Abstract
The purpose of this study was to investigate the relationship between the expression of CD147 and Lewis y antigen in epithelial ovarian carcinoma tissues and resistance to chemotherapeutic drugs, and its underlying clinical significance, and to analyze the correlation between the expression of CD147 and Lewis y antigen. Ninety-two ovarian cancer patients were divided into a chemotherapeutic-drug-resistant group (34 patients) and a drug-sensitive group (58 patients). Immunohistochemical assays were used to measure CD147, and Lewis y antigen to investigate their correlation with chemotherapy resistance. Multivariate logistic regression was used to analyze the relationships between risk factors and resistance to chemotherapy in ovarian cancer. Cox’s model was used to analyze the relationships between risk factors and prognosis. The proportion of tissues expressing CD147 and Lewis y antigen in the drug-resistant group were 94.12 and 91.67 %, respectively, which were significantly higher than those in the sensitive group (77.59 and 60.34 %, respectively). The multivariate analysis indicated that the expression of CD147 and Lewis y antigen and the pathological stage of the ovarian cancer were all independent risk factors for drug resistance. Expression of CD147 and Lewis y antigen was high in the resistant group, and they correlated positively with each other. The expression of CD147 and Lewis y antigen was significantly higher in the drug-resistant group and their expression correlated positively in the ovarian epithelium. The expression of CD147 and Lewis y antigen and the pathological stage of ovarian cancer were all independent risk factors for drug resistance and prognosis in ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer has the highest mortality of all female genital neoplasms. Chemotherapeutic drug resistance is an important factor in the treatment outcome. Therefore, it is very important to identify the risk factors for drug resistance and to identify new treatment targets.
CD147 is an inducer of matrix metalloproteinases (MMPs). As an adhesion molecule, it is strongly expressed in many malignant tumor cells. CD147 is considered to be an important molecule in the progression of tumors and influences the biological behavior of tumor cells, including their metastasis and invasion, by inducing MMPs and angiogenesis factors in both tumor and stromal cells.
Many studies have demonstrated that the expression of CD147 is associated with the drug resistance of tumors, and high expression of CD147 can increase the drug resistance of tumor cells [1]. Small interfering RNA obstruction of CD147 expression can increase the sensitivity of tumor cells to chemotherapeutic drugs [1–3]. Studies have indicated that the drug-resistance mechanism of CD147 acts through its interaction with the hyaluronan receptor, CD44, which affects the expression of downstream factors in the signaling pathway [4–7]. CD147 can also influence the expression and functions of many drug-resistance-associated proteins [8], thereby affecting the drug resistance of tumor cells.
Glycosylation plays an important role in the functions of the CD147 molecule [9, 10]. Lewis y antigen is a tumor-associated carbohydrate antigen. In a previous study, we showed that Lewis y antigen is related to the proliferation, adhesion, and drug resistance of tumors [11–15]. Lewis y antigen, as part of the receptors on the surfaces of cells, influences the biological behaviors of tumors [8, 10, 12]. Wang et al. [16] found that obstructing the N-glycosylation of CD147 and P-glycoprotein (P-gp) or increasing their ubiquitination and degradation enhanced the sensitivity of MCF7/Adr cells to doxorubicin.
Although studies of the effects of CD147 on ovarian cancer biology and drug resistance have increased in recent years, the effect of modifying the glycosylation of CD147 on drug resistance remains unclear. In this study, based on previous experiments, we used immunohistochemical methods to investigate the expression of CD147 and Lewis y antigen and their correlation in histological samples from patients with drug-resistant or drug-sensitive ovarian cancer. The relationships between these molecules and chemotherapeutic drug resistance in ovarian cancer and their clinical significance are discussed.
Materials and methods
Materials
Surgical samples
Ninety-two paraffin samples were obtained from operations performed in 2006–2010 in the Department of Gynecology and Obstetrics of Shengjing Hospital Affiliated to the China Medical University. Following cytoreductive surgery and 6–8 cycles of systematic chemotherapy, each patient was followed up for at least 1 year. Of the 92 cases of primary epithelial ovarian cancer studied, 58 were serous cystadenocarcinoma, eight were mucinous cystadenocarcinoma, four were endometrioid carcinoma, seven were clear cell carcinoma, and 15 were poorly differentiated adenocarcinoma. There were 15 highly differentiated, 35 moderately differentiated, and 27 poorly differentiated tumors, according to their histological grades. The sample included 19 stage I tumors, 13 stage II tumors, and 60 stage III tumors [according to the International Federation of Gynecology and Obstetrics (FIGO) criteria]. All the tumors were primary, and the patient information and follow-up data were complete. No chemical treatment was used in any patient before surgery.
Drug-resistance-related clinical and pathological parameters
Tissues were obtained between 2006 and 2010 from 92 patients with ovarian cancer who met the inclusion criteria and for whom the follow-up data were complete. The clinical and pathological parameters obtained for the ovarian cancer patients included age, clinical stage, differentiation, histological subtype, and chemotherapy scheme (paclitaxel + carboplatin). The patients were divided into the chemotherapy-resistant group (34 patients) and the chemotherapy-sensitive group (58 patients), according to the guideline of the National Comprehensive Cancer Network: recurrence during the chemotherapy period or within 6 months after chemotherapy was defined as drug resistance; recurrence 6–12 months after chemotherapy was defined as partial sensitivity; and recurrence beyond 12 months after chemotherapy or no recurrence was defined as drug sensitivity.
Main reagents
Rabbit polyclonal anti-CD147 antibody and mouse monoclonal anti-Lewis y antibody (clone A 70-C/C8) were purchased from Abcam Company (UK). Bovine serum albumin and the diaminobenzidine kit were purchased from Zhong shan Biotechnology Company (China). Other reagents were supplied by our laboratory.
Methods
This study was conducted at the Shengjing Hospital Affiliated to the China Medical University, China. The Ethics Committee of the Shengjing Hospital Affiliated to the China Medical University approved the study (Approval No. 2013PS166K).
Immunohistochemistry
Streptavidin–biotin–peroxidase (SP) immunohistochemistry was performed. The tissues were fixed in 4 % formaldehyde and embedded in paraffin, and 4-mm-thick serial sections were prepared. The working dilutions of the CD147 and Lewis y antibodies were 1:100 and 1:200, respectively. The staining procedure was according to the SP kit manual. Phosphate-buffered saline replaced the primary antibody in the negative control. A liver cancer sample was used the positive control for CD147, and a colon cancer sample as the positive control for Lewis y antigen.
Analysis of immunohistochemistry results
The presence of brown-colored granules on the cell membrane or in the cytoplasm was taken to be a positive signal and was classified according to the color intensity as follows: not colored = 0, light yellow = 1, brown = 2, and tan = 3. We chose five high-power fields in a series from each slice, scored them for color intensity, and then calculated the average percentage cell chromatosis: positive cell rate of <5 % = 0, 5–25 % = 1, 26–50 % = 2, 51–75 % = 3, and more than 75 % = 4. The final score was determined by multiplying the positive cell rate and the positive signal score: 0–2 was equal to negative expression (−), 3–4 was equal to weakly positive (+), 5–8 was equal to moderate positive (++), and 9–12 was equal to strong positive (+++). The results were read by two independent observers to control for variability.
Statistical analyses
The statistical analyses were performed with the SPSS version 13 software. Data expressed as mean ± SD were used for the statistical analysis. Student’s t test was applied to compare data between two groups, and analysis of variance was used to compare data among multiple groups.
The χ2 test was used to analyze the expression of CD147 and Lewis y antigen and the clinicopathological parameters. Spearman’s correlation analysis was used to calculate the coefficient R of the indices and to analyze their correlations. Cox’s model was used to analyze prognoses. A P < 0.05 was considered statistically significant.
Results
Expressions of CD147 and Lewis y antigen in the ovarian tissues of each group
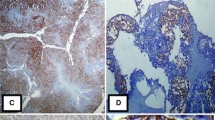
The expression of CD147 was mainly detected in the cell membrane in the 92 cases of malignant ovarian tumor. Its expression was low in the cytoplasm and nuclei, as indicated by staining. The expression rate of CD147 in the drug-resistant group was 94.12 %, which was significantly higher than that in the drug-sensitive group (77.59 %; P < 0.05). The expression intensity of CD147 in the drug-resistant group was also significantly higher than that in the drug-sensitive group. In the former, 17 tumors showed strong positivity, representing 50 % of the group members, whereas in the latter, only seven tumors (12 %) showed strong positive CD147 expression (Table 1; Fig. 1).
The expression of Lewis y antigen was similar to that of CD147. It was mainly localized to the cell membrane, and the cytoplasm was rarely stained. Its positive expression rate in the drug-resistant group was 91.67 %, which was significantly higher than that in the drug-sensitive group (60.34 %; P < 0.05) (Table 1; Fig. 1).
Drug Resistance-Related Risk Factors Univariate Analysis
Univariate analysis of the pathological stage, pathological grade, and pathological type of the cancer patients in the drug-resistant and drug-sensitive groups indicated that the two groups differed significantly only in terms of their pathological stage (P = 0.01; Table 2).
Multivariate analysis of drug resistance in ovarian cancer
The age, pathological stage, pathological grade, pathological type of the patient, and the expression of CD147 and Lewis y antigen were included as the dependent variables in a multivariate logistic regression analysis (forward stepwise regression). The results indicated that CD147, Lewis y antigen, and the pathological stage of the cancer were all independent risk factors for drug resistance in ovarian cancer (Table 3).
Correlation between the expression of CD147 and Lewis y antigen in ovarian cancer tissues
In the 92 ovarian cancer tissues examined, 58 showed simultaneous positive expression of CD147 and Lewis y antigen and four showed their simultaneous negative expression.
In tissues with high-intensity CD147 expression, the expression of Lewis y antigen was also high. The expression of the two molecules correlated linearly (R s = 0.293, P < 0.05, Table 4).
Prognostic analysis
Survival curves
A follow-up survey of the ovarian cancer patients in the two groups was conducted (until December 2013). The survey data were analyzed with a Kaplan–Meier analysis, and the log-rank test was used to test the results. The mortality of patients with strong CD147 expression, drug resistance, and pathological stages III–IV was significantly higher than that of patients with weak CD147 expression, drug sensitivity, and pathological stages I–II (P = 0.027, 0.000, and 0.018, respectively, Fig. 2). The mortality of patients with positive expression of Lewis y antigen was higher than that of patients with no Lewis y antigen expression, but the difference was not statistically significant. When pathological differentiation and pathological type were used to divide the patients, the mortality of the groups did not differ significantly (Fig. 3).
Cox’s model
The Cox’s model analysis indicated that CD147, Lewis y antigen, and the pathological stage of the cancer were all independent risk factors for the prognoses of ovarian cancer patients (Table 5).
Discussion
CD147 was first discovered in pulmonary tissues in 1984, as a secreted factor of human fibroblast-activated collagenase [17]. It belongs to the type I transmembrane glycoproteins of immunity superfamily [18]. CD147, also called extracellular matrix (ECM) metalloproteinase inducer, is so named because of its activity in inducing the secretion of extracellular MMPs. CD147 is strongly expressed in many tumor tissues, whereas it is not expressed or is expressed at low levels in normal tissues. It participates in many biological processes in cells.
Matrix metalloproteinases are Zn2+-dependent proteolytic enzymes that can degrade the ECM and facilitate the metastasis of tumor cells. In tumor cells, CD147 stimulates the secretion of MMP-1 and MMP-2. It also forms complexes with MMP-1 on the surfaces of tumors, which cause the MMPs to accumulate around the tumor cells, thereby facilitating the degradation of the matrix surrounding them and facilitating their migration [19].
Studies have found that the expression of CD147 in liver transplantation patients is closely associated with CD34, which is a marker of microvessel density [20]. In tumor tissues, the expression of CD147 correlates positively with the expression of vascular endothelial growth factor [21], which suggests that CD147 is closely related to the growth, invasion, and angiogenesis of tumors. CD147 also interacts with integrins α3β1 and α6β1 and participates in the formation of the blood–brain barrier. This implies that CD147 plays a role in the process of cell adhesion [22].
The molecular mechanism of drug resistance in tumor cells is very complex and can involve the following factors: a reduction in drug uptake or an increase in drug excretion; drug deactivation; changes in drug localization; recovery from drug-induced damage; and the inhibition of apoptosis [23]. Changes in the cycles of tumor cells also affect drug resistance [24]. In recent years, a new drug-resistance mechanism in tumors, cell-adhesion-mediated drug resistance (CAM-DR), has drawn wide attention [25–27]. Cell–ECM adhesion plays an important role in the metastasis of tumors [26]. Tumor cells have greater survival potential and a greater capacity to resist apoptosis when they adhere to their surrounding environment. The growth and metastasis of tumor cells are closely related to drug resistance. Chemotherapeutic drug resistance has long been a difficult problem in the treatment of ovarian cancer. Research into the underlying drug-resistance mechanism and targeted countermeasures has been highly topical issues in ovarian cancer studies. In recent years, CD147 has been recognized as a drug-resistance-associated protein, and its role in tumor multidrug resistance and the underlying mechanism have attracted increasing attention.
It has been confirmed that CD147 is related to the drug resistance of many tumors. Pan et al. [28], using a proteomic analysis, found that the expression of CD147 is elevated in drug-resistant tissues. After CD147 knockout in the ovarian cancer cell line HO-8910PM, the invasive capacity of the cells and their tumorigenic potential in nude mice were reduced and the cells were more sensitive to paclitaxel [29]. Yang et al. [30] found that the expression of MDR1/P-gp and CD147/CD98hc complexes was high in a cisplatin-resistant ovarian cancer cell line (SKOV3/DDP), whereas in the maternal cells (SKOV3), their expression was low. When the expression of CD98hc or CD147 was reduced with an RNA interference technique, both the expression of the target genes and P-gp and the dose of cisplatin required for 50 % survival of drug-resistant tumor cells (IC50) were reduced [30]. Hao et al. [6] found that the expression of MCT4 and MRP2 decreased after CD147 knockout in prostate cancer cells. The sensitivity of cells to docetaxel increased, and the expression of p-AKT and p-ERK, which are closely related to cell survival and growth, was downregulated. CD147 knockout in a human oral squamous cell line increased the cells’ sensitivity to vincristine, all-transretinoic acid, and taxol [31].
In this study, we have confirmed using clinical samples that the expression of CD147 is significantly higher in chemotherapeutic-drug-resistant ovarian cancer than in chemotherapy-sensitive ovarian cancer. As a newly discovered drug-resistance-associated protein in tumors, CD147 is a candidate target molecule for strategies that inhibit tumor metastasis and reduce drug resistance in tumors. Although the relationship between CD147 and the drug resistance of tumors has been explained in a preliminarily way, the mechanism of multidrug resistance remains unclear. It may involve the CD44 hyaluronan ligand and its downstream signaling pathway [4–7], or CD147 could also affect the expression and functions of multiple drug-resistance-associated proteins [8].
The most common form of CD147 is a single-stranded transmembrane glycoprotein, with three N-linked glycosylation sites. CD147 exists as both low-glycosylated (LG-CD147) and high-glycosylated (HG-CD147) molecules. The N-linked glycosylation of CD147 is crucial for the induction of MMPs, because only HG-CD147 self-crosslinks on the surface of the cell membrane and induces the synthesis of MMPs [32]. In contrast, deglycosylated or low-glycosylated CD147 cannot induce the formation of MMPs or has some inhibitory effect on it.
Our previous studies showed that the fucosylation of receptors on the surface of the cell membrane can increase the adhesion of cells and the CAM-DR of cells [14, 33–35]. Core fucosylation is essential for cell migration and signal transduction and plays a key role in the interaction between intercellular and extracellular matrices. In this way, it influences tumor cell migration. Lewis y antigen is a doubly fucosylated oligosaccharide. As a cancer-associated antigen, it has received attention because of its role in the genesis and development of tumors. Wang et al. [16] showed that obstruction of the N-glycosylation of CD147 and P-gp and their increased ubiquitination and degradation enhanced the sensitivity of MCF7/Adr cells to doxorubicin. CD147 is involved in the regulation of tumor cell growth, with an antiapoptotic effect that acts via different pathways in breast cancer, oral squamous cancer, and head and neck squamous cell cancer. The downregulation of CD147 expression in liver cancer cells with RNAi interference or the treatment of liver cancer cells with anti-CD147 antibody substantially inhibited the adhesion and invasion of liver cancer cells [36, 37].
Our previous experiments confirmed that Lewis y antigen is part of the CD147 structure. The Lewis y modification of CD147 increases the malignant behavior of cells, such as the proliferation and adhesion of ovarian cancer cells and the drug resistance of cells (results to be published). The present study has shown that the expression of Lewis y antigen and CD147 is similar. The expression of both was high in drug-resistant ovarian cancers, and the expressions of CD147 and Lewis y antigen correlated positively in the ovarian epithelium. The expression of CD147 and Lewis y antigen and the pathological stage of ovarian cancer were all independent risk factors for drug resistance. Cox’s multivariate regression analysis indicated that the expression of CD147 and that of Lewis y antigen in tissues was independent risk factors for the prognosis of ovarian cancer. The results of this study indicate that the expression of CD147 and Lewis y antigen in histopathological samples is important in predicting the drug resistance and prognosis of ovarian cancers.
Li et al. [8] found that in breast cancer, CD147 regulates P-gp, MMP2, and MMP9 via the ERK1/2 pathway, thereby affecting the invasion and drug resistance of the cells. In a previous study, we demonstrated that the expression of MMP-2 and MMP-9 is elevated in the RMG-1-H cell line, together with high expression of Lewis y antigen. After treatment with Lewis y antigen, the expression of MMP-2 and MMP-9 decreased significantly [32]. Because Lewis y antigen influences the biological behavior of cells via the ERK1/2 pathway [12], it is assumed that, as part of the CD147 structure, Lewis y antigen influences the drug resistance of ovarian cancer via the ERK1/2 pathway.
The results of this study show that the immunohistochemical assay of CD147 and Lewis y antigen in ovarian cancer tissues can be used as an important index of the appropriate clinical chemotherapy and the prognostic outcome. An increased understanding of CD147 and the signal transduction pathway involved in the chemotherapeutic drug resistance induced by its glycosylation should provide the foundation for chemosensitization strategies and the development of new chemotherapeutic methods.
References
Kuang YH, Chen X, Su J, Wu LS, Liao LQ, Li D, Chen ZS, Kanekura T. RNA interference targeting the CD147 induces apoptosis of multi-drug resistant cancer cells related to XIAP depletion. Cancer Lett. 2009;276(2):189–95.
Wang B, Xu YF, He BS, Pan YQ, Zhang LR, Zhu C, Qu LL, Wang SK. RNAi-mediated silencing of CD147 inhibits tumor cell proliferation, invasion and increases chemosensitivity to cisplatin in SGC7901 cells in vitro. J Exp Clin Cancer Res. 2010;29:61.
Jia L, Wei W, Cao J, Xu H, Miao X, Zhang J. Silencing CD147 inhibits tumor progression and increases chemosensitivity in murine lymphoid neoplasm P388D1 cells. Ann Hematol. 2009;88(8):753–60.
Misra S, Ghatak S, Toole BP. Regulation of MDR1 expression and drug resistance by a positive feedback loop involving hyaluronan, phosphoinositide 3-kinase, and ErbB2. J Biol Chem. 2005;280(21):20310–5.
Torre C, Wang SJ, Xia W, Bourguignon LY. Reduction of hyaluronan-CD44-mediated growth, migration, and cisplatin resistance in head and neck cancer due to inhibition of Rho kinase and PI-3 kinase signaling. Arch Otolaryngol Head Neck Surg 2010;136(5):493–501.
Hao J, Madigan MC, Khatri A, Power CA, Hung TT, Beretov J, Chang L, Xiao W, Cozzi PJ, Graham PH, et al. In vitro and in vivo prostate cancer metastasis and chemoresistance can be modulated by expression of either CD44 or CD147. PLoS ONE. 2012;7(8):e40716.
Misra S, Ghatak S, Zoltan-Jones A, Toole BP. Regulation of multidrug resistance in cancer cells by hyaluronan. J Biol Chem. 2003;278(28):25285–8.
Li QQ, Wang WJ, Xu JD, Cao XX, Chen Q, Yang JM, Xu ZD. Involvement of CD147 in regulation of multidrug resistance to P-gp substrate drugs and in vitro invasion in breast cancer cells. Cancer Sci. 2007;98(7):1064–9.
Tang W, Chang SB, Hemler ME. Links between CD147 function, glycosylation, and caveolin-1. Mol Biol Cell. 2004;15(9):4043–50.
Jia L, Zhou H, Wang S, Cao J, Wei W, Zhang J. Deglycosylation of CD147 down-regulates Matrix Metalloproteinase-11 expression and the adhesive capability of murine hepatocarcinoma cell HcaF in vitro. IUBMB Life. 2006;58(4):209–16.
Zhang F, Liu J, Lin B, Liu Q, Zhao Y, Zhu L, Hao Y, Zhang S, Iwamori M. Increase in docetaxel-resistance of ovarian carcinoma-derived RMG-1 cells with enhanced expression of Lewis Y antigen. Int J Mol Sci. 2011;12(11):7323–34.
Liu JJ, Lin B, Hao YY, Li FF, Liu DW, Qi Y, Zhu LC, Zhang SL, Iwamori M. Lewis (y) antigen stimulates the growth of ovarian cancer cells via regulation of the epidermal growth factor receptor pathway. Oncol Rep. 2010;23(3):833–41.
Iwamori M, Tanaka K, Kubushiro K, Lin B, Kiguchi K, Ishiwata I, Tsukazaki K, Nozawa S. Alterations in the glycolipid composition and cellular properties of ovarian carcinoma-derived RMG-1 cells on transfection of the alpha1,2-fucosyltransferase gene. Cancer Sci. 2005;96(1):26–30.
Yan LM, Lin B, Zhu LC, Hao YY, Qi Y, Wang CZ, Gao S, Liu SC, Zhang SL, Iwamori M. Enhancement of the adhesive and spreading potentials of ovarian carcinoma RMG-1 cells due to increased expression of integrin alpha5beta1 with the Lewis Y-structure on transfection of the alpha1,2-fucosyltransferase gene. Biochimie. 2010;92(7):852–7.
Hu Z, Gao S, Gao J, Hou R, Liu C, Liu J, Li B, Liu D, Zhang S, Lin B. Elevated levels of Lewis Y and integrin alpha5beta1 correlate with chemotherapeutic drug resistance in epithelial ovarian carcinoma. Int J Mol Sci. 2012;13(12):15588–600.
Wang WJ, Li QQ, Xu JD, Cao XX, Li HX, Tang F, Chen Q, Yang JM, Xu ZD, Liu XP. Interaction between CD147 and P-glycoprotein and their regulation by ubiquitination in breast cancer cells. Chemotherapy. 2008;54(4):291–301.
Biswas C. Collagenase stimulation in cocultures of human fibroblasts and human tumor cells. Cancer Lett. 1984;24(2):201–7.
Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55(2):434–9.
Guo H, Li R, Zucker S, Toole BP. EMMPRIN (CD147), an inducer of matrix metalloproteinase synthesis, also binds interstitial collagenase to the tumor cell surface. Cancer Res. 2000;60(4):888–91.
Zhang Q, Chen X, Zhou J, Zhang L, Zhao Q, Chen G, Xu J, Qian F, Chen Z. CD147, MMP-2, MMP-9 and MVD-CD34 are significant predictors of recurrence after liver transplantation in hepatocellular carcinoma patients. Cancer Biol Ther. 2006;5(7):808–14.
Zhang Q, Zhou J, Ku XM, Chen XG, Zhang L, Xu J, Chen GS, Li Q, Qian F, Tian R, et al. Expression of CD147 as a significantly unfavorable prognostic factor in hepatocellular carcinoma. Eur J Cancer Prev. 2007;16(3):196–202.
Tang W, Hemler ME. Caveolin-1 regulates matrix metalloproteinases-1 induction and CD147/EMMPRIN cell surface clustering. J Biol Chem. 2004;279(12):11112–8.
Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275–92.
Shapiro GI, Harper JW. Anticancer drug targets: cell cycle and checkpoint control. J Clin Investig. 1999;104(12):1645–53.
Hazlehurst LA, Dalton WS. Mechanisms associated with cell adhesion mediated drug resistance (CAM-DR) in hematopoietic malignancies. Cancer Metastasis Rev. 2001;20(1–2):43–50.
Damiano JS. Integrins as novel drug targets for overcoming innate drug resistance. Curr Cancer Drug Targets. 2002;2(1):37–43.
Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998;17(22):6622–32.
Pan S, Cheng L, White JT, Lu W, Utleg AG, Yan X, Urban ND, Drescher CW, Hood L, Lin B. Quantitative proteomics analysis integrated with microarray data reveals that extracellular matrix proteins, catenins, and p53 binding protein 1 are important for chemotherapy response in ovarian cancers. OMICS J Integr Biol. 2009;13(4):345–54.
Zou W, Yang H, Hou X, Zhang W, Chen B, Xin X. Inhibition of CD147 gene expression via RNA interference reduces tumor cell invasion, tumorigenicity and increases chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett. 2007;248(2):211–8.
Yang H, Zou W, Li Y, Chen B, Xin X. Bridge linkage role played by CD98hc of anti-tumor drug resistance and cancer metastasis on cisplatin-resistant ovarian cancer cells. Cancer Biol Ther. 2007;6(6):942–7.
Kuang YH, Chen X, Su J, Wu LS, Li J, Chang J, Qiu Y, Chen ZS, Kanekura T. Proteome analysis of multidrug resistance of human oral squamous carcinoma cells using CD147 silencing. J Proteome Res. 2008;7(11):4784–91.
Gardner MJ, Jones LM, Catterall JB, Turner GA. Expression of cell adhesion molecules on ovarian tumour cell lines and mesothelial cells, in relation to ovarian cancer metastasis. Cancer Lett. 1995;91(2):229–34.
Wang Y, Liu J, Lin B, Wang C, Li Q, Liu S, Yan L, Zhang S, Iwamori M. Study on the expression and clinical significances of Lewis y antigen and integrin alphav, beta3 in epithelial ovarian tumors. Int J Mol Sci. 2011;12(6):3409–21.
Gao L, Yan L, Lin B, Gao J, Liang X, Wang Y, Liu J, Zhang S, Iwamori M. Enhancive effects of Lewis y antigen on CD44-mediated adhesion and spreading of human ovarian cancer cell line RMG-I. J Exp Clin Cancer Res. 2011;30:15.
Wang C, Yan L, Wang Y, Lin B, Liu S, Li Q, Gao L, Zhang S, Iwamori M. Overexpression of Lewis (y) antigen protects ovarian cancer RMG-1 cells from carboplatin-induced apoptosis by the upregulation of Topo-I and Topo-II beta. Anat Rec (Hoboken). 2011;294(6):961–9.
Xu J, Xu HY, Zhang Q, Song F, Jiang JL, Yang XM, Mi L, Wen N, Tian R, Wang L, et al. HAb18G/CD147 functions in invasion and metastasis of hepatocellular carcinoma. Mol Cancer Res. 2007;5(6):605–14.
Qian AR, Zhang W, Cao JP, Yang PF, Gao X, Wang Z, Xu HY, Weng YY, Shang P. Downregulation of CD147 expression alters cytoskeleton architecture and inhibits gelatinase production and SAPK pathway in human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2008;27:50.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81172491, 81101527); the Research Fund for the Doctoral Program of Higher Education (Nos. 20112104110016, 20112104120019); Shengjing freedom researchers (Nos. 20112104110016, 20112104120019); Scientific research projects of Liaoning province department of education (No. L2011129).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, J., Hu, Z., Liu, J. et al. Expression of CD147 and Lewis y antigen in ovarian cancer and their relationship to drug resistance. Med Oncol 31, 920 (2014). https://doi.org/10.1007/s12032-014-0920-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0920-9