Abstract
Transmembrane protease, serine 4 (TMPRSS4), is a novel type II transmembrane serine protease that is highly expressed on the cell surface in pancreatic, thyroid, lung, and other cancer tissues, although its oncogenic significance and molecular mechanisms are unknown. In a series of 109 primary breast cancer patients, we performed a comprehensive analysis of TMPRSS4 expression using immunohistochemistry. The relationship between TMPRSS4 expression and the clinicopathological characteristics or prognosis was evaluated. Results showed that breast cancer tissues exhibited higher levels of TMPRSS4 expression compared with benign tissues (65.1 versus 17.5 %, P < 0.001). High expression of TMPRSS4 was significantly correlated with lymph node metastasis (P < 0.001), high pathological grade (P = 0.001), and tumor size >2 cm (P = 0.006), but not correlated with other clinicopathological parameters, including the patient’s age (P = 0.289), menopausal status (P = 0.300), histological subtype (P = 0.418), and status of estrogen receptor (ER) (P = 0.913), progesterone receptor (PR) (P = 0.247), and HER-2 (P = 0.882). Patients with high expression of TMPRSS4 had shorter OS and DFS than those with low expression (P = 0.0009 and P = 0.0044, respectively). TMPRSS4 expression and lymph node metastasis were independent prognostic factors for both OS and DFS by multivariate analysis. Based on our results, we propose TMPRSS4 as a putative biological marker for breast cancer and as an indicator of poor prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the most common malignancies in women and has become the second leading cause of death for women worldwide [1]. Despite advances in breast cancer prevention, diagnosis, and therapy, the prognosis and survival for most patients have not dramatically changed [2]. To date, adjuvant systemic therapy in women with early-stage disease is guided by prognostic and predictive factors, including stage, grade, estrogen receptor (ER) and progesterone receptor (PR) status, and HER2 amplification [3]. Due to the heterogeneity within specific subgroups of breast cancer and the inter-observer variability with detection frequencies, not all breast cancers can be successfully classified into specific risk groups based on the expression profile of these traditional markers alone. Therefore, new prognostic and predictive factors are still required to optimize treatments among these patients.
In normal tissues, cell surface proteases are involved in regulating cellular activities, such as cell–cell interaction, adherence to matrix components, motility, and homeostasis. Overexpression of these proteases is linked to cancer progression [4], because malignant cells require a range of proteolytic activities to enable growth, survival, motility, invasion, and digestion of the extracellular matrix. The transmembrane protease, serine 4 (TMPRSS4) gene, initially referred to as TMPRSS3, is located on chromosome 11.q23.3 and encodes a member of the type II transmembrane serine proteases (TTSPs) family [4]. It has been reported that TMPRSS4 is upregulated in pancreatic cancer [5] and has been suggested as a diagnostic marker for the malignant thyroid neoplasms [6]. Jung et al. [7] reported that TMPRSS4 controls the invasive and metastatic potential of human cancer cells by facilitating an epithelial–mesenchymal transition (EMT). Kim et al. [8] also demonstrated that TMPRSS4 induced integrin alpha5 expression and its signal transduction in human colorectal cancer tissues, leading to invasiveness and EMT accompanied by downregulation of E-cadherin. Recent qPCR studies showed that high levels of TMPRSS4 message in non-small-cell lung cancer (NSCLC) patients were associated with a poor prognosis [9]. In addition, siRNA knockdown of TMPRSS4 in cancer cell lines and in metastatic potential mouse model reduced cell invasion and migration, thus implying a role for TMPRSS4 in metastasis [10].
In view of the evidence for the expression of TMPRSS4 at the transcriptional level in pancreatic, colorectal, and thyroid cancers, and NSCLC via Northern blot analyses, microarray gene-chips, and RT-PCR [6, 11–13], endogenous protein expression of TMPRSS4 in normal breast and breast cancer cells has not been examined. The objectives of the present investigation were to study the connection between TMPRSS4 expression and breast cancer progression and evaluate their potential relation to the clinical outcome.
Materials and methods
Patients
This study used archival material from the Department of Pathology, No. 202 Hospital in Shenyang, including the tissues from 109 consecutive patients with histologically confirmed breast cancer and 40 benign tissue samples between January 2005 and August 2007, which was harvested from the patients treated by surgical resection. All the patients included in present study did not receive any chemotherapy and radiation therapy before, and their complete clinical data, including age, menopausal status, histological type, lymph nodes status, tumor size, grade, ER status, PR status, and HER2 status, were available and reviewed. Males were excluded and all patients were females.
Outcome data include survival status, overall survival (OS) time, disease-free survival (DFS) time. DFS and OS times were defined as the time interval from the date of surgery to the date of first recurrence or death, which were the two assessments used for prognostic analyses.
Informed consent was obtained from all patients, and all healthy controls for the use of their samples to detect TMPRSS4 expression. The present study conformed to the ethical standards of the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee of No. 202 Hospital.
Immunohistochemical staining
Immunohistochemical analysis of breast tissue was performed as described previously before. Briefly, paraffin sections were cut at 4 μm thickness, mounted on silane coated slides, and incubated overnight at 37 °C. Sections were washed with distilled water after two changes of xylene and three changes of ethanol. Antigen retrieval was performed using citrate buffer (pH 6.0), and sections were held in Tris buffered saline (TBS). Endogenous peroxidase activity was blocked by incubation in 3 % hydrogen peroxide. The sections were incubated overnight in primary antibody (Proteintech Group, Inc., China) diluted with 1/50 in 1 % BSA in Tris buffer (100 mM, pH 7.6) at room temperature. Antibody binding was amplified using horseradish peroxidase-conjugated goat anti-rabbit IgG for 15 min each, and the complex was visualized using DAB Horseradish Peroxidase Color Development Kit ((Maixin Co., Fuzhou, China).
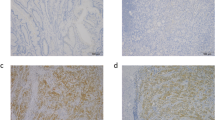
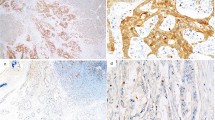
Sections were assessed microscopically for positive DAB staining. Two observers independently evaluated the immunostaining results. Semiquantitative expression levels were based on staining intensity and distribution. The percentage of positive-staining tumor cells was scored as follows: 0 (no positive tumor cells), 1 (<15 % positive tumor cells), 2 (15–50 %positive tumor cells), and 3 (>50 % positive tumor cells). In cytoplasm, staining intensity was graded as follows: 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The staining intensity score plus the percentage of positive staining was used to define the TMPRSS4 expression levels: 0–2, low expression and 3–6, high expression, which classified breast cancer patients into two groups.
Statistical analysis
All data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). The two-sided Pearson’s χ2 test and the Fisher’s exact test were used to compare the clinicopathological parameters between low- and high-expression groups. For analysis of the follow-up data, DFS and OS were evaluated using the Kaplan–Meier method and log-rank tests. Univariate and multivariate Cox proportional hazards regression analyses were performed to evaluate the impact of expression of TMPRSS4 and other categorical factors on DFS and OS, respectively.
Results
Expression of TMPRSS4 in breast cancer tissue
Patients and tumor characteristics were summarized in Table 1. All patients were women with a mean age of 48.5 years (range 28–69 years). Expression of TMPRSS4 was examined in the breast cancer and benign tissues. Immunohistochemical examination showed that TMPRSS4 was located in the cytoplasm and cell membrane in breast cancer tissue (Fig. 1). The high expression of TMPRSS4 was 65.1 % (71/109) in 109 breast cancer specimens and 17.5 (7/40) in 40 benign tissue (P < 0.001). The different intensities of the staining were shown in Fig. 1.
Analysis of correlation of TMPRSS4 with clinicopathologic parameters
We analyzed the associations between the levels of TMPRSS4 expression and a series of clinicopathological characteristics, including age, histological type, tumor size, lymph node stage, grade, menopausal status, and status of ER, PR, and HER-2 in breast cancer patients (Table 2). High expression of TMPRSS4 was significantly correlated with, lymph node metastasis (P < 0.001), high pathological grade (P = 0.001), and tumor size >2 cm (P = 0.006), but not correlated with other clinicopathological parameters, including the patient’s age (P = 0.289), menopausal status (P = 0.300), histological subtype (P = 0.418), and status of ER (P = 0.913), PR (P = 0.247), and HER-2 (P = 0.882).
Association of TMPRSS4 expression with overall survival and disease-free survival in patients with breast cancer
The Kaplan–Meier 5-year survival curves stratified for TMPRSS4 expression were shown in Fig. 2a, b. Patients with high expression of TMPRSS4 had shorter OS and DFS than those with low expression (P = 0.0009 and P = 0.0044, respectively). Moreover, these data revealed that 5-year OS and DFS were 65.2 and 59.7 % in patients with high TMPRSS4 expression level, and 90.0 and 85.0 % in patients with low TMPRSS4 expression level, respectively. Univariate and multivariate analyses were carried out using Cox proportional hazard model to evaluate the impact of TMPRSS4 expression and pathological factors on the prognosis of breast cancer patients (Table 3). Univariate analysis of OS rate showed four statistically significant variables: lymph node metastasis (P = 0.003), tumor size (P = 0.029), grade (P = 0.036), and TMPRSS4 expression (P = 0.006). Univariate also showed that DFS rate was consistent with OS rate in terms of statistically significant variables: lymph node metastasis (P = 0.002), tumor size (P = 0.037), grade (P = 0.031), and TMPRSS4 expression (P = 0.011). In multivariate analyses, both TMPRSS4 expression (P = 0.007 and P = 0.026, respectively) and lymph node metastasis (P = 0.004 and P = 0.016, respectively) were associated with poor OS and DFS.
Kaplan–Meier curves for overall survival (OS) and disease-free survival (DFS) of patients with breast cancer stratified by TMPRSS4 expression. a OS curves of breast cancer patients according to TMPRSS4 immunostaining; b DFS curves of breast cancer patients according to TMPRSS4 immunostaining. P values were obtained by log-rank test
Discussion
Breast cancer is a major public health problem and the most common malignant tumor for women worldwide [14, 15]. Although the incidence of breast cancer is lower in China compared to that in western countries, it has increased by 80 % in young women in the past two decades, with a total increase in 50–100 % [16]. To date, established prognostic indicators for breast cancer include parameters such as tumor size, lymph node status, vascular invasion, ER status, PR status, Her2 gene amplification, and Ki67 status, which are derived from careful histological analysis of primary breast cancer samples [17]. These parameters help physicians to select adjuvant systemic therapy. However, these remain imperfect tools, in that some patients receive systemic chemotherapy even though they can be cured by surgery alone. In contrast, those who were categorized in low-risk group had short disease-free survival without receiving adjuvant chemotherapy. Therefore, it is necessary to identify some new prognostic and predictive factors for a more definitive insight into tumor biology and to substantiate the importance of the existing biomarkers.
The key observations obtained in the present study were (1) TMPRSS4 expression in breast cancer tissue was higher than benign breast tissue. (2) TMPRSS4 expression was associated with tumor size, lymph node metastasis, and grade, which reflects tumor progression. (3) DFS and OS time were significantly shorter for patients with increased TMPRSS4 expression. These findings may imply that the increased expression of TMPRSS4 plays an essential role in tumor progression and metastasis. Furthermore, high expression of TMPRSS4 may also predict the long-term outcome after surgery in breast cancer.
TMPRSS4, as a member of TTSPs family, was identified through its strong upregulation in pancreatic cancer, and its deduced sequence of 437 amino acids contains a serine protease domain with putative trypsin-like activity and a transmembrane domain [5]. TMPRSS4 is highly expressed in pancreatic, thyroid, lung, and colorectal cancers, but the biological functions of TMPRSS4 and its underlying mechanisms are not, however, well understood. Kim semi et al. reported that TMPRSS4 induces invasion, migration, and metastasis of cancer cells by facilitating the epithelial–mesenchymal transition (EMT) events, which EMT is a process implicated in the progression of early-stage noninvasive tumors to invasive malignancies [18, 19]. Jung et al. [7] found that depleting TMPRSS4 in cell lines established from lung and colon cancers using siRNA affected cell proliferation via regulation of cell cycle progression, invasion, and adhesion in vitro, which may be a result of downregulation of ERK1/2 and p38 MAPK activation. Further analysis of TMPRSS4-mediated signaling in cancer cells suggested that multiple downstream signaling pathways are activated including focal adhesion kinase (FAK) and extracellular signal regulated kinase (ERK) resulting in the downregulation of E-cadherin and induced expression of integrin α5, a critical molecule implicated in tumor cell invasion, migration, and tumor progression. Analyzing the relationship between TMPRSS4 expression and clinicopathological parameters of breast cancer, our study also showed that TMPRSS4 was highly expressed in breast cancer tissues compared with benign tissues and was significantly correlated with tumor size, tumor grade, and lymph node metastasis, suggesting that TMPRSS4 maybe participated in the progression of breast cancer and involved in EMT of breast cancer.
The next question we addressed was whether the expression of TMPRSS4 was associated with the clinical outcome in breast cancer. In our study, high TMPRSS4 expression was associated with poor DFS and OS in breast cancer, which is consistent with the evidence provided by Larzabal et al. [9], indicating that TMPRSS4 protein expression is involved in the formation and proliferation of breast cancer. Univariate and multivariate Cox regression analysis revealed that TMPRSS4 could serve as an independent prognostic factor for breast cancer. These results show for the first time (to the best of our knowledge) an association between high TMPRSS4 and poor prognosis.
A limitation of the present study is that it involves only correlative observations between TMPRSS4 expression and clinicopathological parameters in breast cancer, without direct evidence of the function and mechanism of TMPRSS4. Furthermore, validation of the predictive significance of TMPRSS4 requires large-scale studies on homogenous populations. It is unlikely that clinicopathological subgroups of adequate size could be pooled within a single institution.
Conclusion
In conclusion, our study provides evidence that TMPRSS4 has an important role in the progression of breast cancer. Based on our results in patients, we propose TMPRSS4 as a putative biological marker for breast cancer and as an indicator of poor prognosis. Further in-depth studies are still needed to expand samples and elucidate the molecular mechanisms of TMPRSS4 in breast cancer.
References
Jemal A, Bray F, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Wei G, Wang Y, Zhang P, Lu J, Mao JH. Evaluating the prognostic significance of FBXW7 expression level in human breast cancer by a meta-analysis of transcriptional profiles. J cancer sci ther. 2012;4(9):299–305.
Dechaphunkul A, Phukaoloun M, Kanjanapradit K, Graham K, Ghosh S, Santos C, et al. Prognostic significance of tissue inhibitor of metalloproteinase-1 in breast cancer. Int J breast cancer;2012:290854.
Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH, et al. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer metastasis rev. 2003;22(2–3):237–58.
Wallrapp C, Hahnel S, Muller-Pillasch F, Burghardt B, Iwamura T, Ruthenburger M, et al. A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res. 2000;60(10):2602–6.
Kebebew E, Peng M, Reiff E, Duh QY, Clark OH, McMillan A. ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve the accuracy of fine needle aspiration biopsy. Ann Surg. 2005;242(3):353–61. discussion 61–3.
Jung H, Lee KP, Park SJ, Park JH, Jang YS, Choi SY, et al. TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial-mesenchymal transition. Oncogene. 2008;27(18):2635–47.
Kim S, Kang HY, Nam EH, Choi MS, Zhao XF, Hong CS, et al. TMPRSS4 induces invasion and epithelial-mesenchymal transition through upregulation of integrin alpha5 and its signaling pathways. Carcinogenesis. 2010;31(4):597–606.
Larzabal L, Nguewa PA, Pio R, Blanco D, Sanchez B, Rodriguez MJ, et al. Overexpression of TMPRSS4 in non-small cell lung cancer is associated with poor prognosis in patients with squamous histology. Br J Cancer. 2011;105(10):1608–14.
Li T, Zeng ZC, Wang L, Qiu SJ, Zhou JW, Zhi XT, et al. Radiation enhances long-term metastasis potential of residual hepatocellular carcinoma in nude mice through TMPRSS4-induced epithelial-mesenchymal transition. Cancer Gene Ther. 2011;18(9):617–26.
Grutzmann R, Pilarsky C, Ammerpohl O, Luttges J, Bohme A, Sipos B, et al. Gene expression profiling of microdissected pancreatic ductal carcinomas using high-density DNA microarrays. Neoplasia. 2004;6(5):611–22.
Iacobuzio-Donahue CA, Ashfaq R, Maitra A, Adsay NV, Shen-Ong GL, Berg K, et al. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63(24):8614–22.
Ma XJ, Patel R, Wang X, Salunga R, Murage J, Desai R, et al. Molecular classification of human cancers using a 92-gene real-time quantitative polymerase chain reaction assay. Arch Pathol Lab Med. 2006;130(4):465–73.
Bener A, Ayub H, Kakil R, Ibrahim W. Patterns of cancer incidence among the population of Qatar: a worldwide comparative study. Asian Pac J Cancer Prev: APJCP. 2008;9(1):19–24.
Bener A, El Ayoubi HR, Basha B, Joseph S, Chouchane L. Breast cancer screening barriers: knowledge, attitudes and practices of women toward breast cancer. Breast J. 2011;17(1):115–6.
Huang CS, Chang KJ, Shen CY. Breast cancer screening in Taiwan and China. Breast dis. 2001;13:41–8.
Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer. 2010;17(4):R245–62.
Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–42.
Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–29.
Acknowledgments
This work was supported by a grant from the Liaoning Nature Science Fund (201102279).
Conflict of interest
The authors declare that they have no conflict of interest relating to the publication of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, B., Wu, M., Bu, Y. et al. Prognostic value of TMPRSS4 expression in patients with breast cancer. Med Oncol 30, 497 (2013). https://doi.org/10.1007/s12032-013-0497-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0497-8