Abstract
Among the gliomas, glioblastoma (GBM) is the highest grade and the most malignant glioma tumor. GATA2 is a hematopoietic factor that has been intensely studied in hematopoietic malignancies. Recently, the functions of GATA2 as an oncogene in other types of human cancer have been reported. However, no role for GATA2 in the development and progression of glioma has been reported to date. In the present study, we found that the expression level of GATA2 is upregulated in GBM and is correlated with GBM outcome. Ectopic expression of GATA2 or RNAi-mediated knockdown of GATA2 significantly enhanced or inhibited proliferation, migration and invasion of glioma cells. Moreover, we found that epidermal growth factor receptor and extracellular signal-regulated kinase, as upstream components of the signaling pathway, upregulate GATA2 expression; moreover, GATA2 promotes Elk-1 expression. Therefore, a genetic approach or pharmacological intervention targeting GATA2 could potentially serve as an effective strategy for treating glioma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytoma gliomas are the most common type of human primary brain tumors [1]. Based on histopathologic criteria, astrocytoma gliomas can be divided into I, II, III and IV grades [2]. Glioblastoma (GBM) is the highest grade (grade IV) and the most malignant glioma tumor. GBM is difficult to treat and is therefore associated with an unfavorable prognosis. In the past decade, some molecular markers have been intensively explored for the evaluation and management of GBM patients [3]. For instance, the promoter methylation of the O6-methylguanine methyltransferase (MGMT) gene has been shown to predict a longer survival in GBM patients [4]. We know that the overexpression of epidermal growth factor receptor (EGFR) is often found in GBM patients [5]. These markers have been used clinically to predict survival and to evaluate the efficacy of novel molecular drugs. Therefore, identifying some novel molecular markers or targets in glioma and understanding the molecular mechanism of the development and progression of glioma are critical for predicting outcomes and treating this disease.
The GATA families of transcription factors play key roles in organ development and lineage specification [6–8]. The families include six members that have clear-cut and qualified tissue expression patterns. Among them, GATA2 is mainly regarded as a hematopoietic factor that has been intensely studied in hematopoietic malignancies [9]. Recently, the functions of GATA2 as an oncogene in other types of human cancer have been reported. For example, GATA2 is overexpressed in human breast carcinomas; it promotes breast cancer cell growth by inhibiting expression of PTEN, a well-known tumor suppressor gene [10]. In a Kras-driven non-small cell lung cancer (NSCLC) mouse model, GATA2 loss reduced tumor development, suggesting a key tumor-promoting role for GATA2 [11]. In prostate cancer, GATA2 can be a metastasis-driving gene, suggesting that it is a potential therapeutic target for metastatic prostate cancer [12]. Unfortunately, no role for GATA2 in the development and progression of glioma has been reported to date.
In the present study, we analyzed the prognostic significance of GATA2 protein expression in a set of GBM patients. In addition, the functional role of GATA2 was characterized in two glioma cell lines. Moreover, we found that EGFR and ERK, as upstream components of the signaling pathway, upregulate GATA2 expression; moreover, GATA2 promotes Elk-1 expression. Overall, our findings indicate that GATA2 plays an essential role in glioma progression and suggest GATA2 as a novel prognostic indicator and potential therapeutic target in glioma patients.
Materials and methods
Ethics statement
Patient information and samples were obtained with written informed consent. Each patient in this study gave written informed consent to publish these case details. The research was approved by the ethics committee of Second Affiliated Hospital, Soochow University.
Patients and tumor samples
We enrolled a total of 56 patients with GBM who had undergone surgical tumor resection between June 2006 and June 2012 in the Department of Neurosurgery, Second Affiliated Hospital, Soochow University, China. All specimens were collected under an institutional review board-approved protocol and de-identified for patient confidentiality. Clinical information was available for all patients. Additionally, we collected six non-neoplastic brain tissue samples with corresponding clinical information from epileptogenic patients within our department.
Immunohistochemistry assay
Immunohistochemistry (IHC) studies were performed on formalin-fixed paraffin-embedded GBM tumor samples using anti-GATA2 antibody at a dilution of 1:100 (sc-9008, Santa Cruz). The slides were then applied in the detection system of Elivision™ Plus Kit and DAB following counterstaining with hematoxylin. A 4-tiered semiquantitative scale was used to assess the degree of staining based on the average percentage of positive staining cells: 0 = negative cells, 1 = 1–25 %, 2 = 26–50 %, and 3 = 51–100 %. To analyze the prognosis between groups, staining of either <50 or ≥50 % of the tumor cells was used to assign patients to a low-score or high-score group, respectively.
Cell culture
Human glioma cell lines SW1783 and U87 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA), cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA), supplemented with 10 % fetal bovine serum (Invitrogen), and maintained in a humidified atmosphere with 5 % CO2 at 37 °C.
Transfection of small interfering RNA
Small interfering RNA (siRNA) targeting GATA2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. After 6 h, the cultures were replaced with fresh medium.
Western blot analysis
Cells were lysed by ice-cold protein extraction buffer (150 mM Tris–HCl, pH 7.4, 120 mM NaCl, 2 mM EDTA, 50 mM sodium fluoride, 0.2 % SDS, 1 % Nonidet P-40, 100 mM sodium vanadate and 1 mM phenylmethylsulfonyl fluoride). Proteins were quantitated using the BCA kit. The protein samples were separated in 10 % SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA). The membranes were blocked in 5 % nonfat milk for 1 h at room temperature and then incubated with the following primary antibodies: Elk-1 and phospho-EGFR (Tyr1068) antibodies (Cell Signaling Technology, Inc., Beverly, MA, USA); GATA-2 and GAPDH antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4 °C. After washing, the membranes were incubated with the secondary antibody for 1 h at room temperature. Detection was performed using a chemiluminescence-based detection system (ECL Western blotting kit; Pierce Biotechnology, Inc., Rockford, IL, USA).
MTT assay
Cells were plated in 96-well plates and allowed to grow for 24 h. After transfection, 50 μL MTT solution was added and incubated at 37 °C for 4 h. After dissolving the formazine granulars with 150 μL DMSO, the optical density (OD) at 570 nm was measured using a microplate reader.
Cell migration and invasion assays
Cell migration and invasion were analyzed using transwell migration and extracellular matrix-coated invasion chambers (Millipore, CA, USA) according to the manufacturer’s instructions. In short, cells were plated into the top well of a transwell migration chamber (for migration assay) or an extracellular matrix-coated invasion chamber (for invasion assay). After 16-h incubation, the non-migrating/non-invading cells were removed with a cotton swab, and the migrating/invading cells on the underside of the membrane were stained with cell stain solution (Millipore) for 15 min. After washing two times with PBS, the stain of each membrane was removed with 100 mL extraction buffer (Millipore) and quantitated with a colorimetric microplate reader at 570 nm.
Luciferase reporter assay
For luciferase reporter assays, SW1783 cells were seeded in 24-well plates and transfected with the indicated plasmids. Cells were harvested 36 h after transfection. Luciferase activities were measured using the Dual Luciferase Reporter Assay System (Promega, USA).
Statistical analysis
Statistical significance of differences between mean values was assessed with the Student’s t test for unpaired data and analysis of variance. All reported significance levels represent two-tailed P values. A P value of <0.05 was used to indicate statistical significance.
Results
Expression levels of GATA2 are upregulated in GBM and correlated with prognosis
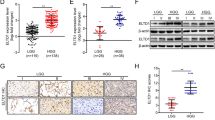
First, we detected the expression levels and subcellular localization of GATA2 protein. Immunohistochemical staining was performed on 56 archived paraffin-embedded GBM tumor samples and six control brain tissues. GATA2 was highly expressed in 73.2 % (41/56) of GBM samples compared with only 16.7 % (1/6) of normal brain samples (Fig. 1a). GATA2 was mainly localized in the nucleus of GBM tumor cells (Fig. 1a). Based on the staining intensity in GBM samples, we divided them into a low-score group (<50 % staining) and a high-score group (≥50 % staining).
Expression levels of GATA2 are upregulated in GBM and correlated with prognosis. a Representative images of IHC staining of GATA2 expression in human normal brain or GBM tissues. Scale bar 100 μm. b Western blot analysis of GATA2 expression in GBM and controlled brain tissues. C indicates non-neoplastic control brain tissues. T indicates GBM tumor tissues. ***P < 0.001. c Correlation analysis of GATA2 protein expression with patient overall survival (n = 56). The low- and high-score groups indicate the patients whose staining intensity in GBM tissues determined by IHC was <50 or ≥50 %, respectively
Next, we performed immunoblot analysis to assess the expression level of GATA2 using GBM tissue from three randomly selected GBM patients and three samples of normal brain. The level of GATA2 in the tumor tissues was significantly higher than that in the control brain tissues (Fig. 1b).
To investigate the clinical significance of GATA2 expression in GBM, the Kaplan–Meier analysis was performed to study the correlation between GATA2 expression and patient outcome. Patients in the high-score group had notably shorter survival than patients in the low-score group, suggesting that GATA2 expression was negatively correlated with survival (Fig. 1c). These findings suggest that higher GATA2 expression is associated with poor prognosis in GBM patients.
GATA2 is necessary for glioma cell proliferation, migration and invasion
To evaluate the role of GATA2 in glioma, we utilized two human glioma cell lines from SW1783 (grade III astrocytoma) and U87 (grade IV astrocytoma; glioblastoma) cells. SW1783 cells were transiently transfected by GATA2 plasmid, while U87 cells were transfected with siRNA to knock down GATA2 expression. The growth curves determined by MTT assay showed that GATA2 overexpression promoted the proliferation of SW1783 cells; however, GATA2 knockdown significantly blocked proliferation of U87 cells (Fig. 2a).
GATA2 is necessary for glioma cell proliferation, migration and invasion. a Effect of GATA2 on glioma cell proliferation. Cells were cultured for the indicated days, and relative cell growth was determined by MTT assay. Left panel growth curve for SW1783 cells. Right panel growth curve for U87 cells. b Effect of GATA2 on the migration and invasion of U87 cells. Upper panels representative microscopic images of migrated or invaded cells. Lower panels the number of migrating/invading cells counted from five randomly selected fields. c Effect of GATA2 on the migration and invasion of SW1783 cells. Upper panels representative microscopic images of migrated or invaded cells. Lower panels the number of migrating/invading cells counted from five randomly selected fields
Next, we performed migration and invasion assays on the two cell lines to examine the effect of GATA2 on cell migration and invasion. siGATA2-transfected U87 cells were cultured on the transwell apparatus. After 16-h incubation, the percentage of migrated cells was significantly lower in siGATA2-treated group (Fig. 2b). Using a Boyden chamber coated with Matrigel, we analyzed changes in cell invasiveness after 16-h incubation. When compared to the control groups, siGATA2-transfected U87 cells exhibited low invasive ability (Fig. 2b). Meanwhile, the overexpression of GATA2 in SW1783 cells dramatically increased migration and invasion compared with the control cells (Fig. 2c).
Together, these results suggest an essential role for GATA2 in the proliferation, migration and invasion of glioma cells in vitro.
The expression of GATA2 is regulated by EGFR/ERK signaling pathway
EGFR induces proliferation and promotes progression of many tumors [13]. EGFR gene amplification and overexpression are very common in GBM patients [14, 15]. To determine the role of EGFR signaling in the regulation of GATA2, U87 cells were treated with the EGFR inhibitor AG1478 for 1 h. AG1478 inhibited EGFR phosphorylation and the expression of GATA2 (Fig. 3a). We also employed the ERK inhibitor U0126 to treat the cell line because of the role ERK plays as a direct EGFR downstream effector. Similar changes in the status of EGFR phosphorylation and GATA2 levels were observed (Fig. 3b). Next, we detected the levels of phospho-EGFR and GATA2 in SW1783 cells with 100 ng/mL EGF treatment. As shown in Fig. 3c, the phosphorylation of EGFR on Tyr1068 was increased by EGF treatment and a significant upregulation of GATA2 was observed accordingly. Furthermore, increased GBM cell proliferation stimulated by EGF treatment could be dramatically suppressed by GATA2 knockdown (Fig. 3d), suggesting that GATA2 acts as a downstream effector in the EGFR signaling pathway.
The expression of GATA2 is regulated by EGFR/ERK signaling pathway. a U87 cells were pretreated with EGFR inhibitor AG1478 at 100 nM for 1 h. The indicated protein expression levels were determined by Western blot analysis. b U87 cells were pretreated with ERK1/2 inhibitor U0126 at 100 nM for 1 h. The indicated protein expressions were determined by Western blot analysis. c SW1783 cells were treated with 100 ng/mL EGF for 1 h. The indicated protein expressions were determined by Western blot analysis. d EGF (100 ng/mL) was applied to U87 cells, and cell proliferation was determined by MTT cell proliferation assay
These observations confirm that EGFR/ERK pathway signaling plays a role in regulating the expression of GATA2.
GATA2 promotes Elk-1 expression
It is well known that Elk-1 is an important downstream signaling molecule of the EGFR/ERK signaling pathway [16–18]. We therefore explored the idea that GATA2 regulates the expression of Elk-1. Ectopic expression of GATA2 increased the Elk-1 expression in SW1783 cells, while knockdown of GATA2 reduced the Elk-1 expression in U87 cells (Fig. 4a). We then assessed whether GATA2 increases the activity of the ELK-1 gene promoter. The Elk-1 luciferase reporter constructs (Elk-1-Luc) transiently transfected into SW1783 cells with or without GATA2 expression plasmids. Luciferase reporter activity increased in a dose-dependent manner in cells with GATA2 ectopic expression compared with the control cells (Fig. 4b).
The tumor-promotion role of GATA2 depends on EGFR/ERK/Elk-1 signaling pathway
To examine whether GATA2 promotes glioma cell proliferation, migration and invasion through the EGFR/ERK/Elk-1 pathway, the overexpression of GATA2 in SW1783 cells was treated with the EGFR inhibitor AG1478 for 1 h. We found that the proliferation, migration and invasion of these cells were completely blocked compared with GATA2 overexpression SW1783 cells (Fig. 5a–c). Similar to the EGFR inhibitor AG1478 treatment results, the knockdown of Elk-1 by siRNA also thoroughly inhibited GATA2 overexpression-induced tumor-promotion effects in SW1783 cells (Fig. 6a–c). These results demonstrated that GATA2 promotes glioma cell proliferation, migration and invasion through the EGFR/ERK/Elk-1 pathway.
Discussion
In the present study, we found that high GATA2 expression in patients with GBM is positively correlated with poor patient survival. In vitro evidence showed that the overexpression of GATA2 or the knockdown of GATA2 in glioma cell lines leads to corresponding promotion or inhibition of tumor growth and progression.
Glioma is a common type of primary brain tumor that originates from the glial cells in the brain [19–21]. Therefore, characterization of the proliferation, migration and invasion of glioma cells is critical for understanding the development of glioma. In this study, we focused on GATA2 because it is a well-known oncogene in the hematopoietic system. Furthermore, several studies have reported that GATA2 promoted tumor growth and metastasis in other types of cancers [10–12]. Consistent with these studies, we found that knockdown of GATA2 in U87 cells decreased cell proliferation, migration and invasion, whereas forced expression of GATA2 in SW1783 cells caused the opposite effects. Taken together, the results support our hypothesis that GATA2 overexpression is positively correlated with GBM progression and poor prognosis; it might therefore serve as a potential therapeutic target in patients with GBM.
EGFR (also known as ErbB1 or HER1) is a member of the HER family of receptor tyrosine kinases [22]. Activation of downstream signaling molecules such as ERK and Elk-1 by EGFR stimulation promotes cell proliferation [23]. Amplifications and mutations of EGFR often occur in GBM, accounting for approximately 50 % of GBM; therefore, EGFR has recently been seen as a promising therapeutic target for treating GBM [24, 25]. The molecular mechanisms underlying this process remain unknown, and no report has yet described the association of GATA2 with EGFR. In this study, we found that GATA2 may be one of the downstream effector molecules of EGFR signaling to regulate the malignant behavior of GBM cells. We demonstrated that upon EGF stimulation, the upregulation of GATA2 expression was temporally followed by the EGFR phosphorylation level, whereas the inhibition of EGFR signaling by EGFR or ERK inhibitor decreased GATA2 expression. This finding suggests that expression of GATA2 is regulated by EGFR/ERK signaling.
Transcription factor GATA2 has been intensively studied in hematopoietic development over the past decade [26]. Recent studies have begun to clarify its role in tumorigenesis. Elk-1 is in the family of Ets domain-containing transcription factors, which can activate the ERK signaling pathway [27]. However, it is not yet clear whether GATA2 regulates the expression of Elk-1. In the present study, we found that GATA2 promoted the expression of Elk-1 by Western blotting and luciferase assay. Combined with the fact that EGFR/ERK as upstream molecules stimulates the level of GATA2, we considered that GATA2 plays a key role in the EGFR/ERK/Elk-1 pathway. Furthermore, inhibiting the EGFR/ERK or knocking down the expression of Elk-1 could block the tumor-promotion function of GATA2. These results suggest that GATA2 promotes glioma progression via the EGFR/ERK/Elk-1 pathway and that GATA2 is an important molecular component of the EGFR/ERK/Elk-1 pathway.
In conclusion, for the first time, we evaluated the expression of GATA2 and its prognostic potential in GBM. Our results showed that the expression level of GATA2 is upregulated in GBM and correlated with GBM outcome. Ectopic expression of GATA2 or RNAi-mediated knockdown of GATA2 significantly enhanced or inhibited proliferation, migration and invasion of glioma cells. Therefore, a genetic approach or pharmacological intervention targeting GATA2 could potentially serve as an effective strategy for glioma treatment.
References
Ahluwalia MS, Chang SM. Medical therapy of gliomas. J Neurooncol. 2014;119:503–12.
Prosniak M, Harshyne LA, Andrews DW, Kenyon LC, Bedelbaeva K, Apanasovich TV, Heber-Katz E, Curtis MT, Cotzia P, Hooper DC. Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res. 2013;19:3776–86.
Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomark Prev. 2014;23:1985–96.
Martinez R, Schackert G, Yaya-Tur R, Rojas-Marcos I, Herman JG, Esteller M. Frequent hypermethylation of the DNA repair gene MGMT in long-term survivors of glioblastoma multiforme. J Neurooncol. 2007;83:91–3.
Simmons ML, Lamborn KR, Takahashi M, Chen P, Israel MA, Berger MS, Godfrey T, Nigro J, Prados M, Chang S, Barker FG 2nd, Aldape K. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61:1122–8.
Zheng R, Blobel GA. GATA transcription factors and cancer. Genes Cancer. 2010;1:1178–88.
Bresnick EH, Katsumura KR, Lee HY, Johnson KD, Perkins AS. Master regulatory GATA transcription factors: mechanistic principles and emerging links to hematologic malignancies. Nucleic Acids Res. 2012;40:5819–31.
Chlon TM, Crispino JD. Combinatorial regulation of tissue specification by GATA and FOG factors. Development. 2012;139:3905–16.
Vicente C, Conchillo A, García-Sánchez MA, Odero MD. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit Rev Oncol Hematol. 2012;82:1–17.
Wang Y, He X, Ngeow J, Eng C. GATA2 negatively regulates PTEN by preventing nuclear translocation of androgen receptor and by androgen-independent suppression of PTEN transcription in breast cancer. Hum Mol Genet. 2012;21:569–76.
Kumar MS, Hancock DC, Molina-Arcas M, Steckel M, East P, Diefenbacher M, Armenteros-Monterroso E, Lassailly F, Matthews N, Nye E, Stamp G, Behrens A, Downward J. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell. 2012;149:642–55.
Chiang YT, Wang K, Fazli L, Qi RZ, Gleave ME, Collins CC, Gout PW, Wang Y. GATA2 as a potential metastasis-driving gene in prostate cancer. Oncotarget. 2014;5:451–61.
Kancha RK, von Bubnoff N, Peschel C, Duyster J. Functional analysis of epidermal growth factor receptor (EGFR) mutations and potential implications for EGFR targeted therapy. Clin Cancer Res. 2009;15:460–7.
Liu KJ, Chen CT, Hu WS, Hung YM, Hsu CY, Chuang BF, Juang SH. Expression of cytoplasmic-domain substituted epidermal growth factor receptor inhibits tumorigenicity of EGFR-overexpressed human glioblastoma multiforme. Int J Oncol. 2004;24:581–90.
Carlsson J, Ren ZP, Wester K, Sundberg AL, Heldin NE, Hesselager G, Persson M, Gedda L, Tolmachev V, Lundqvist H, Blomquist E, Nistér M. Planning for intracavitary anti-EGFR radionuclide therapy of gliomas. Literature review and data on EGFR expression. J Neurooncol. 2006;77:33–45.
Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–87.
Mut M, Lule S, Demir O, Kurnaz IA, Vural I. Both mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinases (ERK) 1/2 and phosphatidylinositide-3-OH kinase (PI3 K)/Akt pathways regulate activation of E-twenty-six (ETS)-like transcription factor 1 (Elk-1) in U138 glioblastoma cells. Int J Biochem Cell Biol. 2012;44:302–10.
Wu P, Wee P, Jiang J, Chen X, Wang Z. Differential regulation of transcription factors by location-specific EGF receptor signaling via a spatio-temporal interplay of ERK activation. PLoS ONE. 2012;7:e41354.
Menon LG, Pratt J, Yang HW, Black PM, Sorensen GA, Carroll RS. Imaging of human mesenchymal stromal cells: homing to human brain tumors. J Neurooncol. 2012;107:257–67.
Terasaki M, Sugita Y, Arakawa F, Okada Y, Ohshima K, Shigemori M. CXCL12/CXCR4 signaling in malignant brain tumors: a potential pharmacological therapeutic target. Brain Tumor Pathol. 2011;28:89–97.
Broniscer A, Baker SJ, Stewart CF, Merchant TE, Laningham FH, Schaiquevich P, Kocak M, Morris EB, Endersby R, Ellison DW, Gajjar A. Phase I and pharmacokinetic studies of erlotinib administered concurrently with radiotherapy for children, adolescents, and young adults with high-grade glioma. Clin Cancer Res. 2009;15:701–7.
Ménard S, Casalini P, Campiglio M, Pupa SM, Tagliabue E. Role of HER2/neu in tumor progression and therapy. Cell Mol Life Sci. 2004;61:2965–78.
Adachi T, Kar S, Wang M, Carr BI. Transient and sustained ERK phosphorylation and nuclear translocation in growth control. J Cell Physiol. 2002;192:151–9.
Labussière M, Boisselier B, Mokhtari K, Di Stefano AL, Rahimian A, Rossetto M, Ciccarino P, Saulnier O, Paterra R, Marie Y, Finocchiaro G, Sanson M. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83:1200–6.
Taylor TE, Furnari FB, Cavenee WK. Targeting EGFR for treatment of glioblastoma: molecular basis to overcome resistance. Curr Cancer Drug Targets. 2012;12:197–209.
Moriguchi T, Yamamoto M. A regulatory network governing Gata1 and Gata2 gene transcription orchestrates erythroid lineage differentiation. Int J Hematol. 2014;100:417–24.
Goncharenko-Khaider N, Matte I, Lane D, Rancourt C, Piché A. Ovarian cancer ascites increase Mcl-1 expression in tumor cells through ERK1/2-Elk-1 signaling to attenuate TRAIL-induced apoptosis. Mol Cancer. 2012;11:84.
Acknowledgments
This work was supported by University Graduate Students’ Scientific Research Innovative Program of Jiangsu Province, China (CXLX12-0843); National Natural Science Foundation of China (81172400, 81272799 and 81472739); Scientific and Technological Development Program of Suzhou, China (SYS201477, SYSD2012090); and Priority Academic Program Development of Jiangsu Higher Education Institutions, China.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zhongyong Wang, Hui Yuan and Chao Sun have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, Z., Yuan, H., Sun, C. et al. GATA2 promotes glioma progression through EGFR/ERK/Elk-1 pathway. Med Oncol 32, 87 (2015). https://doi.org/10.1007/s12032-015-0522-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0522-1