Abstract
To assess the outcomes of a cohort of bone oligometastatic prostate cancer patients treated with PSMA-PET guided stereotactic body radiotherapy (SBRT). From April 2017 to January 2021, 40 patients with oligorecurrent prostate cancer detected by PSMA-PET were treated with SBRT for bone oligometastases. Concurrent androgen deprivation therapy was an exclusion criterion. A total of 56 prostate cancer bone oligometastases were included in the present analysis. In 28 patients (70%), oligometastatic disease presented as a single lesion, two lesions in 22.5%, three lesions in 5%, four lesions in 2.5%. 30.3% were spine-metastases, while 69.7% were non-spine metastases. SBRT was delivered for a median dose of 30 Gy (24–40 Gy) in 3–5 fractions, with a median EQD2 = 85 Gy2 (64.3—138.9Gy2). With a median follow-up of 22 months (range 2–48 months), local control (LC) 1- and 2-years rates were 96.3% and 93.9%, while distant progression-free survival (DPFS) rates were 45.3% and 27%. At multivariate analysis, the lower PSA nadir value after SBRT remained significantly related to better DPFS rates (p = 0.03). In 7 patients, a second SBRT course was proposed with concurrent ADT, while 11 patients, due to polymetastatic spread, received ADT alone, resulting in 1- and 2-years ADT-free survival rates of 67.5% and 61.8%. At multivariate analysis, a lower number of treated oligometastases maintained a correlation with higher ADT-free survival rates (p = 0.04). In our experience, PSMA-PET guided SBRT resulted in excellent results in terms of clinical outcomes, representing a helpful tool with the aim to delay the start of ADT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advanced or metastatic prostate cancer (PC) represents a challenging clinical entity due to the complexity and heterogeneity of the disease [1, 2].
Oligometastatic PC represents a favorable prognostic phase characterized by a limited metastatic growth potential. This specific clinical/biologic behavior is mainly related to several factors including the poor properties of circulating cancer cells to colonize target organs [3].
In the recent years, oligometastatic PC has represented a sort of in vivo laboratory in which the role of focal treatments has assumed a strong rationale [4].
More specifically, several advances have been made in terms of common terminology, study design and evidence-based medicine useful to support the routine use of the so-called metastases directed therapy (MDT) in oligorecurrent PC [5, 6].
Some concerns remain regarding the ideal oligometastatic PC patient suitable for MDT approach. Several variables have been investigated such as the number of metastases, PSA doubling time and location of oligometastases [7, 8].
On the other hand, due to the heterogeneity of the disease, few widespread mutations have been identified, thus complicating the use of genotypic precision medicine [9].
For this reason, in advanced PC, it is recently adopted the term of phenotypic precision medicine, assessed through non-invasive diagnostics, such as PSMA-PET exams. This last treatment decision strategy could overcome some limitations of biologic samples for genetic sequencing such as the morbidity associated with biopsy, specifically for bone metastases. Lastly, biopsies of a single metastases may not capture the intra- and inter metastatic heterogeneity [2, 10,11,12,13].
There are limited published experience regarding phenotypic precision medicine by means of PSMA-guided MDT in the case of bone castration sensitive oligometastatic PC. Herein, we report the preliminary findings of a homogeneous series of bone oligometastatic castration sensitive PC patients.
Methods
Written informed consent was obtained from all the patients. From April 2017 to January 2021, 40 patients with castration-sensitive bone oligorecurrent prostate cancer detected by means of PSMA-PET were treated with SBRT. PET-CT was considered positive based on a qualitative visual assessment (i.e., metabolic activity moderately or markedly increased relative to comparable surrounding normal tissues). A lesion with no or faint uptake (less than the surrounding tissues) of the tracer was defined as negative. Lesions with increased uptake suspicious for secondary lesions were considered suitable for metastasis-directed approach also in the case of absence of morphologic bone changes, taking also into account PSA-kinetics. No biopsy was performed in the case of PET positivity. Oligometastatic disease was defined as any presentation with up to five lesions amenable for local treatment, with a maximum diameter ≤ 5 cm. Concurrent androgen deprivation therapy was an exclusion criterion for the purpose of this study.
SBRT was proposed to all patients with a minimum Karnofsky Performance Status ≥ 70 and a life expectancy of at least 6 months. Table 1 collects patients’ characteristics.
Radiotherapy protocol
A 1–2.5 mm slice thickness computed tomography (CT) was acquired for treatment planning purposes. Immobilization procedures for treatment simulation were performed in supine position with the aid of a knee-ankle device in the case of pelvic targets. For thoracic or upper limbs targets, immobilization was performed with the arms up above the head in order to replicate the PSMA-PET positioning. For spine SBRT treatment, immobilization was performed using an abdominal thermoplastic mask customized on patient’s body.
For target volume delineation, the gross tumor volume (GTV) was defined as the radiologically evident disease and contoured with co-registered diagnostic PET-imaging. Clinical target volume (CTV) was considered equivalent to the GTV. The planning target volume (PTV) was generated by adding an isotropic margin ranging from 5 to 7 mm, according to the tumor site. In the case of spine metastases, target volume delineation was performed according to literature guidelines [14].
The dosimetric goal for treatment planning was to guarantee at least 95% of the prescribed dose to the 95% of the PTV. Dose constraints for organs at risk were derived from peer-reviewed literature [15,16,17].
All patients received SBRT delivered with Cone Beam CT-based image guided volumetric modulated arc therapy (IGRT-VMAT).
Clinical outcomes assessment
Follow-up visits were planned with quarterly periodicity after SBRT for the first year, and every 6 months starting from the second year. Treatment response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria v1.1 and PET Response Evaluation Criteria in Solid Tumors (PERCIST) criteria v1.0 in the case of metabolic imaging. Toxicity was prospectively collected and assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0
Statistical analysis
Descriptive statistics were collected for baseline patients’ characteristics. Local control (LC), distant progression-free survival (DPFS), androgen deprivation therapy (ADT)-free survival and overall survival (OS) were assessed using Kaplan–Meier method. Univariate and multivariate analyses were performed to assess any potential predictive factor for clinical outcomes. A p < 0.05 was assumed as statistically significant; a p-value ≤ 0.20 was considered as reference for multivariate analysis. All statistical analyses were carried out using MedCalc Statistical Software v.20.009 (MedCalc Software bv, Ostend, Belgium; https://www.medcalc.org).
Results
A total of 56 prostate cancer bone oligometastases treated with SBRT between April 2017 and January 2021 in 40 patients were included in the present analysis. All lesions were detected by means of PSMA-PET. Median age was 69.5 years (range 54–85 years).
In 27 subjects (67.5%), primary treatment consisted of surgery, while 6 patients received definitive radiotherapy (15%). In the remaining cases (17.5%), primary treatment consisted of surgery and post-operative radiotherapy.
Oligometastatic disease occurred after a median interval of 39 months (range 2–244 months) from the primary treatment, with a median PSA doubling time of 6.7 months (range 1.1–40.8 months) and a median baseline PSA = 0.60 ng/ml (range 0.16–15.2 ng/ml). In 28 patients (70%), oligometastatic disease presented as a single bone lesion, two lesions in 9 cases (22.5%), three lesions in 2 subjects (5%), four lesions in one patient (2.5%). Among the 56 lesions, 17 (30.3%) were spine-metastases, while the remaining 39 (69.7%) were non-spine metastases. In the entire cohort, SBRT was delivered alone, without androgen deprivation therapy, for a median dose of 30 Gy (range 24–40 Gy) delivered in 3–5 fractions, with a median equivalent-dose in 2 Gy/fraction (EQD2) = 85 Gy2 (range 64.3—138.9Gy2).
Patients’ characteristics are displayed in Table 1.
Clinical outcomes
The median follow-up for the entire cohort was 30 months (range 10–56 months). At the last follow-up, all patients are alive.
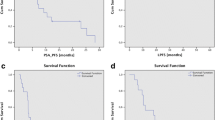
Median local control (LC) was 18 months (range 2–48), resulting in 1- and 2-years rates of 96.3% and 93.9%. (Fig. 1).
The median value of nadir PSA after SBRT was 0.9 ng/ml (0.36–13.8 ng/ml), with twelve patients who did not report a PSA drop after the treatment. Consequently, 1- and 2-years distant progression-free survival (DPFS) rates were 45.3% and 27% for a median time interval of 9 months (range 3–37 months) (Fig. 1).
At univariate analysis, a longer time to the evidence of oligometastastic disease was found to favorably impact on DPFS (p-value = 0.0003); similarly, for lower number of metastases treated (p = 0.003), lower PSA pre-SBRT (p = 0.0013) and PSA nadir values after SBRT (p < 0.0001). Interestingly, also those patients who kept LC of the treated lesions maintained an advantage in terms of DPFS (p = 0.017). Nonetheless, at multivariate analysis, only the lower PSA nadir value after SBRT remained significantly related to better DPFS rates (p = 0.03) (Tables 2–3).
Eleven patients developed a further oligoprogression (10 new bone lesions and one lymph node oligometastasis) for which a new course of SBRT was proposed after a median interval of 8 months (range 3–25 months). In 7 patients, a second course of SBRT was proposed with concurrent ADT, while 11 patients, due to the evidence of polymetastatic spread, received ADT alone, thus resulting in 1- and 2-years ADT-free survival rates of 67.5% and 61.8%, for a median ADT-free survival time of 13.5 months (range 2–45 months).
At univariate analysis, ADT-free survival was found to be significantly related to lower number of oligometastases treated (p = 0.0001), a longer disease-free interval (i.e. the time between primary treatment and the onset of oligometastatic disease, p = 0.0097) and lower PSA values both before (p < 0.0001) and after SBRT (p = 0.004). At multivariate analysis, only the number of oligometastases treated maintained a significant correlation with higher ADT-free survival rates (p = 0.04) (Tables 2–3).
Toxicity
All patients completed the planned sessions without any interruption. No acute or late grade 2 or higher adverse events were observed until the last follow-up.
Discussion
Literature data regarding the clinical outcomes by MDT for bone metastases are generally affected by several confounding factors, such as: administration of concomitant ADT, heterogeneous populations including synchronous and metachronous metastatic PC, several diagnostic tools used to propose MDT. We conducted a multi-institutional retrospective study to explore the clinical outcomes following exclusive PSMA-guided MDT for bone-only PC oligometastases. In the current study, only metachronous castration-sensitive bone oligorecurrent PC were collected and analyzed. We aimed to assess the impact of this approach on a homogeneous cohort of patients, for which very few data are currently available in the literature. For the purpose of the study, concurrent ADT was an exclusion criterion. The current results showed high rates of LC with optimal tolerability profile. 1- and 2-years LC rates were 96.3% and 93.9%, respectively. We did not find any statistically significant correlation with the BED1.5 Gy, likely due to the paucity of local failure events. No acute or late grade 2 or higher adverse events were observed. On the other hand, 1- and 2-years distant progression-free survival (DPFS) rates were 45.3% and 27%. Several factors were statistically related to a more favorable DPFS, such as: the longer disease-free interval between the primary tumor treatment and the onset of oligorecurrent disease, the limited number of treated metastases, the low-levels of PSA pre- and post-SBRT. At multivariate analysis, only the low-value of PSA nadir revealed to be a predictive factor of better DPFS. This last finding indicates that microscopic and, thus, untreated disease was present at the time of molecular imaging, and it also might be the reason for reporting a higher value of PSA nadir post-SBRT when compared to PSA pre-SBRT.
Patients affected by bone metastatic PC have unfavorable prognosis. Bone tissue could provide a favorable microenvironment (so-called “niches”) where PC cells can nestle, survive and re-growth [18].
Probably, ADT could modify the homing of cancer cells to the bone with a subsequent dormancy status of bone PC cells [19].
Clinically, it remains a field of investigation which bone oligometastatic PC patient could benefit of MDT alone compared to combined ADT + MDT approaches. Few experiences evaluated the potential impact of a tailored strategy by means of PSMA-guided MDT for bone metastases. Of these, the TROD 09–004 study retrospectively evaluated the outcomes of MDT for bone-only oligometastases detected by means of 68 Ga-PSMA PET/CT in a quite heterogeneous population. In fact, 36.5% had synchronous diseases whereas 63.5% had metachronous oligometastatic castration-sensitive PC [20].
Additionally, synchronous oligometastatic PC patients were more likely to receive ADT during and after SBRT. Similarly, Rogowski and colleagues recently published their data regarding PSMA-guided MDT for bone-only oligometastatic PC [21].
Concomitant ADT was administered in 69% of patients. In both experiences, concomitant ADT related to better DPFS comparing to the current data.
Some uncertainties related to the ADT administration are related to the possible side effects and impairment of patients’ quality of life. Therefore, clinicians aim to defer ADT by means of MDT. About that, ADT-free survival (ADT-FS) was recently introduced as an interesting clinical endpoint, ranging between 7–66 months in several studies. Herein, 1- and 2-years ADT-free survival rates were 67.5% and 61.8%, for a median ADT-free survival time of 13.5 months (range 2–45 months).
The real challenge in the future will be to distinguish, well in advance, patients with occult polymetastatic disease from those with a “true” oligometastatic state. This last distinction could open to possible personalized therapies in terms of MTD alone or combined with ADT. Surely, biomarkers as well as modern molecular imaging could help to detect oligometastatic disease with great sensitivity and specificity. In a comparative analysis of effectiveness between MDT guided by PET-CT 18F-choline versus PET-CT 68 Ga-PSMA in the setting of castration-sensitive oligorecurrent PC, ADT administration was greater after PET-choline guided MDT. This effect was related to the higher incidence of polymetastatic disease after first-course SBRT compared with 68 Ga-PSMA-based SBRT [22, 23].
In conclusion, exclusive PSMA-guided MDT for bone-only castration sensitive oligometastatic PC is safe and guarantees high rates of LC. At 1 year 45% of patients were free-from disease progression with a median ADT-FS time of 13.5 months.
References
Testa U, Castelli G, Pelosi E (2019) Cellular and molecular mechanisms underlying prostate cancer development: therapeutic implications. Medicines 6(3):82. https://doi.org/10.3390/medicines6030082
Haffner MC, Zwart W, Roudier MP, True LD, Nelson WG, Epstein JI, De Marzo AM, Nelson PS, Yegnasubramanian S (2021) Genomic and phenotypic heterogeneity in prostate cancer. Nat Rev Urol 18(2):79–92. https://doi.org/10.1038/s41585-020-00400-w
Chalkidou A, Macmillan T, Grzeda MT, Peacock J, Summers J, Eddy S, Coker B, Patrick H, Powell H, Berry L, Webster G, Ostler P, Dickinson PD, Hatton MQ, Henry A, Keevil S, Hawkins MA, Slevin N, van As N (2021) Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: a prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol 22(1):98–106. https://doi.org/10.1016/S1470-2045(20)30537-4
Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, Dingemans AC, Fournier B, Hurkmans C, Lecouvet FE, Meattini I, Méndez Romero A, Ricardi U, Russell NS, Schanne DH, Scorsetti M, Tombal B, Verellen D, Verfaillie C, Ost P (2020) Characterisation and classification of oligometastatic disease: a European society for radiotherapy and oncology and European organisation for research and treatment of cancer consensus recommendation. Lancet Oncol 21(1):e18–e28. https://doi.org/10.1016/S1470-2045(19)30718-1
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, Lambert B, Delrue L, Bultijnck R, Claeys T, Goetghebeur E, Villeirs G, De Man K, Ameye F, Billiet I, Joniau S, Vanhaverbeke F, De Meerleer G (2018) Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol 36(5):446–453. https://doi.org/10.1200/JCO.2017.75.4853
Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, Rowe SP, Ross AE, Gorin MA, Deville C, Greco SC, Wang H, Denmeade SR, Paller CJ, Dipasquale S, DeWeese TL, Song DY, Wang H, Carducci MA, Pienta KJ, Pomper MG, Dicker AP, Eisenberger MA, Alizadeh AA, Diehn M, Tran PT (2020) Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol 6(5):650–659. https://doi.org/10.1001/jamaoncol.2020.0147
Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K, Tangen CM, Rosenthal M, Petrylak DP, Hussain M, Vogelzang NJ, Thompson IM, Chi KN, de Bono J, Armstrong AJ, Eisenberger MA, Fandi A, Li S, Araujo JC, Logothetis CJ, Quinn DI, Morris MJ, Higano CS, Tannock IF, Small EJ (2016) Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol 34(14):1652–1659. https://doi.org/10.1200/JCO.2015.65.7270
Ost P, Decaestecker K, Lambert B, Fonteyne V, Delrue L, Lumen N, Ameye F, De Meerleer G (2014) Prognostic factors influencing prostate cancer-specific survival in non-castrate patients with metastatic prostate cancer. Prostate 74(3):297–305. https://doi.org/10.1002/pros.22750
Cancer Genome Atlas Research Network (2015) The molecular taxonomy of primary prostate cancer. Cell 163(4):1011–1025. https://doi.org/10.1016/j.cell.2015.10.025
Ku SY, Gleave ME, Beltran H (2019) Towards precision oncology in advanced prostate cancer. Nat Rev Urol 16(11):645–654. https://doi.org/10.1038/s41585-019-0237-8
Evans R, Loeb A, Kaye KS, Cher ML, Martin ET (2017) Infection-related hospital admissions after prostate biopsy in United States men. Open Forum Infect Dis. 4(1):ofw265
Mullane SA, Van Allen EM (2016) Precision medicine for advanced prostate cancer. Curr Opin Urol 26(3):231–239. https://doi.org/10.1097/MOU.0000000000000278.PMID:26909474;PMCID:PMC4955574
Mazzoni A, Oddo CM, Valle G, Camboni D, Strauss I, Barbaro M, Barabino G, Puddu R, Carboni C, Bisoni L, Carpaneto J, Vecchio F, Petrini FM, Romeni S, Czimmermann T, Massari L, di Iorio R, Miraglia F, Granata G, Pani D, Stieglitz T, Raffo L, Rossini PM, Micera S (2020) Morphological neural computation restores discrimination of naturalistic textures in trans-radial amputees. Sci Rep 10(1):527. https://doi.org/10.1038/s41598-020-57454-4.Erratum.In:SciRep.2021Aug11;11(1):16662.PMID:31949245;PMCID:PMC6965126
Cox BW, Spratt DE, Lovelock M, Bilsky MH, Lis E, Ryu S, Sheehan J, Gerszten PC, Chang E, Gibbs I, Soltys S, Sahgal A, Deasy J, Flickinger J, Quader M, Mindea S, Yamada Y (2012) International spine radiosurgery consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 83(5):e597-605. https://doi.org/10.1016/j.ijrobp.2012.03.009
Sahgal A, Chang JH, Ma L, Marks LB, Milano MT, Medin P, Niemierko A, Soltys SG, Tomé WA, Wong CS, Yorke E, Grimm J, Jackson A (2019) Spinal cord dose tolerance to stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys S0360–3016(19):33862–33863. https://doi.org/10.1016/j.ijrobp.2019.09.038
Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, Purdie T, Sadagopan R, Schell MC, Salter B, Schlesinger DJ, Shiu AS, Solberg T, Song DY, Stieber V, Timmerman R, Tomé WA, Verellen D, Wang L, Yin FF (2010) Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 37(8):4078–4101. https://doi.org/10.1118/1.3438081
Moraes FY, Chen X, Yan M et al (2020) Evolving role of stereotactic body radiation therapy in the management of spine metastases: defining dose and dose constraints. Neurosurg Clin N Am 31(2):167–189. https://doi.org/10.1016/j.nec.2019.12.001
Wang N, Docherty FE, Brown HK, Reeves KJ, Fowles AC, Ottewell PD, Dear TN, Holen I, Croucher PI, Eaton CL (2014) Prostate cancer cells preferentially home to osteoblast-rich areas in the early stages of bone metastasis: evidence from in vivo models. J Bone Miner Res 29(12):2688–2696. https://doi.org/10.1002/jbmr.2300
Zhang X (2019) Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun (Lond) 39(1):76. https://doi.org/10.1186/s40880-019-0425-1
Onal C, Ozyigit G, Akgun Z, Atalar B, Igdem S, Oymak E, Agaoglu F, Selek U, Guler OC, Hurmuz P, Mustafayev TZ, Akyol F (2021) Oligometastatic bone disease in castration-sensitive prostate cancer patients treated with stereotactic body radiotherapy using 68Ga-PSMA PET/CT: TROD 09–004 study. Clin Nucl Med 46(6):465–470. https://doi.org/10.1097/RLU.0000000000003558
Rogowski P, Trapp C, von Bestenbostel R, Schmidt-Hegemann NS, Shi R, Ilhan H, Kretschmer A, Stief C, Ganswindt U, Belka C, Li M (2021) Outcomes of metastasis-directed therapy of bone oligometastatic prostate cancer. Radiat Oncol 16(1):125. https://doi.org/10.1186/s13014-021-01849-8
Mazzola R, Francolini G, Triggiani L, Napoli G, Cuccia F, Nicosia L, Livi L, Magrini SM, Salgarello M, Alongi F (2021) Metastasis-directed therapy (SBRT) guided by PET-CT 18F-choline versus PET-CT 68Ga-PSMA in castration-sensitive oligorecurrent prostate cancer: a comparative analysis of effectiveness. Clin Genitourin Cancer 19(3):230–236. https://doi.org/10.1016/j.clgc.2020.08.002
Alongi F, Fersino S, GiajLevra N, Mazzola R, Ricchetti F, Fiorentino A, Ruggieri R, Malfatti V, Cavalleri S, Salgarello M (2015) Impact of 18F-Choline PET/CT in the decision-making strategy of treatment volumes in definitive prostate cancer volumetric modulated radiation therapy. Clin Nucl Med 40(11):e496-500. https://doi.org/10.1097/RLU.0000000000000841
Funding
No fundings received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mazzola, R., Cuccia, F., Pastorello, E. et al. PSMA-guided metastases directed therapy for bone castration sensitive oligometastatic prostate cancer: a multi-institutional study. Clin Exp Metastasis 39, 443–448 (2022). https://doi.org/10.1007/s10585-022-10157-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-022-10157-8