/113 and IG-14300 “Carbon ions boost followed by pelvic photon radiotherapy for high risk prostate cancer” and by a research grant from Accuray Inc. entitled “Data collection and analysis of Tomotherapy and CyberKnife breast clinical studies, breast physics studies and prostate study.” The Sponsors did not play any role in the study design, collection, analysis and interpretation of data, nor in the writing of the manuscript, nor in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Ethical approval
The study was a part of general SBRT and image-guided radiation therapy (IGRT) research notified to the Institutional Ethical Committee (Notifications No. 79/10, 86/11, 87/11, 93/11).
Informed consent
In this research, no animals were involved. All patients signed a written informed consent for stereotactic body radiation therapy (SBRT) and written informed consent for the use of the anonymized data for research or educational purpose.
Rights and permissions
About this article
Cite this article
Fanetti, G., Marvaso, G., Ciardo, D. et al. Stereotactic body radiotherapy for castration-sensitive prostate cancer bone oligometastases. Med Oncol 35, 75 (2018). https://doi.org/10.1007/s12032-018-1137-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1137-0