Abstract
Overexpression of extracellular matrix metalloproteinase inducer (EMMPRIN or CD147), a member glycoprotein enriched on the surface of many malignant tumor cells, promotes tumor progression and confers resistance to some chemotherapeutic drugs. To investigate the possible role of CD147 in the macrophage-like lymphoid neoplasm P388D1 cells progression, we used RNA interference approach to silence CD147 expression. The results showed that silencing of CD147 in P388D1 cells impeded the expression of MMP11 at both mRNA and protein levels. The reduced CD147 expression also resulted in reductions in tumorigenicity, as well as decreased in regional lymph node metastasis. Furthermore, the down-regulation of CD147 expression sensitized cells to be more sensitive to chemotherapeutic drugs. Treatment of tumor cells with U-0126, an inhibitor of mitogen-activated protein kinase/Erk, also down-regulated the expression of MMP11. Our current results indicate that the expression of CD147 functionally mediates tumor progression and is a potential target for therapeutic anti-cancer drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CD147 is a transmembrane glycoprotein of the immunoglobulin superfamily expressed on the surface of many malignant tumor cells and acts as an upstream modulator of multiple matrix metalloproteinases production in the local tumor environment [1]. The expression of CD147 plays an important role in tumor formation and invasion/metastasis in both animal models [2] and cancer patients [3].
Overexpression of CD147 is shown to promote tumorigenicity, invasion, and metastasis of malignant cells [6]. CD147 expression is associated with poor prognosis, higher tumor grade, increases tumor size, and a higher mitotic index [3]. Also, significantly higher expression of CD147 is found in malignant effusions than in primary tumors in patients with breast cancer [4]. CD147 is also involved in resistance of cancer cells to a variety of chemotherapeutic agents [5]. Inhibition of CD147 gene expression via RNA interference (RNAi) could increase chemosensitivity to paclitaxel in the human ovarian cancer cell line [6]. Although the detailed mechanisms whereby CD147 regulates these numerous phenomena are not yet known, it is clear that CD147 is a major mediator of malignant cell behavior.
It is well understood that matrix metalloproteinase (MMPs) play a critical role in cancer invasion and metastasis. Blocking their activities is a possible strategy for controlling cancer spread. CD147 could be a target for this purpose because it causes MMPs production, not only in heterotypic cell interactions between tumor cells and fibroblasts but also in homotypic cell interactions [7–9]. Moreover, the fact that CD147 stimulates tumors progression through the same cell interactions makes CD147 more attractive as a target. MMPs are a family of zinc-dependent endopeptidases that are believed to affect tumor angiogenesis, lymphangiogenesis, tumor growth, local invasion, and subsequent distant metastasis [10]. MMPs are often overproduced in the tumor local environment, and high levels of MMPs have been correlated with tumor metastasis [11]. MMP11 (stromelysin-3) is member of the MMP family, which belongs to the tumor microenvironmental factor and has been observed in almost all human carcinomas.
Several previous studies showed that tyrosine kinases were required for CD147 induction of MMPs [12, 13]. Since tyrosine kinases are integrally involved in mitogen-activated protein kinase (MAPK) signaling pathways, we attempted to further clarify whether MMPs expression could be mediated by CD147 via MAPK pathways. MAPK superfamily, including extracelluar regulated protein kinase 1/2 (ERK1/2), p38, and Jun N-terminus kinase, regulate cell mobility by distinct mechanisms [14]. Li et al. have shown that CD147 regulates the expression of MDR1, MMP2, and MMP9 via an Erk1/2-dependent signaling pathway in breast cancer cells, which is consistent with their alterations in cellular invasiveness in vitro and MDR [5]. Lim et al. showed that tumor-derived CD147 stimulates collagenase transcription through MAPK p38 [13].
The purpose of the present research was to investigate whether CD147 participated in the regulation MMP11 and determined the mechanism of tumor progression. Our findings showed that the expression of CD147 is responsible for tumor progression and regulates chemosensitivity to anti-tumor drugs through the regulation of MMP11 expression in mouse lymphoma.
Materials and methods
Cell culture and animals
Murine macrophage-like lymphoid neoplasm P388D1 cells (obtained from Chinese Academy of Sciences, Shanghai, China) were cultured in 90% RPMI 1640 (Gibco) supplemented with antibiotics (1× penicillin/streptomycin 100 U/ml, Gibco), 10% FBS (Gibco). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. DBA/2 type mice were (males and 6 week old) obtained from Animal Facility of Dalian Medical University.
RNAi assay
RNAi assay was performed to silence CD147 expression. P388D1 cells were incubated in appropriate antibiotic-free medium with 10% fetal bovine serum (Gibco), transferred to a six-well tissue culture, and incubated at 37°C, CO2 incubator to obtain 60–80% confluence. The cell cultures were transfected with siRNA control and CD147 specific siRNA Transfection Reagent Complex (Santa Cruz Biotech, sc-35299), which was prepared according to the protocol. Cells were transfected twice at 3-day intervals and collected 72 h after the second transfection. Specific silencing of the targeted gene was confirmed by reverse transcription-polymerase chain reaction (RT-PCR) and Western blot analysis. Cells were harvested and experimented as described below.
Reverse transcription-polymerase chain reaction analysis
Total RNA was isolated from cells using Trizol (Invitrogen, Carlsbad, CA, USA), and cDNA was synthesized using RT-PCR kit (TaKaRa, Japan) according to the manufacturer’s instruction. The sequences of the primers were as follows: 5′-GAGAGCTTGCGAAACTGGTC-3′ (F) and 5′-AACCAACACCAGGACCTCA G-3′(R) for CD147; 5′-GCCAGATTTGGTTCTTCCAA-3′and 5′-CACGGGATC AAACTT CCAGT-3′ for MMP-11; 5′-G GCCGTGAAGTCGTCAGAAC-3′ (F) and 5′-GCCACGATGCCCAGGAA-3′ (R) for GAPDH, respectively. PCR analysis was performed under the following conditions: denaturation at 94°C for 1 min, and then 30 cycles of denaturation for 20 s at 97°C, annealing for 20 s at 64°C, and extension for 20 s at 72°C. The amplified products were analyzed by agarose gel electrophoresis using 1.5% gel, followed by ethidium bromide staining.
Western blot analysis
Cells were harvested and lysed in 2× concentrated electrophoresis sample buffer (125 mmol/L Tris–HCl, pH 6.8, 2% sodium dodecyl sulfate, 5% glycerol, and 1% β-mercaptoethanol). Equal amounts of denatured proteins (40 μg/well) were resolved by 8% SDS-PAGE and then blotted onto PVDF membranes (Pall Corporation). After blocking for 2 h with phosphate-buffered saline containing 0.1% Tween 20 and 5% powdered skim milk, the blots were incubated with rabbit anti-CD147, anti-MMP-11, polyclonal antibody (Santa Cruz Biotech, 1:200 dilution) overnight in 5% powdered skim milk buffer, washed thrice with phosphate-buffered saline with 0.1% Tween 20, and then incubated with secondary antibody anti-rabbit-HRP (Santa Cruz Biotech, 1:1000 dilution). GAPDH antibody (Santa Cruz Biotech, 1:200 dilution) was used as a control. Band intensities were measured using BioImaging systems (UVP, labworksTM, ver 4.6) and were normalized to those for GAPDH.
MTT assay
About 1 × 106 cells in 200 μl RPMI 1640 was seeded in duplicate into each well of the 96-well culture plates, and 100 μl MTT (5 mg/ml, Sigma) was added at 24, 48, 72, 96, and 120 h, respectively. After 4-h incubation at 37°C in 5% CO2, 100 μl DMSO (Gibco) was pipetted to solubilize the formazan product for 30 min at room temperature. The absorbency A490 was measured using microplate reader (Bio-Rad). Growth rate (%) = Absorbency A490 of transfectants/Absorbency A490 of Hca-F control × 100%.
Soft agar colony formation assay
The abilities of cells to form macroscopically visible colonies in soft agar were determined according to standard protocol [15]. Cells (1 × 104) were suspended in a complete RPMI 1640 medium containing 0.3% of agar and overlaid onto a bottom layer of solidified 0.6% agar in a complete RPMI 1640 medium in 35 mm dishes. Cells were incubated at 37°C in 5% CO2 for 14 days. The dishes were then scanned and photographed, and the number of colonies (greater than 0.5 mm size) was quantified by Quantity one v.4.0.3 software (Bio-Rad, Hercules, CA, USA).
In vivo migration assay
Cells (5 × 106) were incubated with 5 μM vital dye carboxyfluorescein diacetate succinimidyl ester (CFSE, Sigma) at 37°C for 10 min. Labeled cells were washed thrice, counted, and inoculated into the footpad of mice subcutaneously. After 1–48 h, lymph nodes were removed from mice and incubated with 0.1% (w/v) collagenase IV (Sigma) in 90% RPMI 1640 (Gibco) and 10% FBS (Gibco) for 30 min at 37°C. The ratio of green fluorescence positive P388D1 cells in whole lymph node digest mixture was detected by flow cytometry.
In vivo tumor progression assay
For the tumorigenicity assay, P388D1, control RNAi and P388D1/RNAi cells (5 × 106) were suspended in 0.1 ml PBS then injected subcutaneously on either side of the DBA/2 mice’s back. After 2 weeks, tumors were weighted.
For the tumor lymphatic metastasis assay, P388D1, control RNAi and P388D1/RNAi cells (5 × 106) were inoculated into the footpad of DBA/2 mice subcutaneously. After 2 weeks, lymph nodes were removed from mice and weighed.
Tumor and lymph nodes were collected and fixed in phosphate-buffered formalin and embedded in paraffin. Histological examination was performed with hematoxylin and eosin (H&E) staining, and immunohistochemistry analysis was used to detect CD147 protein expression. Goat anti-mouse CD147 polyclonal antibody (1:50 dilution, Santa Cruz Biotech) was used in standard indirect immunoperoxidase procedures.
Drug sensitivity assay
To assess chemosensitivity to anti-tumor drugs, P388D1, control RNAi and P388D1/RNAi cells (1 × 104) were plated in 96-well plates and incubated with different anti-tumor drug paclitaxel (Sigma), docetaxel (Sigma), vincristine (Sigma), and adriamycin (Sigma) for 48 h, respectively. Then, cells were treated with 100 μl MTT (5 mg/ml, Sigma). After 4-h incubation at 37°C in 5% CO2, 100 μl DMSO (Gibco) was pipetted to solubilize the formazan product for 30 min at room temperature. Spectrometric absorbance at 490 nm was measured with a microplate reader. Each group contained three wells and was repeated three times. The IC50 value was determined by the dose of drug that caused 50% cell viability.
Statistical analysis
SPSS13.0 software was used. Each assay was performed at least three times. The data were expressed as mean ± SD, and Student’s t test was used to determine the significance of differences in multiple comparisons. p < 0.05 was considered to be statistically significant.
Results
Inhibition of MMP11 expression in P388D1 cells by down-regulation of CD147
A number of molecules have been shown to be associated with CD147, and some of them are involved in tumor progression. To test directly whether down-regulation of CD147 influenced MMP11 expression, we performed a gene-silencing experiment utilizing double-stranded siRNA to silence the CD147 gene in P388D1 cells. As illustrated in Fig. 1a and b, RT-PCR and Western blot analysis revealed a significant decrease in CD147 expression at both mRNA and protein levels after transfection. Further observation showed that CD147 expression did not recover to original level until 10 days after siRNA transfection (result not shown). At the same time, the MMP11 expressions at both mRNA and protein levels were obviously decreased in the siRNA-transfected cells compared to the control cells (Fig. 1a, b). These results showed that CD147 silencing down-regulated the expression of MMP11.
Inhibition of MMP11 expression in P388D1 cells by down-regulation of CD147. The P388D1 cells were transfected with CD147 siRNA and control siRNA. Cells were collected on the indicated day (RNAi-3, 3 days; RNAi-7, 7 days). Total RNA was extracted and analyzed by RT-PCR using primers specific to CD147 and MMP11 (a). The expressions of CD147 and MMP11 were analyzed by Western blot analysis using anti-CD147 and anti-MMP11 antibodies (b). GAPDH was also examined and served as controls for sample loading
Inhibition of P388D1 cell proliferation and tumorigenicity by down-regulation of CD147
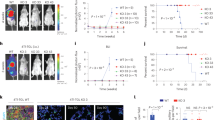
The proliferative ability of tumor cell is important in tumor progression. The in vitro proliferative ability of CD147 deficient cells was determined by MTT assay. The results indicated that CD147 depletion suppressed cell growth in regular medium. About 45% inhibition rate of CD147/RNAi cells at 120 h time point is shown in Fig. 2a. Meanwhile, growth of P388D1 cells transfected with RNAi control was not affected. Subsequent soft agar colony formation assay was then performed to evaluate the capacity of siRNA-treated cells to form macroscopically visible colonies. The colony formation rates were 12%, 29%, and 31% in CD147/RNAi, control RNAi, and parental P388D1, respectively. Compared to the control, cell clones CD147/RNAi exhibited not only less amount but also smaller size of colonies (Fig. 2b, c).
Inhibition of P388D1 cells proliferation and tumorigenicity by down-regulation of CD147. a Cells were harvested at 24, 48, 72, 96, and 120 h post-transfection, and cell proliferation was measured by MTT assay. Decreased growth ability was detected in CD147/RNAi cells compared with RNAi control cells. b Colony formation numbers were compared between transfected and control RNAi cells. Significant down-regulation of colony formation numbers was confirmed in CD147/RNAi cells compared with control RNAi cells (*p < 0.05). c Colony-forming activities of CD147/RNAi cells in soft agar. The cell colonies were photographed (×100). d CD147/RNAi groups showed a significant decrease in mean tumor weight (n = 10) and volume (n = 10; *p < 0.05) compared with control groups. e Histological analysis was performed in implanted tumors generated from P388D1, control RNAi, and CD147/RNAi cells with H&E staining, respectively, (×400). f CD147 protein expression was determined by immunohistochemistry staining in solid tumors derived from P388D1, control RNAi, and CD147/RNAi cells (400×). Differences in CD147 protein expression were shown. The data were obtained from three independent experiments
Next, to directly assess whether silencing of CD147 expression could impede the tumorigenicity of P388D1 cells in nude mice, P388D1, control RNAi, and CD147/RNAi cells were injected subcutaneously on either side of the mice back, respectively. After cell inoculation in mice for 2 weeks, we found a decrease in the weight (2.3-fold) and volume (2.5-fold) of tumors (n = 10) derived from CD147/RNAi cells (Fig. 2d), indicating that the down-regulation of CD147 markedly impacts the ability of murine lymphoid neoplasm cells to form tumors.
Histological examination did not reveal obvious morphological changes between the tumors generated from the three groups (Fig. 2e). Immunohistochemistry analysis showed that CD147 protein expression was almost not detectable in tumor derived from CD147/RNAi cells, but was highly expressed in tumor derived from control RNAi, parental P388D1 cells (Fig. 2f). As described above, silencing of CD147 in P388D1 cells reduced their tumorigenic potential.
Inhibition of P388D1 cell migration and lymphatic metastasis by down-regulation of CD147 in vivo
To explore the role of CD147 mediating tumor cells migration to peripheral lymph nodes in vivo, P388D1, control RNAi, and CD147/RNAi cells were labeled with CFSE, a green fluorescence dye, which can be transported across plasma membrane to react covalently with free amino group of intracellular macromolecules. We found that the positive ratio of CFSE-tagged P388D1 cells in whole lymph node digest mixture was quite different. About only 12.6% CD147 /RNAi positive cells at 48-h time point was shown (Fig. 3a), supporting that loss of CD147 played an important role in migration in vivo, and therefore contributed to tumor lymphatic metastasis.
Inhibition of P388D1 cell migration and lymphatic metastasis by down-regulation of CD147 in vivo. a The number of CFSE++ P388D1 cells migration to lymph nodes was measured by flow cytometry. Surface labeling was expressed as the percentage of positive cells in P388D1, control RNAi, and CD147/RNAi cells relative to the total number of analyzed cells (*p < 0.05). b Three groups of DBA/2 mice were injected subcutaneously with P388D1, control RNAi, and CD147/RNAi cells. After 2 weeks, mice were sacrificed, and their lymph nodes were isolated, weighed, and photographed. CD147/RNAi groups showed a significant decrease in mean tumor-metastasized lymph nodes weight (*p < 0.05) compared with controls. c Histological analysis was performed in tumor-metastasized lymph nodes from P388D1, control RNAi, and CD147/RNAi cells with H&E staining, respectively (×400). d CD147 protein expression was determined by immunohistochemistry staining in tumor-metastasized lymph nodes from P388D1, control RNAi, and CD147/RNAi cells (×400). Differences in CD147 protein expression were shown. The data were obtained from three independent experiments
Next, we also sought to determine whether the down-regulation of CD147 affected the metastatic capability of P388D1 cells to peripheral lymph nodes in vivo. P388D1, control RNAi, and CD147/RNAi cells were injected into the footpad of DBA mice, respectively. By 2 weeks, we detected that a significant reduction of mean lymph nodes weight of MMP-11/siRNA groups was observed, as compared with control groups. The average lymph nodes weight (n = 10) of the CD147/RNAi group, control RNAi, and P388D1 group was 0.21 ± 0.04, 0.63 ± 0.08, and 0.65 ± 0.09g, respectively (p < 0.05, Fig. 3b). Histological analysis revealed that there were not obvious morphological changes between the three groups (Fig. 3c). Immunohistochemistry analysis showed that CD147 protein expression was almost not detectable in tumor derived from CD147/RNAi cells, but was highly expressed in tumor derived from control RNAi, parental P388D1 cells (Fig. 3d). As described above, silencing of CD147 in P388D1 cells inhibited tumor metastatic potential to peripheral lymph nodes.
Silencing of CD147 in P388D1 cells results in increased chemosensitivity to anti-tumor drugs
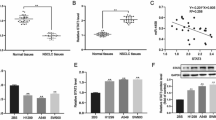
It had been reported that CD147 expression was increased in MDR tumor cells, and CD147 could confer resistance to some anti-tumor drugs. To test directly whether CD147 silencing affected the resistance of P388D1 cells to the chemotherapeutic drugs, we down-regulated CD147 expression in P388D1 cells using RNAi approach. The results showed that the CD147/RNAi cells showed increased sensitivity to these chemotherapeutic drugs, including paclitaxel, docetaxel, vincristine, and adriamycin as compared with control RNAi groups (Fig. 4, p < 0.05). The IC50 values for the drugs were significantly less in the CD147/RNAi group than in the control groups. Thus, silencing of CD147 on tumor cells increased the sensitivity of P388D1 cells to chemotherapeutic drugs.
Silencing of CD147 in P388D1 cells results in increased chemosensitivity to anti-tumor drugs. P388D1, control RNAi, and CD147/RNAi cells were treated with different anti-tumor drugs. Cell viability was determined by MTT chronometry. The IC50 (milligrams per liter) value was determined by the dose of drug that caused 50% cell viability. The data were obtained from three independent experiments
U-0126 effects on MMP-11 expression in P388D1 cells
Previous studies have showed that MMP works are in conjunction with the MAPK pathway. To determine whether MAPK signaling also mediates the CD147-stimulated production of MMP-11 in P388D1 cells, we tested the effects MAPK inhibitors on MMP-11 expression by Western blot analysis. As seen in Fig. 5a, U-0126, an inhibitor of MAPK/Erk, inhibited the expression of MMP-11 in P388D1 cells in a dose-dependent manner (1–10 μM; Fig. 5b).
Effect of U-0126 on the expression of MMP11 in P388D1 cells. Cells were treated with various concentrations of U-0126 for 24 h in serum-free media. At the end of the incubation, the expression of MMP11 was analyzed by Western blot (a). Relative signal intensities of MMP-11 protein (b) level was normalized against those of GAPDH, respectively (*p < 0.05). Results are representative of three similar experiments
Discussion
The CD147 gene has garnered attention because of its high expression in many malignant tumor cells and stimulating several matrix metalloproteinase (MMPs) production, which play very important roles in several aspects of tumor progression, including growth, invasion, metastasis, and angiogenesis [16, 17]. Since the initial cloning of MMP11 from human breast cancer, extensive studies have been carried out a close association between MMP11 expression and tumor metastasis. Overexpression of MMP11 modulates cervical cancer progression and is related to the lymph node involvement in non-small cell lung cancer [18]. The down-regulation MMP11 expression suppresses primary tumor of gastric cancer cell line BGC823 proliferation both in vitro and in vivo [19]. Our previous reports have also shown that CD147 depletion down-regulates MMP-11 expression and the lymphatic metastasis potential of mouse hepatocarcinoma Hca-F cells with highly metastatic potential in the lymph nodes [20]. Here, we used RNAi to down-regulate directly the expression of CD147 in P388D1 cells. Further observation showed that CD147 silencing in P388D1 cells obviously down-regulated MMP11 expression at both mRNA and protein levels, suggesting a regulatory function of CD147 molecule on murine macrophage-like lymphoid neoplasm cells progression via MMP11 activity.
Numerous studies have shown that CD147 promotes metastasis of tumor cells by stimulating stromal cells or tumor cells themselves to produce elevated levels of MMPs. Moreover, supporting its key role in the processes of tumorigenesis and metastasis, CD147 was reported as one of the most constantly up-regulated mRNA in metastatic cells [21]. Zou et al. [6] already reported that inhibition of CD147 expression via RNAi reduces tumor cell invasion and tumorigenicity in a highly metastatic human ovarian cancer cell line HO-8910pm. Here, our consistent results from experiments using MTT, soft agar colony formation, and tumorigenicity assay all indicated that CD147 depletion resulted in the suppression of proliferation of P388D1 cells both in vitro and in vivo. Furthermore, silencing of CD147 led to significant reductions in tumor-metastasized lymph nodes burden, as well as decreases in lymph node metastases in vivo. Taken together, these results delineate a direct causal relationship between CD147 expression and the development of more advanced lymphoma. Our findings also validated the use of CD147 as a marker for lymphoma progression and identified CD147 as a potential target for gene therapy-based interventions.
Drug resistance and tumor metastasis are the main causes of treatment failure and mortality in tumor patients. Overexpression of CD147 in breast cancer cells MCF7 promotes tumor cells metastasis and confers the multidrug resistance to P-gp substrate drugs [22]. CD147 expression is increased in multidrug resistant cancers cells MCF-7/AdrR, KBV-1 (the MDR human oral carcinoma line), and A2780Dx5 (the MDR human ovarian carcinoma line) [23]. In this study, down-regulation of CD147 not only influenced tumor progression both in vitro and in vivo but also increased chemosensitivity to some anti-tumor drugs in P388D1 cells. This result showed that CD147 is an adjuvant chemotherapy target of murine macrophage-like lymphoid neoplasm cells.
Matrix metalloproteinases production, cell motility, and invasion can be influenced by phospho-activation of a variety of key regulators of signal transduction pathways. MAPK pathway plays an important role in regulating many fundamental processes, such as cell growth, mobility, differentiation, and multidrug resistance [24]. MAPK transmits the signal that it receives from Ras to the nucleus, where regulation of gene transcription determines the fate of cells [25]. Activation of MAPK pathways result in alterations in the expression levels of either MMPs, which in turn are responsible for the degradation of ECM and are required for cell mobility [26]. Our current results indicated that inhibition of MAPK/Erk by U-0126 decreased the expression of MMP11 in a dose-dependent manner, suggesting that MAPK/Erk pathway was involved in the regulation of MMP11 production in P388D1 cells, whereas MAPK/p38 does not appear to be essential. This difference may reflect the distinct signaling pathways involved in the regulation of MMP production.
In summary, our results demonstrate that silencing of CD147 inhibits MMP11 production via MAPK/Erk-dependent signaling pathway in murine macrophage-like lymphoid neoplasm P388D1 cells, which is consistent with tumor progression and drug resistance. Our findings provide further insight into the mechanism underlying the functional linkage between tumor progression and drug resistance.
References
Gabison EE, Hymowitz M, Rollo EE, Mann R, Conner CE, Cao J et al (2005) EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie 87:361–368 doi:10.1016/j.biochi.2004.09.023
Zucker S, Hymowitz M, Rollo EE, Mann R, Conner CE, Cao J et al (2001) Tumorigenic potential of extracellular matrix metalloproteinase inducer. Am J Pathol 158:1921–1928
Reimers N, Zafrakas K, Assmann V, Egen C, Riethdorf L, Riethdorf S et al (2004) Expression of extracellular matrix metalloproteases inducer on micrometastatic and primary mammary carcinoma cells. Clin Cancer Res 10:3422–3428 doi:10.1158/1078-0432.CCR-03-0610
Davidson B, Konstantinovsky S, Nielsen S, Dong HP, Berner A, Vyberg M et al (2004) Altered expression of metastasis-associated and regulatory molecules in effusions from breast cancer patients: a novel model for tumor progression. Clin Cancer Res 10:7335–7346 doi:10.1158/1078-0432.CCR-04-0183
Misra S, Ghatak S, Zoltan-Jones A, Toole BP (2003) Regulation of multidrug resistance in cancer cells by hyaluronan. J Biol Chem 278:25285–25288 doi:10.1074/jbc.C300173200
Zou W, Zhu H, Wu X (2006) Inhibition of CD147 gene expression via RNA interference reduces tumor cell invasion, tumorigenicity and increases chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett 248:211–218 doi:10.1016/j.canlet.2006.07.005
Caudroy S, Polette M, Nawrocki-Raby B, Cao J, Toole BP, Zucker S et al (2002) EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin Exp Metastasis 19:697–702 doi:10.1023/A:1021350718226
Li R, Huang L, Guo H, Toole BP (2001) Basigin (murine EMMPRIN) stimulates matrix metalloproteinase production by fibroblasts. J Cell Physiol 186:371–379 doi:10.1002/1097-4652(2000)9999:999<000::AID-JCP1042>3.0.CO;2-8
Sun J, Hemler ME (2001) Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res 61:2276–2281
Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM (2000) Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 18:1135–1149
Illemann M, Bird N, Majeed A, Sehested M, Laerum OD, Lund LR et al (2005) MMP-9 is differentially expressed in primary human colorectal adenocarcinomas and their metastases. Mol Cancer Res 4:293–302 doi:10.1158/1541-7786.MCR-06-0003
Davidson B, Givant-Horwitz V, Laranovici P, Risberg B, Nesland JM, Trope CG et al (2002) Matrix metalloproteinases (MMP), EMMPRIN (extracellular matrix metalloproteinase inducer) and mitogen-activated protein kinases (MAPK): co-expression in metastatic serous ovarian carcinoma. Clin Exp Metastasis 20:621–631 doi:10.1023/A:1027347932543
Lim M, Martinez T, Jablons D, Cameron R, Guo H, Toole B et al (1998) Tumor-derived EMMPRIN (extracellular matrix metalloproteinase inducer) stimulates collagenase transcription through MAPK p38. FEBS Lett 441:88–92 doi:10.1016/S0014-5793(98)01474-4
Khunkeawla P, Moonsom S, Staffler G, Kongtawelert P, Kasinrer KW (2001) Engagement of CD147 molecule-induced cell aggregation through the activation of protein kinases and reorganization of the cytoskeleton. Immunobiology 203:659–669
Jia L, Wang S, Cao J, Zhou H, Wei W, Zhang J (2007) siRNA targeted against matrix metalloproteinase 11 inhibits the metastatic capability of murine Hepatocarcinoma cell Hca-F to lymph nodes. IJBCB 39:2049–2062
Kanekura T, Chen X, Kanzaki T (2002) Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int J Cancer 99:520–528 doi:10.1002/ijc.10390
Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P et al (2005) Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res 65:3193–3199
Delebecq TJ, Porte H, Zerimech F, Copin MC, Gouyer V, Dacquembronne E et al (2000) Overexpression level of stromelysin 3 is related to the lymph node involvement in nonsmall cell lung cancer. Clin Cancer Res 6:1086–1092
Deng H, Guo RF, Li WM, Zhao M, Lu YY (2005) Matrix metalloproteinase 11 depletion inhibits cell proliferation in gastric cancer cells. BBRC 14:274–281
Jia L, Cao J, Wei W, Wang S, Zuo Y, Zhang J (2007) CD147 depletion down-regulates matrix metalloproteinase-11, vascular endothelial growth factor-A expression and the lymphatic metastasis potential of murine Hepatocarcinoma Hca-F. IJBCB 39:2135–2142
Klein CA, Seidl S, Petat-Dutter K, Offner S, Geigl JB, Schmidt-Kittler O et al (2002) Combined transcriptome and genome analysis of single micrometastatic cells. Nat Biotechnol 20:387–392 doi:10.1038/nbt0402-387
Li QQ, Wang WJ, Xu JD, Cao XX, Chen Q, Yang JM et al (2007) Involvement of CD147 in regulation of multidrug resistance to P-gp substrate drugs and in vitro invasion in breast cancer cells. Cancer Sci 98:1064–1069 doi:10.1111/j.1349-7006.2007.00487.x
Yang JM, Xu Z, Wu H, Zhu H, Wu X, Hait WN (2003) Overexpression of extracellular matrix metalloproteinase inducer in multidrug resistant cancer cells. Mol Cancer Res 1:420–427
Yang JM, Vassil AD, Hait WN (2001) Activation of phospholipase C induces the expression of the multidrug resistance (MDR1) gene through the Raf-MAPK pathway. Mol Pharmacol 60:674–680
Huang C, Jacobson K, Schaller MD (2004) MAP kinases and cell mobility. J Cell Sci 117:4619–4628 doi:10.1242/jcs.01481
Brauchle M, Gluck D, Padova FD, Han JH, Gram H (2000) Independent role of p38 and ERK1/2 mitogen-activated kinases in the upregulation of matrix metalloproteinase-1. Exp Cell Res 258:135–144 doi:10.1006/excr.2000.4913
Acknowledgment
This work was supported by grants from National Natural Science Foundation of China (30670466).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, L., Wei, W., Cao, J. et al. Silencing CD147 inhibits tumor progression and increases chemosensitivity in murine lymphoid neoplasm P388D1 cells. Ann Hematol 88, 753–760 (2009). https://doi.org/10.1007/s00277-008-0678-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-008-0678-2