Abstract
Accelerated hypofractionated whole-breast radiotherapy (WBRT) is considered a standard therapeutic option for early breast cancer (EBC) in the postoperative setting after breast conservation (BCS). A boost to the lumpectomy cavity may further increase local control. We herein report on the 10-year results of a series of EBC patients treated after BCS with hypofractionated WBRT with a concomitant photon boost to the surgical bed over 4 weeks. Between 2005 and 2007, 178 EBC patients were treated with a basic course of radiotherapy consisting of 45 Gy to the whole breast in 20 fractions (2.25 Gy daily) with an additional boost dose of 0.25 Gy delivered concomitantly to the lumpectomy cavity, for an additional dose of 5 Gy. Median follow-up period was 117 months. At 10-year, overall, cancer-specific, disease-free survival and local control were 92.2% (95% CI 88.7–93.4%), 99.2% (95% CI 96.7–99.7%), 95.5% (95% CI 91.2–97.2%) and 97.3% (95% CI 94.5–98.9%), respectively. Only eight patients recurred. Four in-breast recurrences, two axillary node relapses and two metastatic localizations were observed. Fourteen patients died during the observation period due to other causes while breast cancer-related deaths were eight. At last follow-up, ≥G2 fibrosis and telangiectasia were seen in 7% and 5% of patients. No major lung and heart toxicities were observed. Cosmetic results were excellent/good in 87.8% of patients and fair/poor in 12.2%. Hypofractionated WBRT with concomitant boost to the lumpectomy cavity after BCS in EBC led to consistent clinical results at 10 years. Hence, it can be considered a valid treatment option in this setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The standard treatment option for early-stage breast cancer (EBC) comprises conservative surgery, radiotherapy, chemotherapy, and hormone therapy, differently combined according to patient and tumor characteristics [1]. Breast-conserving surgery (BCS) and subsequent adjuvant whole-breast radiotherapy (WBRT) showed equivalent outcomes compared to mastectomy in this subset of patients [2,3,4]. A total dose of 45–50 Gy, given with conventional fractionation (1–8–2 Gy daily) over 5 weeks, is generally delivered for WBRT. A subsequent 10–16 Gy boost dose to the lumpectomy cavity can be added sequentially to reduce the rate of local relapse, over 1–2 adjunctive weeks of treatment [5]. To optimize patient and healthcare system resources, hypofractionated regimens (radiation delivered with higher dose per fraction given over a shorter treatment period) were implemented [6]. Clinical results of hypofractionated WBRT were found to be consistent, especially if the total nominal dose is lowered compared to conventionally fractionated schedules [7,8,9,10,11,12,13]. Prospective randomized studies contributed to create clinical evidence in this setting [14,15,16]. Thus, we started treating EBC patients with WBRT after BCS, employing a 20 fraction schedule delivered over 4 weeks with a concomitant boost to the lumpectomy cavity [8]. We implemented this approach in several settings including both invasive and in situ disease and results at 5 years have already been reported [17–19]. We herein present 10-year results in terms of local control, survival, toxicity profile and cosmetic results.
Methods and materials
Data of 178 EBC patients were retrieved from the medical records of our institution hospital and reviewed. Those patients were treated with WBRT at the Ivrea Hospital between March 2005 and May 2007.
Cohort characteristic
Patients had a histologically proven diagnosis of breast adenocarcinoma, received prior conservative surgery (lumpectomy or quadrantectomy) with negative surgical margins and pathological stage pT1 or pT2, pN0-N1 according to America Joint Committee-Union Internationale Contre le Cancer staging system (6th Edition 2002).
Setup, simulation and target definition
All the 178 patients were positioned on a wingboard with both arms raised above the head and radiopaque markers placed along the clinical borders of the breast. Subsequently, 5-mm slice thickness axial images were acquired from the lower aspect of the mandible to the bases of the lung. An isocenter was then found in virtual simulation using the CT-simulation software Oncentra Masterplan v 3.0 (Nucletron, Veendhal, The Netherlands). The whole-breast clinical target volume (WB-CTV) encompassed the palpable remaining glandular tissue of the breast, with a superior and inferior border delimited within the extent of the radiopaque catheters, which marked the palpable breast gland. Moreover, a uniform limit of 5 mm separated the WB-CTV from the skin surface and the thoracic wall. The whole-breast planning target volume (WB-PTV) was generated by adding a 5-mm isotropic margin around the WB-CTV and subsequently refined to account for the skin and thoracic surface and organs at risk. The definition of the lumpectomy cavity was driven by the position of radioopaque clips placed at time of surgery. The concomitant boost clinical target volume (CB-CTV) was generated by adding a 5-mm isotropic margin around the lumpectomy cavity, while the concomitant boost planning target volume (CB-PTV) required a further margin of 5 mm around the CB-CTV. The heart and ipsilateral lung were separately contoured as organs at risk; particularly, the heart was outlined up to the level of the pulmonary trunk superiorly, with the inclusion of the pericardium and the exclusion of the major vessels.
Treatment schedule and delivery
The basic course for WBRT consisted of 45 Gy, prescribed to the ICRU reference point dose and delivered to the whole breast in 20 fractions with 2.25 Gy per fraction with two opposing 6 MV tangential fields. An additional boost dose of 0.25 Gy was concomitantly delivered, on a daily basis, to the lumpectomy cavity (CB-PTV), for a total additional dose of 5 Gy, using 2–3 adjunctive 6 MV-photon fields. The cumulative nominal dose was 50 Gy. Finally, the isocenter and the dose normalization point were identical either for the breast or for the boost plan. For setup verification purposes, tangential fields portal images were taken before the first treatment session and quantitatively compared with digitally reconstructed radiographs (DRRs) obtained from planning computed tomography.
Follow-up, toxicity and cosmesis
During follow-up time, patients were examined at 3 and 6 months after the end of WBRT and twice a year afterward. Surveillance for disease recurrence at any site included a clinical examination at every time point, plan chest X-ray and mammography once a year, complete blood cell count and serological markers twice a year. Acute toxicity was assessed at the completion of treatment and after 3 months; conversely, late toxicity was scored starting from 6 months after the end of the treatment course. The maximal detected toxicity was scored according to the Common Terminology Criteria for Adverse Events, version 3.0, using the RTOG/EORTC toxicity scale associated with radiation as Refs. [20, 21]. Cosmetic results were assessed at the end of the radiation course and thereafter at 3 and 6 months of follow-up time, using a cosmetic evaluation scale consisting of different categories, namely excellent, good, fair or poor in agreement with the Harvard criteria [22]. At each follow-up examination, patients were asked to judge the cosmetic appearance of the irradiated breast as follows: “excellent,” designating little or no change; “good,” minimal but noticeable change; “fair,” significant change; “poor,” severe change. Similarly, physicians were asked to judge the cosmetic results as follows: an “excellent” cosmetic result score was assigned when the treated breast looked essentially the same as the contralateral breast; a “good” cosmetic score was assigned for minimal but identifiable radiation effects of the treated breast; a “fair” score meant significant radiation effects were readily observable; a “poor” score was used for radiation-induced severe late effects of breast tissue.
Statistical analysis
Descriptive statistical analysis was provided for all 178 patients. Overall (OS), cancer-specific (CSS), disease-free (DFS) survival and local control (LC) were assessed. Disease recurrence was defined as local (LR) if occurring in the ipsilateral breast or overlying skin, as regional (RR) if involving the ipsilateral axillary, supraclavicular or internal mammary lymph nodes and as systemic with distant metastasis (DM) if arising at other sites. Local relapse, RR and DM were evaluated to calculate the disease-free survival (DFS). Multivariate analyses were also performed on the available dataset. Cox proportional hazard model was used to develop a regression model for survival based on pretreatment characteristics, and Kaplan–Meier estimate was used to evaluate survival. Groups were compared with the log-rank test. A p value <0.05 between groups was considered significant. Time was calculated starting from the end of WBRT. Deaths from disease recurrence were considered as an event for disease-specific mortality, while the others were censored. In descriptive statistics, mean values, standard deviation and statistical range were reported for numerical variables, while for ordinal and categorical variables data were arranged as rates. In multivariate analysis, tumor and nodal stage, grading, estrogen/progesterone receptor status, Ki-67, histology, age, chemotherapy, hormonal therapy and surgery were included. Kaplan–Meier analysis was stratified according to these variables and plotted against overall and disease-free survival. All statistical analyses were performed using IBM SPSS® Statistical Software, version 20.0.
Results
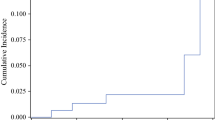
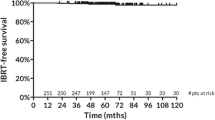
Baseline characteristics of all 178 patients are reported in Table 1. Almost 92% were aged >50; most represented pathological stages were pT1b/pT1cN0. Up to 23% of the patients received adjuvant chemotherapy, while up to 91% was given hormonal therapy. The most common surgical approach was quadrantectomy and sentinel lymph node biopsy (66.3%), while almost 22% of the patients received also axillary dissection. All patients completed the planned radiotherapy program, with no interruptions needed. In Table 2, the average PTV and boost volumes and average doses to ipsilateral lung and heart (for left-sided tumors) are reported. With a median follow-up of 117 months (range 4–140), 10-year OS, CSS, DFS and LC were 92.2% (95% CI 88.7–93.4%), 99.2% (95% CI 96.7–99.7%), 95.5% (95% CI 91.2–97.2%) and 97.3% (95% CI 94.5–98.9%), respectively (Figs. 1, 2). Only eight patients experienced disease recurrence during the observation period with four in-breast recurrences, two axillary node relapses and two metastatic localizations. Fourteen patients died during follow-up due to other causes while breast cancer-related deaths were seen in eight patients; among the latter, four LR, two RR and two DM were seen. At last follow-up examination, ≥ G2 fibrosis and telangiectasia were 7% and 5%, respectively. No major lung and heart toxicities could be detected. Cosmetic results were scored as excellent/good in 87.8% of patients and fair/poor in 12.2%. Cox multivariate analysis was performed on the pretreatment with respect to DFS (T, N, G, PGR, ER, KI67, histology, age, chemotherapy, hormone therapy and surgery). As seen in Table 3, nodal stage and estrogen receptor status had a significant correlation with DFS (p = 0.037 and p = 0.026, respectively).
Discussion
Hypofractionation, with the delivery of a daily dose per fraction higher than 1–8–2 Gy, is a valid option to treat EBC patients in a postoperative setting after breast conservation, shortening the overall treatment time [23]. Long-term follow-up data coming from randomized phase III trials confirmed consistent clinical results for hypofractionated schedules in this setting [24, 25]. Concerning the UK studies, the START-A trial employed 41.6 or 39 Gy in 13 fractions over 5 weeks, while the START-B trial used a regimen of 40 Gy in 15 fractions over 3 weeks, as experimental arms [15, 16]. Conversely, the Canadian trial used a pragmatic regimen of 42.5 Gy in 16 fractions over 22 days, in a similar manner as the START-B study [14]. These reported relapse rates of 3.5% (START-A for the 41.6 Gy/13 fraction schedule), 2% (START-B) and 2.8% (Canadian trial) at 5 years and of 4.3% (START trials) and 6.2% (Canadian study) at 10 years. [14,15,16, 24, 25]. In the Canadian study, no patients received a boost dose to the lumpectomy cavity [14]. In the UK trials, the boost strategy was left to discretion of enrolling institutions, outside the study protocol. Notably, in the START-B study, almost 44% of the enrolled patients received a boost [16]. In our series of patients, LC rate was 97.3% (95% CI 94.5–98.9%), with a percentage of local relapse below 3% (namely 2.7%). This is reasonable, considering the low-risk profile of our cohort where most of the tumors were below 2 cm (76.9%), pN0 (79.8%), G1–G2 (83.2%), with expression of estrogen receptor (88.8%). These findings are consistent with clinical series available in the literature [26]. A positive nodal status and a negative estrogen receptor status were found as significantly correlated with the likelihood to develop tumor recurrence. These clinical and biological factors are well-established prognostic variables for breast cancer patients [1]. In our series, we employed a boost to the lumpectomy cavity, in order to provide a further potential benefit in terms of LC. The EORTC “boost vs no boost” trial demonstrated a 10-year cumulative incidence of local relapse of 10.2% for the boost arm compared to 6.2% for the no boost arm [27]. The largest absolute risk reduction (23.9 vs 13.5%; p = 0.0014) was seen for patients ages ≤40 [27]. Risk factors for local relapse were young age (≤50 years) and high-grade invasive ductal carcinoma. A 16 Gy sequential boost dose to the surgical bed significantly reduced the rate recurrence (19.4 vs 11.4%; HR 0.51; p = 0.0046; 18.9 vs 8.6%; HR 0.42; p = 0.01) [27]. Hence, not all cases do benefit from an additional dose to the surgical bed, but only a subset of patients stratified according to clinical characteristics and tumor features. In general, the boost dose to the lumpectomy cavity can be delivered after the WBRT phase, employing a sequential approach or incorporated within the irradiation of the whole breast with a concomitant boost or simultaneous integrated boost strategy [28, 29]. Incorporating the boost dose in the whole-breast phase can shorten the overall treatment time, with a further acceleration of treatment [30]. Moreover, dosimetric advantages in terms of normal tissue avoidance and dose homogeneity and conformity within the lumpectomy cavity can be seen [26]. The role of boost integration within a hypofractionated WBRT schedule is being explored in few prospective trials. In the USA, the RTOG 1005 trial was launched as a phase III prospective study exploring accelerated WBRT for EBC, comparing standard RT (50 Gy/25 fractions) (with the option of 42.7/16 fr; 2.67 Gy daily) followed by a sequential boost of 12–14 Gy/6–7 fractions vs an hypofractionated accelerated WBRT schedule of 40 Gy/15 fractions (2.67 Gy daily) with a concomitant boost of 3.2 Gy to the tumor bed (up to 48 Gy/15 fractions) [26]. This trial has terminated accrual, and results are awaited. In the UK, the IMPORT High trial tested dose escalated RT delivered with IMRT in early breast cancer patients with higher than average risk of local recurrence, with the primary end-point of palpable induration inside boost volume of irradiated breast [26]. The standard arm comprised 40.5 Gy/15 fractions (2.7 Gy daily) and a sequential tumor bed boost of 16 Gy/8 fractions for adjunctive 1.6 weeks (23 fractions for total of 4.6 weeks). Two different experimental arm were chosen: in addition to 2.4 Gy × 15 fractions to the whole breast and 2.67 Gy × 15 fractions to the index quadrant, the first arm received 3.2 Gy × 15 fractions (up to 48 Gy), while the second arm got 3.5 Gy × 15 fractions (up to 53 Gy) to the tumor bed. These schedules were calculated (considering an α/β ratio = 3 Gy for tumor control) as isoeffective to 60 and 69 Gy, respectively [26]. The planned global sample size has been reached, and results are awaited. Since the first results of the aforementioned trials have yet to be published, our data provide long-term evidence on the clinical consistency in terms of local control, survival toxicity profile and cosmetic results of hypofractionated WBRT after BCS for EBC delivered with a concomitant boost to the lumpectomy cavity. This approach can be safely implemented in routine clinical practice.
Change history
12 December 2017
An error inadvertently occurred in the discussion of the original publication when citing the local relapse rates of the EORTC 22881-10882 trial (‘boost vs no boost trial’).
References
Poortmans P. Evidence-based radiation oncology: breast cancer. Radiother Oncol. 2007;84:84–101.
Veronesi U, Cascinelli N, Mariani I, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–32.
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow up of a randomized trial comparing total mastectomy, lumpectomy and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41.
Early Breast Cancer Trialists Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local control and 15-year survival: an overview of the randomized trials. Lancet. 2005;366:2087–106.
Whelan TJ, Do-Hoon K, Sussman J. Clinical experience using hypofractionated radiation schedules in breast cancer. Semin Radiat Oncol. 2008;18:257–64.
Williams MV, James ND, Summers ET, et al. National survey of radiotherapy fractionation practice in 2003. Clin Oncol (R Coll Radiol). 2006;18:3–14.
Olivotto IA, Weir LM, Kim-Sing C, Bajdik CD, Trevisan CH, Doll CM, et al. Late cosmetic results of short fractionation for breast conservation. Radiother Oncol. 1996;41:7–13.
Cante D, La Porta MR, Casanova-Borca Sciacero P, Girelli G, Pasquino M, et al. Accelerated hypofractionated adjuvant whole breast radiotherapy with concomitant photon boost after conserving surgery for early stage breast cancer: a prospective evaluation on 463 patients. Breast J. 2011;17:586–93.
Ash DV, Benson EA, Sainsbury JR, Round C, Head C. Seven-year follow up on 334 patients treated by breast conserving surgery and short course radical postoperative radiotherapy: a report of the Yorkshire Breast Cancer Group. Clin Oncol (R Coll Radiol). 1995;7:93–6.
Clark RM, Whelan T, Levine M, Roberts R, Willan A, McCulloch P, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. Ontario clinical Oncology Group. J Natl Cancer Inst. 1996;88:1659–64.
Magee B, Stewart AI, Swindell R. Outcome of radiotherapy after breast conserving surgery in screen detected breast cancer. Clin Oncol (R Coll Radiol). 1999;11:40–5.
Shelley W, Brundage M, Hayter C, Paszat L, Zhou S, Mackillop W. A shorter fractionation schedule for postlumpectomy breast cancer patients. Int J Radiat Oncol Biol Phys. 2000;47:1219–28.
McBain CA, Young EA, Swindell R, Magee B, Stewart AL. Local recurrence of breast cancer following surgery and radiotherapy: incidence and outcome. Clin Oncol (R Coll Radiol). 2003;15:25–31.
Whelan T, Mackenzie R, Julian J, Levine M, Shelley W, Grimard L, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph-node negative breast cancer. J Natl Cancer Inst. 2002;94:1143–50.
The START Trialists’ Group. The UK Standardization of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet Oncol. 2008;9:331–41.
The START Trialists’ Group. The UK Standardization of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet. 2008;371:1098–107.
Cante D, Franco P, Sciacero P, Girelli G, Pasquino M, Casanova Borca V, et al. Hypofractionated whole-breast radiotherapy and concomitant boost after breast conservation in elderly patients. Tumori. 2016;102:196–202.
Cante D, Franco P, Sciacero P, Girelli G, Marra AM, Pasquino M, et al. Hypofractionation and concomitant boost to deliver adjuvant whole-breast radiation in ductal carcinoma in situ (DCIS): a subgroup analysis of a prospective case series. Med Oncol. 2014;31:838.
Cante D, Franco P, Sciacero P, Girelli G, Marra A, Pasquino M, et al. Five-year results of a prospective case series of accelerated hypofractionated whole breast radiation with concomitant boost to the surgical bed after conserving surgery for early stage breast cancer. Med Oncol. 2013;30:518.
National Cancer Institute Cancer Therapy Evaluation Program. Common Toxicity Criteria for Adverse Events. Version 3.0. http://ctep.cancer.gov. Accessed 1 July 2017.
Cox JD, Stetz J, Pajak TF. Toxicity Criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341–6.
Rose MA, Olivotto I, Cady B, Koufman C, Osteen R, Silver B, et al. Conservative surgery and radiation therapy for early breast cancer. Long-term cosmetic result. Arch Surg. 1989;124:153–7.
Arcadipane F, Franco P, De Colle C, Rondi N, Di Muzio J, Pelle E, et al. Hypofractionation with no boost after breast conservation in early-stage breast cancer patients. Med Oncol. 2016;33:108.
Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–20.
Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–94.
Franco P, Cante D, Sciacero P, Girelli G, La Porta MR, Ricardi U. Tumor bed boost integration during whole breast radiotherapy: a review of the current evidence. Breast Care. 2015;10:44–9.
Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259–65.
Franco P, Zeverino M, Migliaccio F, Sciacero P, Cante D, Casanova Borca V, et al. Intensity-modulated adjuvant whole breast radiation delivered with static angle tomotherapy (TomoDirect): a prospective case series. J Cancer Res Clin Oncol. 2013;139:1927–36.
Franco P, Zeverino M, Migliaccio F, Cante D, Sciacero P, Casanova Borca V, et al. Intensity-modulated and hypofractionated simultaneous integrated boost adjuvant breast radiation employing statics ports of tomotherapy (TomoDirect): a prospective phase II trial. J Cancer Res Clin Oncol. 2014;140:167–77.
Rovea P, Fozza A, Franco P, De Colle C, Cannizzaro A, Di Dio A, et al. Once-weekly hypofractionated whole-breast radiotherapy after breast-conserving surgery in older patients: a potential alternative treatment schedule to daily 3-week hypofractionation. Clin Breast Cancer. 2015;15:270–6.
Acknowledgments
The Authors would like to thank all the professionals involved in the Breast Unit of the Ivrea Community Hospital and ASL Torino 4, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Ethical Approval
The present study has been reviewed and approved by the Ethical Review Board of Ivrea Community Hospital, ASLTO4, Ivrea, Italy.
Additional information
A correction to this article is available online at https://doi.org/10.1007/s12032-017-1056-5.
Rights and permissions
About this article
Cite this article
Cante, D., Petrucci, E., Sciacero, P. et al. Ten-year results of accelerated hypofractionated adjuvant whole-breast radiation with concomitant boost to the lumpectomy cavity after conserving surgery for early breast cancer. Med Oncol 34, 152 (2017). https://doi.org/10.1007/s12032-017-1020-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-017-1020-4