Abstracts
Transmembrane protease/serine 4 (TMPRSS4), a member of the type II transmembrane serine protease family, is highly expressed in some human cancers and involved in the EMT regulation of cancer cells. The prognostic value of TMPRSS4 in colorectal cancer (CRC) has not been discussed. This study aims to evaluate the association between TMPRSS4 expressions and survival in CRC patients. Immunohistochemistry revealed high expression of TMPRSS4 in 69/122 CRC samples, compared with 14/47 in normal tissues (P < 0.01). Correlation analysis showed high expression of TMPRSS4 was significantly associated with advanced TNM stage (P = 0.011), pT (P = 0.019), pN (P = 0.035), and pM status (P = 0.004). Higher TMPRSS4 predicted shorter overall survival (OS) and disease-free survival (DFS) in CRC patients (P < 0.01, both). Moreover, both TMPRSS4 expression and TNM stage were independent predictive factors of OS and DFS in Cox regression analysis. The findings in our study demonstrated the potential value of TMPRSS4 expression level as a prognostic biomarker for CRC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protease plays an important role in the regulation of physical and pathological processes, including tumorigenesis of a variety of cancers [1]. Type II transmembrane serine proteases (TTSPs), a newly discovered subfamily of serine proteases, have gained more attentions as their emerging roles in carcinogenesis and progression [2]. Transmembrane protease/serine 4 (TMPRSS4) is a member of the TTSPs family and was first found overexpressed in pancreatic cancer [3]. Subsequent studies showed that TMPRSS4 was also highly expressed in other tumors, including non-small cell lung cancer [4, 5], malignant thyroid neoplasm [6–8], breast cancer [9, 10], and colon cancer [11], suggesting a possible role for TMPRSS4 in tumor development and progression. Jung et al. [12] demonstrated the pro-invasion and pro-migration role of TMPRSS4 in human tumor cells by promoting epithelial–mesenchymal transition (EMT) while Kim et al. [11] went a step further showing the induction of EMT by TMRPSS4 was via upregulation of integrin α5 and its signaling pathways. Moreover, TMRPSS4 was found to be involved in regulation of EMT in pancreatic, lung [13], and hepatocellular cancer cells [14]. Hence, TMPRSS4 may promote tumor progression by facilitating EMT and thus presents as a candidate marker for outcomes of cancer patients.
The prognostic role of TMPRSS4 has been elucidated in some cancers. In non-small cell cancer, TMPRSS4 overexpression correlated with reduced overall survival (OS) of patients with squamous cell carcinoma histology[4]; in breast cancer, higher expression of TMPRSS4 was associated with positive lymph node metastasis, larger tumor size, and advanced pathological grade, and it was an independent prognostic factor for OS and disease-free survival (DFS) [9, 10]. Although highly expressed in colorectal cancer (CRC), the prognostic value of TMPRSS4 has not been evaluated before. Hereby, in this study, we showed that high expression of TMPRSS4 was associated with advanced TNM stage and independently predicted poor OS and DFS of CRC patients.
Materials and methods
Clinical specimens and patient data
One hundred and twenty-two CRCs of different stages and 47 normal colon mucosa collected between December 2006 and January 2008 were obtained from a prospectively maintained tumor database. During the operations, tissues were cut down as soon as the surgical specimens were excised and then washed clean with saline. Cancer tissues were cut in wedge shapes, and normal mucosa was cut at least 5 cm away from tumor margin. Samples were then fixed with formaldehyde for 2 weeks and embedded with paraffin for immunohistochemistry staining. All specimens were collected with the informed consents of the patients, and all procedures complied with the protocol approved by the Ethical Committee of Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. Demographic and clinicopathological parameters were prospectively recorded or retrieved by chart review. Patients were followed up annually by telephone (Huang and Quan) or at outpatient clinic (Feng) till March 2013 or death.
Immunohistochemistry and scoring
Before staining, paraffin embedded tissue blocks were cut at 4 μm thicknesses and mounted on slides (Jinglun, Shanghai, China). Sections were incubated overnight at 37 °C and then washed with distilled water after two and three changes of xylene and ethanol, respectively. Antigen retrieval was performed with microwave retrieval method; endogenous peroxidase activity was inactivated by incubation with 3 % hydrogen peroxide for 5 min at room temperature. Sections were incubated with TMPRSS4 primary antibody (PA5-18871, Pierce Antibodies, USA) at 4 °C overnight, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary rabbit anti-goat antibody (CW0105, CWBIO, Beijing, China) for 1 h at room temperature. Sections were then washed with TBS and treated with the Metal Enhanced DAB Substrate Kit (Thermo Scientific, USA) to visualize the antigen–antibody complex.
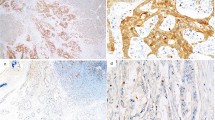
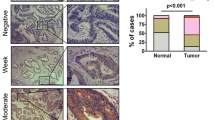
Scoring was performed by two researchers (Huang and Zhou) independently, and discrepancies were resolved by consensus with an additional investigator (Zhao). The number of stained cells on each section was counted and scored as 0 (<10 %), 1 (10–25 %), 2 (26–50 %), and 3 (>50 %) accordingly. Staining intensity was scored as follows: 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The final score of each specimen was determined by multiplying staining intensity score with score of stained cells, with a score range of 0–9. Specimens with final scores of 0–3 were regarded as low expression cases and 4–9 as high expression ones.
Statistical analysis
All analyses were performed with the SPSS 18.0 software (SPSS Inc., Chicago, USA). Correlation between TMPRSS4 expression and clinical parameters was analyzed using Pearson correlation coefficient analysis. OS and DFS were evaluated with the Kaplan–Meier method, and differences were compared by log-rank test. Analyses of predictive factors for OS and DFS were performed with univariate and multivariate Cox proportional hazards regression method. P value <0.05 was considered as statistically significant.
Results
TMPRSS4 is highly expressed in colorectal cancer tissues
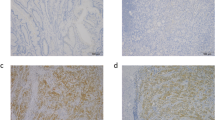
A total of 122 patients were enrolled in this study, and the demographic and clinicopathological parameters were summarized in Table 1. The mean age of all patients was 60.7 (range 38–81 years), and 70 patients were male. Of all tumors, 98 cases were tubular adenocarcinoma, 19 were mucinous ones, and 5 were papillary adenocarcinoma; 79 tumors were located in rectum and sigmoid, 29 in right colon, and 14 in left colon. The numbers of I, II, III, and IV stage cancer were 25, 42, 38, and 17, respectively. The expression of TMPRSS4 in CRC tissue and normal mucosa was evaluated by immunohistochemistry. High expression of TMRPSS4 was detected in 69/122 CRC samples and 14/47 normal tissues (P < 0.01). TMPRSS4 was located predominantly on cell membrane and cancers of different stages showed different staining intensities (Fig. 1).
The correlation of TMPRSS4 with different clinicopathologic parameters
Next, we made an analysis of the correlation between TMPRSS4 expression level of CRC samples and a set of clinicopathologic parameters, including age, gender, histological type, tumor site, and TNM stage. High TMPRSS4 expression was found to be significantly correlated with TNM stage (P = 0.011), pT grade (P = 0.019), pN (P = 0.035), and pM status (P = 0.004). Other characteristics, like age (P = 0.426), gender (P = 0.378), histological type (P = 0.867), and tumor site (P = 0.259) were not associated with TMPRSS4 expression level (Table 2).
High expression of TMPRSS4 correlates with poor overall survival and disease-free survival
The 5-year OS and DFS indicated by Kaplan–Meier survival curve of low and high TMPRSS4 expression were shown in Fig. 2. Patients with low TMPRSS4 had a longer OS and DFS compared with patients showing high expression (P < 0.01, both); the 5-year OS and DFS for low and high TMPRSS4 expression patients were 84.9 % and 67.9, 37.7, and 27.5 %, respectively. Furthermore, univariate analysis and multivariate analysis showed that both high TMPRSS4 expression and advanced clinical stage independently predicted poor prognosis of OS and DFS (Table 3).
Discussion
Colorectal cancer is still a major disease burden, and it is estimated to account for 9 % percent of new cases and deaths among all types of cancers for both sexes in America in 2013 [15]. Despite advances in surgical technique and chemoradiotherapy, local regional relapse and distant metastasis remain to be the major causes for CRC-related deaths [16]. Although widely used in clinical practice for early detection, diagnosis and follow-up, parameters like CEA, CA199, and CA125 are not accurate enough in predicting relapse and metastasis [17], demonstrating less usefulness as prognostic markers. Thus, new markers to predict prognosis of CRC patients are needed.
In recent decades, the role of EMT in epithelial tumor progression has been widely investigated [18]. By undergoing this transition, tumor cells which arise from epithelial tissue gradually lose their epithelial markers, like E-cadherin, cytokeratin, and laminin-1, and acquire some mesenchymal markers, such as N-cadherin, vimentin and β-catenin [19]. Accordingly, epithelial tumor cells get enhanced invasion and migration potential and ultimately would metastasize to distant sites [20]. Moreover, EMT has been linked with cancer stem cell state [21]. Induction of EMT in immortalized human mammary epithelial cells leads to expression of mesenchymal traits and stem cell markers [22]; slug, a transcriptional factor of EMT, could cooperate with sox9 to determine mammary stem cell state [23]. Thus, EMT could further endow tumor cells with self-renewal ability, and this would facilitate micrometastases grow into macrometastases [24]. Considering the accumulating evidences of EMT’s participation in CRC progression [25], it is of importance to investigate regulators of EMT in CRC as potential prognostic markers and therapeutic targets.
TMPRSS4, a member of the TTSPs family, has the extracellular catalytic serine protease domain and takes part in physiological and pathological process via its proteolytic activity. It was found to be involved in normal tissue development [26] and tumor progression [27]. In the zebrafish model, knockdown of TMPRSS4a (homolog of human TMPRSS4) resulted in organ deformity and altered cell–cell contacts, suggesting its vital role in normal physiology, although the underlying mechanism needs further investigation [26]. In human cancers, TMPRSS4 was highly expressed and found to regulate cancer cell invasion and migration: Jung et al. [12] found TMPRSS4 could induce lung and colon cancer cell to undergo EMT and enhance their metastatic ability both in vitro and in vivo; Kim et al. [11] proved this with evidences showing TMPRSS4 was highly expressed in invasive malignancies and could induce invasion and EMT through integrin α5 upregulation. Additionally, inverse association between TMPRSS4 and E-cadherin in colon cancer tumor specimens was also reported, implying the participation of TMPRSS4 in EMT regulation in vivo [11]. Knockdown of TMPRSS4 in several lung cancer lines reduced their invasion potential [4] and TMPRSS4 also regulated EMT of pancreatic adenocarcinoma cells [13]. Hence, TMPRSS4 seems to be associated with tumor development and metastasis, and also a regulator in the EMT. Consequently, we found that TMPRSS4 was highly expressed in CRC tissues compared with normal mucosa, and significantly correlated with high TNM stage, pT, pN, and pM, which in turn support previous finding that TMPRSS4 was involved tumorigenesis and EMT regulation of CRC.
The prognostic value of TMPRSS4 has been validated in other tumors: high expression of TMPRSS4 is an independent predictive factor in lung cancer [4] and breast cancer [9, 10]. Consistently, we found that patients with high expression were associated with a shorter OS and DFS; what’s more, both univariate and multivariate Cox regression model showed TMPRSS4 was an independent prognostic factor. Thus, it is worthy to further detect its value as a follow-up parameter for the benefits of CRC patients.
Conclusively, the findings in this study showed for the first time that high TMPRSS4 was associated with poor prognosis of colorectal cancer patients. Based on our results and other previous publications, TMPRSS4 has an important role in colorectal cancer progression. Further studies are warranted to validate the practical value of this new biomarker and to reveal its underlying mechanisms in regulation of colorectal cancer progression.
References
Choi SY, Bertram S, Glowacka I, Park YW, Pohlmann S. Type II transmembrane serine proteases in cancer and viral infections. Trends Mol Med. 2009;15(7):303–12.
Szabo R, Bugge TH. Type II transmembrane serine proteases in development and disease. Int J Biochem Cell Biol. 2008;40(6–7):1297–316.
Wallrapp C, Hahnel S, Muller-Pillasch F, Burghardt B, Iwamura T, Ruthenburger M, et al. A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res. 2000;60(10):2602–6.
Larzabal L, Nguewa PA, Pio R, Blanco D, Sanchez B, Rodriguez MJ, et al. Overexpression of TMPRSS4 in non-small cell lung cancer is associated with poor prognosis in patients with squamous histology. Br J Cancer. 2011;105(10):1608–14.
Nguyen TH, Weber W, Havari E, Connors T, Bagley RG, McLaren R, et al. Expression of TMPRSS4 in non-small cell lung cancer and its modulation by hypoxia. Int J Oncol. 2012;41(3):829–38.
Jarzab B, Wiench M, Fujarewicz K, Simek K, Jarzab M, Oczko-Wojciechowska M, et al. Gene expression profile of papillary thyroid cancer sources of variability and diagnostic implications. Cancer Res. 2005;65(4):1587–97.
Kebebew E, Peng M, Reiff E, Duh Q-Y, Clark OH, McMillan A. ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve the accuracy of fine needle aspiration biopsy. Trans Meet Am Surg Assoc. 2005;123:57–66.
Kebebew E, Peng M, Reiff E, McMillan A. Diagnostic and extent of disease multigene assay for malignant thyroid neoplasms. Cancer. 2006;106(12):2592–7.
Cheng D, Kong H, Li Y. TMPRSS4 as a poor prognostic factor for triple-negative breast cancer. Int J Mol Sci. 2013;14(7):14659–68.
Liang B, Wu M, Bu Y, Zhao A, Xie F. Prognostic value of TMPRSS4 expression in patients with breast cancer. Med Oncol. 2013;30(2):497.
Kim S, Kang HY, Nam EH, Choi MS, Zhao XF, Hong CS, et al. TMPRSS4 induces invasion and epithelial-mesenchymal transition through upregulation of integrin alpha5 and its signaling pathways. Carcinogenesis. 2010;31(4):597–606.
Jung H, Lee KP, Park SJ, Park JH, Jang YS, Choi SY, et al. TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial-mesenchymal transition. Oncogene. 2008;27(18):2635–47.
Cheng H, Fukushima T, Takahashi N, Tanaka H, Kataoka H. Hepatocyte growth factor activator inhibitor type 1 regulates epithelial to mesenchymal transition through membrane-bound serine proteinases. Cancer Res. 2009;69(5):1828–35.
Jia JB, Wang WQ, Sun HC, Liu L, Zhu XD, Kong LQ, et al. A novel tripeptide, tyroserleutide, inhibits irradiation-induced invasiveness and metastasis of hepatocellular carcinoma in nude mice. Invest New Drugs. 2011;29(5):861–72.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Bolocan A, Ion D, Ciocan DN, Paduraru DN. Prognostic and predictive factors in colorectal cancer. Chirurgia (Bucur). 2012;107(5):555–63.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8.
Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–37.
Tiwari N, Gheldof A, Tatari M, Christofori G. EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol. 2012;22(3):194–207.
Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22(5–6):396–403.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15.
Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148(5):1015–28.
Sampieri K, Fodde R. Cancer stem cells and metastasis. Semin Cancer Biol. 2012;22(3):187–93.
Zhu Q-C, Gao R-Y, Wu W, Qin H-L. Epithelial-mesenchymal transition and its role in the pathogenesis of colorectal cancer. Asian Pac J Cancer Prev. 2013;14(5):2689–98.
Ohler A, Becker-Pauly C. Morpholino knockdown of the ubiquitously expressed transmembrane serine protease TMPRSS4a in zebrafish embryos exhibits severe defects in organogenesis and cell adhesion. Biol Chem. 2011;392(7):653–64.
Choi SY, Shin HC, Kim SY, Park YW. Role of TMPRSS4 during cancer progression. Drug News Perspect. 2008;21(8):417–23.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Houmin Zhou is a co-first author
Rights and permissions
About this article
Cite this article
Huang, A., Zhou, H., Zhao, H. et al. High expression level of TMPRSS4 predicts adverse outcomes of colorectal cancer patients. Med Oncol 30, 712 (2013). https://doi.org/10.1007/s12032-013-0712-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0712-7