Abstract
Purpose
To report the 1-year outcomes of a prospective phase II study on hypofractionated whole-breast intensity-modulated radiotherapy (IM-WBRT) with a simultaneous integrated boost (SIB) to the tumor bed delivered with static ports of tomotherapy (TomoDirect) (TD).
Methods
A prospective cohort of 82 patients was enrolled between 2011 and 2012. Treatment schedule consisted of 45 Gy/20 fractions to the whole breast and 50 Gy/20 fractions to the surgical bed delivered concomitantly with TD over 4 weeks. A one-armed optimal two-stage Simon’s design was selected to test the hypothesis that treatment modality under investigation would decrease acute skin toxicity over historical data using conventional fractionation and sequential boost. Primary endpoint was acute skin toxicity. Secondary endpoints included late toxicity, cosmesis, quality of life and local control.
Results
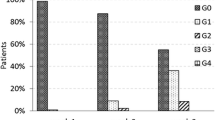
Median follow-up was 12 months (range 6–18). Maximum detected acute skin toxicity was G0 41 %; G1 53 %; G2 6 %; G3 <1 %. With two G2–G3 acute skin toxicity events in the first stage and four in the second, the study fulfilled the requirements for the definition of the treatment approach under investigation as promising. Late skin toxicity was mild with no >G2 events. Cosmesis was good/excellent in 91 % of patients and fair/poor in 9 %. Quality of life was preserved over time, with the exception of fatigue, which was transiently increased.
Conclusions
Hypofractionated IM-WBRT with a SIB to the tumor bed delivered with TD provides consistent clinical results and it is able to reduce acute skin toxicity rate over conventionally fractionated and sequential boost tomotherapy-based IM-WBRT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The standard combination therapy after conserving surgery (BCS) for early-stage breast cancer (EBC) includes adjuvant whole-breast radiotherapy (WBRT), which decreases the rate of local recurrence and increases overall survival (Poortmans 2007; Early Breast Cancer Trialists’ Collaborative Group 2005). Boosting the tumor bed (TB) further raises local control (Bartelink et al. 2007). The most common radiotherapy schedule employs conventional fractionation and a sequential approach where the TB boost dose follows the WBRT phase for a total overall treatment time of 6–7 weeks (Poortmans 2007). Hypofractionation (HF), with a lower nominal dose delivered in larger and fewer fractions generally over a shorter elapsed time interval, has been tested in several randomized controlled trials (RCTs) and it is now considered the standard choice in the United Kingdom (The START Trialists’ Group et al. 2008a, b; Whelan et al. 2010; Harnett 2010). Consequent treatment acceleration might result in improved convenience for patients and decreased costs for individuals and health organizations (Freedman et al. 2013). The incorporation of a daily TB boost dose within the WBRT phase further reduces overall treatment time. In the simultaneous integrated boost (SIB) approach, both whole breast and boost radiation are integrated into a single plan and delivered over the whole treatment course with differential dose per fraction to the different target volumes (Hijal et al. 2010). The association between SIB and inversely planned IMRT has been shown to increase tumor bed conformality and to spare normal tissues (Singla et al. 2006). TomoDirect (TD) is a non-rotational treatment option of the TomoTherapy platform (Accuray Inc., Sunnyvale, CA, USA) allowing for planning and delivery of a series of highly modulated linear beam paths, with up to 12 coplanar static beams and the couch moving through the beam at a constant rate (Franco and Ricardi 2012). The patient is translated along the cranial–caudal axis past the fixed fan beam path during the delivery of each field, and beam intensity is modulated by the binary collimator. After each discrete angle, the gantry is rotated to a different position and the patient is again passed through the bore for the delivery of the subsequent field (Franco et al. 2011). We herein present early results of a prospective phase II trial investigating hypofractionated intensity-modulated whole-breast radiation employing a SIB approach on the surgical bed, delivered with TD for EBC after BCS.
Materials and methods
Study design and sample size determination
Between 2010 and 2012, at the Radiation Oncology Department, Tomotherapy Unit, AUSL Valle d’Aosta (Aosta, Italy; a tomotherapy-based site), we employed tomotherapy to deliver intensity-modulated WBRT after BCS for EBC, using a conventionally fractionated schedule (50 Gy/25 fractions with TD) to the whole breast and a sequential conventionally fractionated boost dose to the surgical bed (10–16 Gy/5–8 fractions) with the helical tomotherapy mode (HT) (Franco et al. 2013). This approach provided consistent clinical results with mild toxicity, promising cosmesis and quality of life (QoL). However, the crude rate of acute skin toxicity we detected (G2–G3 15 %; G0–G1 85 % according to the RTOG scale) required consideration. Among acute skin toxicity predictors (for G2–G3 events), we observed a correlation trend with adjunctive dose received by the whole-breast volume (minus the TB volume) due to the HT boost phase (V 52.5Gy, V 55Gy, V 57.5Gy), with a calculated Pearson’s coefficient around 0.5 (Franco et al. 2013). Thus, in order to supposedly decrease acute skin toxicity, avoiding the delivery of unintended excessive dose outside TB, we decided to run a prospective phase II trial, employing TD to deliver accelerated hypofractionated AWBRT with a SIB to the TB. The treatment schedule was chosen taking into account the previous WBRT LINAC-based experience developed at our Institution, based on acceleration, hypofractionation and a concomitant boost approach (Cante et al. 2011, 2013). A one-armed optimal two-stage Simon’s design was selected to test the hypothesis that treatment modality under investigation (hypofractionated SIB WBRT delivered with TD) would increase the rate of G0–G1 (vs G2–G3) acute skin toxicity (>85 %) over the historical data obtained with the previous approach (conventionally fractionated WBRT with TD and sequential TB boost with HT) [null hypothesis (H0): no difference in acute skin toxicity between treatment modalities] (Simon 1989). The present study was based on the following assumptions: (1) the historical data of success (p0) were represented by the 85 % rate of G0–G1 acute skin toxicity (G2–G3: 15 %) detected in the previous study; (2) the threshold of successful trial (p1) with the treatment schedule under investigation was set to 94 % of G0–G1 acute skin toxicity (G2–G3: 6 %); (3) the α error (one-sided type I error) was set at 5 %; (4) the β error at 20 % (type II error; power 80 %). At the first stage, among 21 enrolled patients, at least 18 (86 %) should have been scored as G0–G1 acute skin toxicity to further proceed with the trial. At the second stage, another 61 patients were accrued for an overall sample size of 82 patients. A minimum of 74 out of 82 (90.2 %) with G0–G1 toxicity represented the threshold for the rejection of H 0 and the fulfillment of the criteria for the definition of a ‘promising’ treatment for the hypofractionated SIB-based TD schedule.
Eligibility criteria
In order to be accrued in the present prospective study, patients should have a histologically proven diagnosis of breast adenocarcinoma; prior BCS (quadrantectomy, lumpectomy or wide excision); pathological stage pTis–pT1–pT2, pN0–N1 stage according to AJCC–UICC staging system (6th edition); negative surgical resection margins (>2 mm); and no evidence of systemic disease. Exclusion criteria included close to positive resection margins (≤2 mm), prior thoracic radiation, synchronous second primary tumor and pregnancy. The Clinical Research and Ethical Review Board of our Institution approved the present study. Written informed consent was obtained from all patients. RT was delivered either immediately after BCS, in patients at low risk of distant failure, or sequentially after adjuvant chemotherapy (CT), whenever high-risk features were present.
Setup, simulation and target definition
Setup consisted of a wingboard with the patient having both arms raised alongside the head and radio-opaque markers along breast borders. Axial images (3–5 mm slice thickness) were acquired from the lower mandible aspect to the base of the lungs. The whole-breast clinical target volume (WB-CTV) included breast palpable tissue, delimited by radio-opaque wires marking clinically detectable breast borders. The whole-breast planning target volume (WB-PTV) was created with a 5-mm margin around WB-CTV, but confined to the interior of the patient’s outer contours reduced by 5 mm (excluding heart and lungs whenever needed). TB definition was driven by radio-opaque clips placed at the time of surgery. The TB clinical target volume (TB-CTV) was obtained with a 5-mm isotropic margin around the TB; the consequent planning target volume (TB-PTV) needed a further margin of 5 mm around the TB-CTV. The heart, bilateral lungs and contralateral breast were separately contoured as organs at risk (OARs): the heart was outlined to the pulmonary trunk superiorly, including pericardium and excluding major vessels.
Dose prescription and dose constraints
Prescription doses of 45 Gy/20 fractions (2.25 Gy daily) and 50 Gy/20 fractions (2.5 Gy daily) were planned to the WB-PTV and TB-PTV, respectively. Both target volumes were integrated in the same treatment plan, and thus, the delivery of a different dose per fraction was concomitant in the same treatment session and performed with the TD modality of the tomotherapy platform. Both RT phases were prescribed to 50 % of respective PTVs. Dose distribution was optimized so that the 95 % of both PTVs received at least 95 % of the prescription dose, minimizing hot-spots occurrence (i.e., D max < 105–107 % of prescribed dose). Dose constraints for OARs were set to V 20Gy < 10 %, V 10Gy < 20 %, V 5Gy < 42 % (ipsilateral lung); V 25Gy < 10 % (heart); maximum dose (D 0.1cc) < 5 Gy (contralateral breast), and V 5Gy < 5 % (controlateral lung). Optimization was addressed to reduce both the mean lung dose (MLD) and mean heart dose (MHD) for ipsilateral lung and heart. Excess irradiation (D 2cc), defined as the percentage of the prescription dose delivered to a volume of 2 cc of normal tissues external to the PTV, was minimized.
Radiobiologic considerations
In order to compare the present treatment schedule, with the conventionally fractionated standard approach (50 Gy/25 fractions WBRT; subsequent TB boost dose of 10 Gy/5 fractions), we performed a conversion into a biologically effective dose (BED), according to the linear quadratic model formalism. Thus, doses per fraction and total doses were calculated to be isoeffective to the standard schedule. An α/β ratio of 4, 10 and 3 Gy was assumed for tumor control, early-responding tissues and late effects, respectively. The hypofractionated regimen employed in the present study delivers BED values of 81, 62.5 and 91.5 Gy for breast adenocarcinoma tumor control, acute and late responding normal tissues, respectively, compared to the conventionally schedule that shows BED values of 90, 72 and 100 Gy.
Tomotherapy planning
Treatment plans were generated using the TomoTherapy Hi-Art (version 4.0.4 or higher) treatment planning software (TPS) (Accuray Inc., Sunnyvale, CA, USA). For each plan, specific field width, pitch (the TD pitch is defined as the distance of couch travel in centimeters per sinogram projection) and modulation factor are chosen. Typically, a number of 4 beams conformed to the WB-PTV were used. In addition, 1 or 2 small beams specifically conformed to the TB-PTV where employed whenever resulting dose distribution deserved improvement in terms of homogeneity and conformality. The 4 wider beams were arranged as follows: two canonical tangential beams, one anterior–posterior (AP) and 1 latero-lateral both with a gantry angle range ±15°. To account for possible breath-related target movements, 3 MLC leaves (approximately 19 mm on the isocenter plane) were opened on the anterior edge of each beam. The additional small beams were usually positioned with oblique incidence in order to reduce the dose spread around TB-PTV. Figure 1 depicts a typical four-beam arrangement for a right-sided whole-breast treatment. For each plan, a 2.5-cm field width was used and the pitch value was set by default to 1/10 of the field width (0.25 cm/projection for the 2.5 cm beam). A 10-mm ring around the WB-PTV was used to help reduce skin overdosage and to improve target dose conformity. Helping structures were created within the body volume outside the WB-PTV where significant hotspots were likely to occur (i.e., at medial/lateral target edges). If necessary, OARs were used as avoidance structures. A 10-mm ring structure was generated around the TB-PTV to avoid unnecessary WB-PTV irradiation. A planning 2–2.5 modulation factor was used in all plans. Patient-specific quality assurance methods included a 2D dose distribution verification in a coronal or sagittal plane of the Cheese Phantom (Gammex RMI, Middleton, WI, USA) by means of GafChromic films EBT2 (ISP Inc, NJ, USA) and/or a 3D diode array evaluation with ArcCHECK (Sun Nuclear, Melbourne, FL, USA) (Catuzzo et al. 2012).
Follow-up, toxicity, cosmesis and quality of life assessment
Follow-up consisted of clinical examination at 3–6 months and twice a year afterward and plain chest X-ray and mammography once a year; other examinations were performed if needed. Acute skin toxicity, the primary endpoint of the study, was assessed at the end of WBRT and after 3 months; late skin toxicity was scored starting from 6 months. The RTOG/EORTC toxicity scale was employed for acute effects; the maximal detected toxicity was scored according to the Common Terminology Criteria for Adverse Events (version 4.02), for late effects. (Cox et al. 1995; NCI-CTEP 2013). Skin toxicity endpoints we considered were the following: erythema, edema, desquamation, ulceration, hemorrhage, necrosis, telangiectasia, fibrosis–induration, hyperpigmentation, retraction and atrophy. Cosmetic results were assessed at the end of RT and at every follow-up time-point, using the standards set forth by the Harvard criteria, a cosmetic evaluation method based on a physician-rated scale consisting of different categories, comparing treated and untreated breast. An “excellent” score was assigned when the treated breast looked essentially as the contralateral; a “good” score for minimal but identifiable radiation effects; a “fair” score if significant radiation effects were readily observable; a “poor” score for radiation-induced severe late effects (Rose et al. 1989). Late skin toxicity and cosmesis are referred to the time of last examination. Quality of life (QoL) was assessed with the EORTC QoL questionnaire QLQ-C30, to measure general cancer QoL, quantifying patient’s capacity to fulfill the activities of daily living. This tool incorporates 30 items exploring global health status/QoL, 5 functioning domains (physical, role, cognition, emotional, social) and 9 symptom scales (fatigue, pain, nausea/vomiting, dyspnea, insomnia, appetite loss, constipation, diarrhea, financial impact). Each item was scored according to the standard scoring rules as in the EORTC QLQ-C30 Scoring Manual (Fayers et al. 1999). We added the EORTC QLQ-BR23, an EORTC QLQ-C30 supplementary module targeted to breast cancer to assess tumor-site-related specific symptoms, treatment-related side effects and disease-specific QoL domains. It is composed of 23 items related to four functioning domains (body image, sexual functioning, sexual enjoyment, future perspective) and to four symptom scales (systemic therapies side effects, breast symptoms, arm symptoms, upset by hair loss). The scoring methods are similar to those of EORTC QLQ-C30. Both EORTC QLQ-C30 and QLQ-BR-23 were assessed at 4 different time-points: before and at the end of RT, 6 months and 1 year after WBRT.
Statistical analysis
Changes in QoL over time were analyzed by the Wilcoxon signed-rank-test. A difference between time-points was considered clinically relevant if >10 points as previously reported (Osoba et al. 1998). Results were considered statistically significant if p < 0.01.
Results
Clinical characteristics
The 82 patients included in the present prospective trial (between May 2011 and November 2012) achieved a mean follow-up time of 12 months (range 6–24). Baseline characteristics are detailed in Table 1. The majority of patients were older than 50 (79 %), with few comorbidities (diabetes 11 %; vasculopathy 27 %; hypertension 40 %), a mean BMI of 25.2, a mean breast and soft tissue thickness (perpendicular distances between rib cage and nipple of non-index breast and between sternum and anterior skin surface, respectively) of 16.2 and 51.2 mm. They were mainly affected with right-sided (59 %) outer quadrants (65 %) tumors, with an invasive primary <2 cm (88 %), node negative (71 %), hormone sensitive (90 %), moderately differentiated (55 %) with ductal histology (74 %), low proliferation index (39 %), no c-erb-B2 amplification (83 %) vascular (87 %) and perineural invasion (91 %). Most of the patients underwent quadrantectomy/lumpectomy and sentinel lymph node biopsy (88 %); six percent had an axillary dissection. We had 6 % of pNx cases. Up to 79 % received concomitant hormonal therapy, 25 % adjuvant CT and 17 % trastuzumab. WBRT was always completed without interruptions due to clinical issues.
Dosimetric results
Dosimetric parameters (Table 2) are reported as mean values and corresponding standard deviations. For target coverage, the boost (TB-PTV) dose received was in average -0.5 % of the prescription dose, while the whole-breast (WB-PTV) dose inhomogeneity was in average -2 % of the prescribed dose; the WB-PTV minimum significant dose (D 98%) and the maximum significant dose (D 2%) were in average approximately 95 and 105 % of the prescription dose, respectively. For TB-PTV, D 98% and D 2% were around 96 % and 102 % of the prescribed dose. The volume of WB-PTV minus TB-PTV receiving 105 % of prescription dose was 2.4 %, while almost no volume (i.e., 0.01 %) received 110 % of prescription dose. Dose to lungs was kept within tolerance levels: ipsilateral lung V 20 and MLD were around 10 % and 6 Gy; maximum dose to contralateral lung was around 2 Gy. Heart did not receive high doses (V 25 around 3 % and MHD around 2 Gy for left-sided tumors). Contralateral breast was adequately spared (D max < 3 Gy).
Tumor control, toxicity, cosmesis and QoL
No local relapse was observed. The maximum acute skin toxicity was Grade 0 in 41 %, Grade 1 in 53 %, Grade 2 in 6 %, Grade 3 in <1 % (Table 3), on the whole cohort. Specifically, the first stage of the present trial (21 patients enrolled) observed a total of 2 acute skin toxicity events ≥G2 according to the RTOG scale, fulfilling the requirements to further proceed with the second stage, which accrued 61 patients and observed other 4 events ≥G2. Thus, a total of 6 events scored as G2–G3 was far under the planned threshold and enabled us to reject H0 (no difference in terms of acute cutaneous toxicity with the historical dataset) and define WBRT delivered with TD using HF and SIB as a ‘promising’ treatment approach. Late skin and subcutaneous toxicity was generally mild (Table 4): no events >G2 were observed. A Grade 1 score was assessed for fibrosis/induration in 5 % of patients, for atrophy in 4 %, telangiectasia in 1 %, hyperpigmentation in 12 % and striae in 2 %. A Grade 2 score was observed only for fibrosis (2 %) and hyperpigmentation (2 %). Cosmetic results (Table 4) were excellent in 69 % of patients, good in 22 %, fair in 5 % and poor in 4 %. QoL was generally preserved over time (Fig. 2). The only difference between time-points was found for fatigue (between pre-RT and 1-year after RT vs the end of RT) with a >10 points decrease (p = 0.001).
Discussion
HF is a common strategy to perform WBRT after BCS for EBC (Mannino and Yarnold 2009). It has been employed in several institutions for decades and tested in randomized controlled trials (The START Trialists’ Group et al. 2008a, b; Whelan et al. 2010; Yarnold et al. 2005). The comprehensive guidelines by the UK’s National Institute of Clinical Excellence (NICE) on the management of EBC recommend HF (40 Gy/15 fractions) as standard (Harnett 2010). Convenience and cost reduction, both for patient and for global health system, are noteworthy (Lievens 2010). From a radiobiological point of view, breast adenocarcinoma holds an α/β ratio of approximately 4 Gy, close to late reacting normal tissues. A larger fraction size achieves the same (or higher) probability of tumor control with a comparable rate of expected late effects, with a therapeutic index widening (Fowler 2010). The 4 randomized trials investigating HF (RMH/GOC, START A and B, Canadian trials) showed at least equivalency for local control between HF and standard schedule (James et al. 2008). Regarding normal tissue toxicity and cosmesis, even if different measuring strategies were employed, the 4 studies globally reported a 25–40 % rate of mild adverse effects, with only 10 % ≥G2, with no fractionation influence (Holloway et al. 2010). For specific endpoints, HF resulted in fewer adverse effects: for example, a lower rate of change in skin appearance was found in the START A and B trials (The START Trialists’ Group et al. 2008a, b). Late effects on ribs, heart, lung and brachial plexus were extremely rare. Thus, consistent evidence supports the use of HF to deliver WBRT. However, none of the 4 RCTs investigated within treatment protocol the use of the TB boost dose, since the Canadian Trial had no boost and the UK trials delivered conventionally fractionated boost dose sequential to WBRT according to institution discretion. Two RCTs strongly support the use of adjunctive dose to the TB with a substantial local control benefit (Bartelink et al. 2007; Romestaing et al. 1997). The TB boost dose might be delivered sequentially after the WBRT phase with conventional fractionation for an overall treatment time of 6–7 weeks or incorporated within WBRT (using HF), with a concurrent delivery (concomitant boost or SIB) allowing for a further reduction in treatment length (in adjunct to overall treatment time decrease due to HF). The concurrent delivery of the boost dose within the whole-breast phase is being tested in prospective clinical trials, with reliable results. Freedman et al. (2012) accrued 75 patients (Tis-T2, clear resection margins) onto a phase II trial of photon-based WBRT delivered in 4 weeks to 45 Gy/20 fractions (2.25 daily) with an IMRT incorporated boost of 2.8 Gy daily to 56 Gy/20 fractions. Five-year LC was 97.3 %. Cosmesis, scored with patient- and physician-reported Breast Cancer Treatment Outcome Scale (BCTOS), was close to excellent with minimal difference between index and non-index breast. Chadha et al. (2013) treated 160 EBC patients (Tis-T2, node negative, clear resection margin and chemotherapy-naïve) with accelerated HF delivering 40.5 Gy/15 fractions (2.7 Gy daily) to the whole breast (over 3 weeks; 19 days) with an adjunctive concurrent 0.3 Gy daily to the TB, to 45 Gy/15 fractions; the 5-year OS and DFS were 90 and 97 %, and local control was 99 %, with a median follow-up of 3.5 years. No late toxicity >G2 according to LENT–SOMA scale was observed with >2-year follow-up. In the UK, the IMPORT High Trial is investigating dose-escalated SIB-IMRT in women with higher than average risk of local recurrence, after BCS (Coles et al. 2006). Similarly, in the USA, the RTOG 1005 phase III RCT started patients accrual, comparing HF WBRT and concomitant boost to conventionally fractionated standard radiation (RTOG 1005). IMRT improves target coverage and dose homogeneity and spares normal tissue over conventional approaches in breast radiation (Hong et al. 1999). Prospective IMRT studies showed consistent clinical long-term results, with a low rate of local relapse and acute toxicity, mild late effects and good/excellent cosmesis (Keller et al. 2012). Freedman et al. (2009) observed a statistically significant reduction with IMRT in the incidence and duration of acute Grade 2/3 dermatitis, compared to conventional radiation, in a retrospective series of 804 consecutive breast cancer patients. In adjunct, researchers showed a decrease in time spent with acute skin reactions with IMRT (analyzing the incidence of acute dermatitis during each treatment week). Canadian researchers confirmed this finding (randomized phase III trial of IMRT vs standard WBRT), showing a significant reduction in terms of moist desquamation with IMRT over conventional RT (Pignol et al. 2008). Regarding late effects and cosmesis, UK researchers reported a reduction in palpable breast induration/negative changes in breast appearance in the IMRT arm, within a randomized phase III trial (2D RT vs IMRT, designed with change in breast appearance as primary endpoint) (Donovan et al. 2007). The 2-year interim results of the Cambridge randomized trial (patients with inhomogeneous plans using standard tangentials were randomized to forward-planned IMRT or standard WBRT) showed a reduction in the telangiectasia rate with IMRT (Barnett et al. 2012). TD allows image-guided IMRT delivery at discrete angles with tomotherapy using a fixed gantry and represents a suitable solution for clinical situation where beam arrangement in constrained to a limited number of restricted directions (Franco et al. 2011). TD had been investigated within planning and clinical studies in breast radiation, but also in other oncological scenarios (Borca et al. 2012; Fiandra et al. 2012; Murai et al. 2013). It provides adequate target coverage of the intact breast, with reduction in high doses to target volumes and OARs over conventional techniques and a decrease in low doses to normal tissue compared to HT (Schubert et al. 2011). Early clinical data on the use of HT in breast cancer patients employing HF and SIB have been recently reported (Van Parijs et al. 2012). Patients enrolled into this phase II prospective trial underwent intensity-modulated hypofractionated WBRT with a SIB to the surgical bed delivered with TD. Our treatment schedule consisted of 45 Gy/20 fractions to the whole breast (2.25 daily) with an adjunctive 0.25 Gy daily dose to the TB to a total nominal dose of 50 Gy (2.5 Gy daily). The whole course was given in 4 weeks (26 days). Assuming α/β ratio values of 4 Gy, 10 Gy and 3 Gy for tumor control, early-responding tissues and late effects, our schedule carries BED2Gy values of 81, 62.5 and 91.5 Gy. Theoretically, this is slightly less than an iso-effective dose regimen compared to WBRT delivered with conventional fractionation and sequential boost (BED2Gy of 90, 72 and 100 Gy). However, we assumed that the reduction in overall treatment time (4 vs 6 weeks) in our study might compensate this issue. It has been demonstrated that the incorporation of the boost dose within the WBRT phase leads to a decrease in unintended excessive dose outside the TB with a favorable toxicity profile and cosmetic outcome (van der Laan et al. 2007; Bantema-Joppe et al. 2012). The treatment for the TB with a SIB approach is able to theoretically improve local control as it reduces treatment time and increases dose per fraction possibly escalating TB BED values. Moreover, in a comparison between 3D conformal RT and HT for WBRT, it has been demonstrated that a HT-based SIB approach leads to the reduction in excess irradiation of the whole breast excluding the TB. With the 3D conformal technique, a large amount of breast tissue outside TB is irradiated in the planes containing the TB (Hijal et al. 2010). This finding is consistent with our results. In our previous study (using TD for WBRT and sequential HT-based TB boost), we demonstrated that adjunctive dose received by the WB-PTV minus TB-PTV (V 52.5Gy, V 55Gy, V 57.5Gy) is correlated with G2–G3 acute skin toxicity. In that patients cohort more than 1/3 of the WB-PTV received 105 % of the prescribed dose, almost 1/5 received 110 % and more than 1/10 got 115 %, due to the sequential boost phase (Franco et al. 2013). In the present study, the TD-based SIB WBRT we used achieved consistent dosimetric results as V 105% (for WB-PTV minus TB-PTV) was very low (2.4 ± 0.9) and V 110% negligible (0.01), strongly limiting unintended irradiation outside TB. These dosimetric results were reflected by a robust reduction in acute skin toxicity, validating the hypothesis under investigation. Late skin toxicity (even with short-term follow-up) was generally mild, and cosmesis seems consistent as assessed with the Harvard criteria, a physician-rated scale comparing the index breast with the contralateral, and not with photographic assessment (more objective as it includes post-surgical/pre-radiotherapy baseline documentation). Quality of life was essentially unaffected by WBRT, apart from transient fatigue increase.
Conclusions
Intensity-modulated and hypofractioned WBRT using a SIB to the TB and delivered with TD after BCS for EBC provides consistent clinical results (mild toxicity, promising cosmesis, QoL). The reduction in unintended excessive dose outside TB decreases acute skin toxicity rate over a sequential approach (conventionally fractionated WBRT with TD and boost dose to TB with HT) and enables us to define the treatment approach under investigation as ‘promising’. A longer follow-up is needed to determine how long-term clinical endpoints might be influenced by the new treatment regimen.
References
Bantema-Joppe EJ, Schilstra C, de Bock GH, Dolsma WV, Busz DM, Langendijk JA, Maduro JH (2012) Simultaneous integrated boost irradiation after breast conserving surgery: physician-rated toxicity and cosmetic outcome at 30 months’ follow up. Int J Radiat Oncol Biol Phys 83(4):e471–e477
Barnett GC, Wilkinson JS, Moody A, Wilson CB, Twyman N, Wishart GC, Burnet NG, Coles CE (2012) Randomized controlled trial of forward-planned intensity modulated-radiotherapy for early breast cancer: interim results at 2 years. Int J Radiat Oncol Biol Phys 82(2):715–723
Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, Jager JJ, Hoogenraad WJ, Oei SB, Warlam-Rodenhuis CC, Pierart M, Colette L (2007) Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10 years results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol 25(22):3259–3265
Borca VC, Franco P, Catuzzo P, Migliaccio F, Zenone F, Aimonetto S, Peruzzo A, Pasquino M, Russo G, La Porta MR, Cante D, Sciacero P, Girelli G, Ricardi U, Tofani S (2012) Does TomoDirect 3DCRT represent a suitable option for post-operative whole breast irradiation? A hypothesis-generating pilot study. Radiat Oncol 7:211
Cante D, Rosa La Porta M, Casanova-Borca V, Sciacero P, Girelli G, Pasquino M, Franco P, Ozzello F (2011) Accelerated hypofractionated adjuvant whole breast radiotherapy with concomitant photon boost after conserving surgery for early breast cancer: a prospective evaluation on 463 patients. Breast J 17(6):586–593
Cante D, Franco P, Sciacero P, Girelli G, Marra AM, Pasquino M, Russo G, Borca VC, Mondini G, Paino O, Barmasse R, Tofani S, Numico G, La Porta MR, Ricardi U (2013) Five-year results of a prospective case series of accelerated hypofractionated whole breast radiation with concomitant boost to the tumor bed after conserving surgery for early breast cancer. Med Oncol 30(2):518
Catuzzo P, Zenone F, Aimonetto S, Peruzzo A, Casanova Borca V, Pasquino M, Franco P, La Porta MR, Ricardi U (2012) Technical note: patient-specific quality assurance methods for TomoDirect(TM) whole breast treatment delivery. Med Phys 39(7):4073–4078
Chadha M, Woode R, Sillanpaa J, Lucido D, Boolbol SK, Kirstein L, Osborne MP, Feldman S, Harrison LB (2013) Early-stage breast cancer treated with 3-week accelerated whole-breast radiation therapy and concomitant boost. Int J Radiat Oncol Biol Phys 86(1):40–44
Coles C, Yarnold J, IMPORT Trials Management Group (2006) The IMPORT trials are launched (September 2006). Clin Oncol 18(8):587–590
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5):1341–1346
Donovan E, Bleakley N, Denholm E, Evans P, Gothard L, Hanson J, Peckitt C, Reise S, Ross G, Sharp G, Symonds-Tayler R, Tait D, Yarnold J, Breast Technology Group (2007) Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol 82(3):254–264
Early Breast Cancer Trialists’ Collaborative Group (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15 years survival: an overview of the randomized trials. Lancet 366(9503):2087–2106
Fayers PM, Aarson NK, Bjordal K, Curran D, Groenvold M, On behalf of the EORTC quality of life study group (1999) EORTC QLQ-C30 scoring manual, 2nd edn. EORTC, Brussels
Fiandra C, Filippi AR, Catuzzo P, Botticella A, Ciammella P, Franco P, Borca VC, Ragona R, Tofani S, Ricardi U (2012) Different IMRT solutions vs 3D-conformal radiotherapy in early stage Hodgkin’s Lymphoma: dosimetric comparison and clinical considerations. Radiat Oncol 7:186
Fowler JF (2010) 21 years of biologically effective dose. Br J Radiol 83(991):554–568
Franco P, Ricardi U (2012) TomoDirect to deliver static angle tomotherapy treatments. J Nucl Med Radiat Ther 3:5
Franco P, Catuzzo P, Cante D, La Porta MR, Sciacero P, Girelli G, Casanova Borca V, Pasquino M, Numico G, Tofani S, Meloni T, Ricardi U, Ozzello F (2011) TomoDirect: an efficient means to deliver radiation at static angles with tomotherapy. Tumori 97(4):498–502
Franco P, Zeverino M, Migliaccio F, Sciacero P, Cante D, Casanova Borca V, Torielli P, Arrichiello C, Girelli G, Numico G, La Porta MR, Tofani S, Ricardi U (2013) Intensity-modulated adjuvant whole breast radiation delivered with static angle tomotherapy (TomoDirect): a prospective case series. J Cancer Res Clin Oncol 139(11):1927–1936
Freedman GM, Li T, Nicolaou N, Chen Y, Ma CC, Anderson PR (2009) Breast intensity-modulated radiation therapy reduces time spent with acute dermatitis for women of all breast sizes during radiation. Int J Radiat Oncol Biol Phys 74(3):689–694
Freedman GM, Anderson PR, Bleicher RJ, Litwin S, Li T, Swaby RF, Ma CM, Li J, Sigurdson ER, Watkins-Bruner D, Morrow M, Goldstein LJ (2012) Five-year local control in a phase II study of hypofractionated intensity modulated radiation therapy with an incorporated boost for early stage breast cancer. Int J Radiat Oncol Biol Phys 84(4):888–893
Freedman GM, White JR, Arthur DW, Li XA, Vicini F (2013) Accelerated fractionation with a concurrent boost for early stage breast cancer. Radiother Oncol 106(1):15–20
Harnett A (2010) Fewer fractions of adjuvant external beam radiotherapy for early breast cancer are safe and effective and can now be the standard of care. Why the UK’s NICE accepts fewer fractions as the standard of care for adjuvant radiotherapy in early breast cancer. Breast 19(3):159–162
Higher-dose radiation therapy or standard radiation therapy in treating patients with early-stage breast cancer that was removed by surgery. RTOG 1005. Located at www.cancer.gov/clinicaltrials/seach/view?cdrid=700069, Accessed 3 Sept 2013
Hijal T, Fourniez-Bodoz N, Castro-Pena P, Kirova YM, Zefkili S, Bollet MA, Dendale R, Campana F, Fourquet A (2010) Simultaneous integrated boost in breast conserving treatment of the breast: a dosimetric comparison of helical and three-dimensional conformal radiotherapy. Radiother Oncol 94(3):300–306
Holloway CL, Panet-Raymond V, Olivotto I (2010) Hypofractionation should be the new ‘standard’ for radiation therapy after breast conserving surgery. Breast 19(3):163–167
Hong L, Hunt M, Chui C, Spirou S, Forster K, Lee H, Yahalom J, Kutcher GJ, McCormick B (1999) Intensity-modulated tangential beam irradiation of the intact breast. Int J Radiat Oncol Biol Phys 44(5):1155–1164
James ML, Lehman M, Hider PN, Jeffery M, Hickey BE, Francis DP (2008) Fraction size in radiation treatment for breast conservation in early breast cancer. Cochrane Database Syst Rev 3:CD003860
Keller LM, Sopka DM, Li T, Klayton T, Li J, Anderson PR, Bleicher RJ, Sigurdson ER, Freedman GM (2012) Five-year results of whole breast intensity modulated radiation therapy for the treatment of early stage breast cancer: the Fox Chase Cancer Center Experience. Int J Radiat Oncol Biol Phys 84(4):881–887
Lievens Y (2010) Hypofractionated breast radiotherapy: financial and economic consequences. Breast 19(3):192–197
Mannino M, Yarnold JR (2009) Shorter fractionation schedules in breast cancer radiotherapy: clinical and economic implications. Eur J Cancer 45(5):730–731
Murai T, Shibamoto Y, Manabe Y, Murata R, Sugie C, Hayashi A, Ito H, Miyoshi Y (2013) Intensity-modulated radiation therapy using static ports of tomotherapy (TomoDirect): comparison with the TomoHelical mode. Radiat Oncol 8:68
National Cancer Institute Cancer Therapy Evaluation Program (2013) Common toxicity criteria for adverse events. 2013 Version 4.02. Available at http://ctep.cancer.gov. Accessed September 2013
Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16(1):139–144
Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckhman W, Vu TT, Truong P, Ackerman I, Paszat L (2008) A multicenter randomized trial of breast intensity modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 26(13):2085–2092
Poortmans P (2007) Evidence-based radiation oncology. Breast cancer. Radiother Oncol 84(1):84–101
Romestaing P, Lehingue Y, Carrie C, Coquard R, Montbarbon X, Ardiet JM, Mamelle N, Gerard JP (1997) Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France. J Clin Oncol 15(3):963–968
Rose MA, Olivotto IA, Cady B, Koufman C, Osteen R, Silver B, Recht A, Harris JR (1989) Conservative surgery and radiation therapy for early stage breast cancer: long-term cosmetic results. Arch Surg 124(2):153–157
Schubert LK, Gondi V, Sengbush E, Westerly DC, Soisson ET, Paliwal BR, Mackie TR, Metha MP, Patel RR, Tomé WA, Cannon GM (2011) Dosimetric comparison of left-sided whole breast irradiation with 3DCRT, forward-planned IMRT, inverse-planned IMRT, helical tomotherapy and topotherapy. Radiother Oncol 100(2):241–246
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10
Singla R, King S, Albuquerque K, Creech S, Dogan N (2006) Simultaneous-integrated boost intensity-modulated radiation therapy (SIBIMRT) in the treatment of early-stage left-sided breast carcinoma. Med Dosim 31(3):190–196
The START Trialists’ Group, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, Brown J, Dewar JA, Dobbs HJ, Haviland JS, Hoskin P, Hopwood P, Lawton PA, Magee BJ, Mills J, Morgan DA, Owen JR, Simmons S, Sumo G, Sydenham MA, Venables K, Yarnold JR (2008a) The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet Oncol 9(4):331–341
The START Trialists’ Group, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, Brown J, Dewar JA, Dobbs HJ, Haviland JS, Hoskin P, Hopwood P, Lawton PA, Magee BJ, Mills J, Morgan DA, Owen JR, Simmons S, Sumo G, Sydenham MA, Venables K, Yarnold JR (2008b) The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet 371(9618):1098–1107
van der Laan HP, Dolsma WV, Maduro JH, Korevaar EW, Hollander M, Langendijk JA (2007) Three-dimensional conformal simultaneously integrated boost technique for breast-conserving radiotherapy. Int J Radiat Oncol Biol Phys 68(4):1018–1023
Van Parijs H, Miedema G, Vinh-Hung V, Verbanck S, Adriaenssens N, Kerkhove D, Reynders T, Schuermans D, Leysen K, Hanon S, Van Camp G, Vincken W, Storme G, Verellen D, De Ridder M (2012) Short course radiotherapy with simultaneous integrated boost for stage I-II breast cancer, early toxicities of a randomized trial. Radiat Oncol 7:80
Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H, Perera F, Fyles A, Schneider K, Gulavita S, Freeman C (2010) Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 362(6):513–520
Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J, Haviland J, Bentzen S, Owen R (2005) Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomized trial. Radiother Oncol 75(1):9–17
Conflict of interest
We declare that we do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franco, P., Zeverino, M., Migliaccio, F. et al. Intensity-modulated and hypofractionated simultaneous integrated boost adjuvant breast radiation employing statics ports of tomotherapy (TomoDirect): a prospective phase II trial. J Cancer Res Clin Oncol 140, 167–177 (2014). https://doi.org/10.1007/s00432-013-1560-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-013-1560-8