Abstract
The aim of this work is to study the expression and regulatory effects of CIP2A protein in breast cancer and the correlation between CIP2A protein expression and the prognosis of breast cancer. The CIP2A protein level was detected by immunohistochemistry staining. The relationship between CIP2A protein and clinicopathological parameters of breast cancer was determined. It was observed that 448 (35.00 %) of the 1,280 cases positively expressed CIP2A protein. Univariate analysis indicated that CIP2A expression was related to histological grade, lymph node metastasis, distant metastasis, and triple-negative breast cancer (P = 0.001, 0.001, 0.001, and 0.001, respectively). Spearman correlation analysis showed that CIP2A expression has line correlation with histological grade, lymph node metastasis, distant metastasis, triple-negative breast cancer, and TNM stage (P = 0.03, 0.001, 0.008, 0.001, and 0.001, respectively). After multivariate analysis, tumor size, histological grade, lymph node metastasis, triple-negative breast cancer, distant metastasis, and TNM stage were related to CIP2A expression (P = 0.035, 0.001, 0.028, 0.001, 0.001, and 0.001, respectively). CIP2A expression also significantly related to chemotherapeutic sensitivity of breast cancer in the neoadjuvant chemotherapy. In the Cox regression test, histological grade, lymph node metastasis, triple-negative breast cancer, and TNM stage were detected as the independent prognostic factors (P = 0.001, 0.006, 0.01, 0.011, and 0.001, respectively). CIP2A expression may be a potential biomarker for chemotherapeutic sensitivity and prognosis of breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast carcinoma is the most common malignancy in women and is the second leading cause of cancer deaths in women [1]. Over the last 30 years, deaths from breast cancer have approximately tripled in Japan, which historically has had low incidence of breast cancer [2]. According to the World Health Organization, more than 1.2 million people will be diagnosed with breast cancer each year worldwide [2]. Although many identified molecules play roles in the way breast cancers progress and metastasize, the mechanisms of breast cancer are far from clear [3, 4]. At this point, few molecules exhibit high efficiency in predicting postoperative distant metastasis for breast cancer.
Protein CIP2A also known as cancerous inhibitor of PP2A (CIP2A) is a protein that in humans is encoded by the KIAA1524 gene [5, 6]. PP2A is a cellular tumor suppressor which inhibits cell proliferation and transformation of normal cells to malignant cancer cells. Inhibition of PP2A activity is a prerequisite for human cell transformation. However, the in vivo mechanisms by which PP2A activity is inhibited in spontaneously transformed human cancer cells have been unclear. More specifically, CIP2A was demonstrated to inhibit PP2A activity toward oncogenic transcription factor c-Myc and thereby prevent c-Myc proteolytic degradation [7].
At present, CIP2A is reported to be overexpressed in prostate cancer, lung cancer, oral squamous cell carcinoma, and gastric cancer [8, 9]. Furthermore, the expression of CIP2A correlates with breast cancer aggressivity [10]. However, there is still no clinical study addressing the clinical implications of CIP2A expression in breast cancer patients. Therefore, we examined 1,280 mastectomy specimens obtained from patients with breast cancers to investigate the expression of CIP2A in relation to clinicopathological features, immunohistochemical markers, and amplification of key genes in breast cancer and to determine the prognostic impact of CIP2A expression.
Materials and methods
Patients and tissue specimens
One thousand two hundred and eighty patients who were histologically confirmed breast cancer and underwent radical operations in the Harbin Medical University between January 2002 and January 2008. The mean age of the enrolled patients was 51.34 ± 8.02 (mean ± SD). The inclusion criteria were as follows: (a) curative operations were performed; (b) resected specimens were pathologically examined; (c) more than 10 lymph nodes were pathologically examined after operation. The Ethics Committee of Harbin Medical University approved this study’s protocol.
Thin slices of tumor tissue of all cases received in our histopathology unit were fixed in 4 % formaldehyde solution (pH 7.0) for periods not exceeding 24 h. The tissues were processed routinely for paraffin embedding, and 4-μm-thick sections were cut and placed on glass slides coated with 3-aminopropyl triethoxysilane for immunohistochemistry. Tissue samples were stained with hematoxylin and eosin to determine histological type and grade of tumors.
Immunohistochemical analysis
Briefly, immunohistochemical staining was performed using the standard streptavidin-peroxidase (SP) method with the UltraSensitive TM S–P Kit (Maixin-Bio, China) according to the manufacturer’s instructions, and signals were visualized using the DAB substrate, which stains the target protein yellow. Briefly, one paraffin-embedded block of the tissue was cut at 4 μm and placed on poly-l-lysine-coated slides. The slides were deparaffinized in xylene, rehydrated in a gradient of ethanol solutions, and then immersed in 10 mM sodium citrate buffer (pH 6.0), pretreated in a microwave oven for 10 min, followed by a 10-min rinse with phosphate-buffered saline (PBS). The sections were incubated with 3 % hydrogen peroxide for 10 min to block endogenous peroxidase activity at room temperature. Nonspecific reactions were blocked by incubating the sections in a solution containing normal serum. Then, the slides were incubated in a humid chamber at 4 °C overnight with primary antibody. Following washings with PBS, sections were incubated for 30 min in the secondary biotinylated antibody (Multilink Swine anti-goat/mouse/rabbit immunoglobulin; Dako, Inc.). Following washings, Avidin Biotin Complex (1:1,000 dilution, Vector Laboratories, Ltd.) was applied to the sections for 30–60 min at room temperature. The immunoreactive products were visualized by the catalysis of 3,3′-diaminobenzidine (DAB) by horseradish peroxidase in the presence of H2O2. Sections were then counterstained in Gill’s hematoxylin and dehydrated in ascending grades of methanol before clearing in xylene, then mounting under a coverslip.
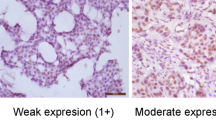
CIP2A expression was classified semiquantitatively according to the following criteria: 0 if <1 % of neoplastic cells discretely expressed CIP2A in their cell cytoplasmic; 1+ if ≥1 and <10 % of morphologically unequivocal neoplastic cells discretely expressed CIP2A in their cell cytoplasmic; and 2+ if ≥10 % of morphologically unequivocal neoplastic cells discretely expressed CIP2A in their cell cytoplasmic. Samples scored as 1+ or 2+ were considered positive.
Statistical analysis
All data were analyzed with SPSS statistics software (version 13.0, Chicago, IL, USA). Relationships between tumor markers and other parameters were studied using chi-square test, Fisher’s extract test, or independent t tests. Disease-specific survival was analyzed using the Kaplan–Meier method. The log-rank test was used to analyze survival differences. Multivariate analysis was performed using the Cox proportional hazards model selected in forward stepwise. A P value of <0.05 was considered statistically significant.
Results
CIP2A staining in breast tissues
Immunochemical staining tests showed that CIP2A protein was located in the cytoplasm as yellow-to-brown staining in the breast tumor tissues (Fig. 1). In normal breast tissue, CIP2A was weakly expressed in a cytoplasm. A stronger staining quality was observed in invasive carcinomas. The expression status data of CIP2A in the 1,280 invasive carcinomas are shown in Table 1.
There was no association observed between CIP2A staining intensity and patient age or tumor size (P = 0.110 and 0.296, respectively). However, we found that patients with high membranous staining of CIP2A demonstrated high histological grade, more lymph node metastasis and triple-negative breast cancer (P = 0.001, 0.001, and 0.001, respectively) (Table 1). Patients with high cytoplasmic staining of CIP2A were prone to have distant metastasis (P = 0.001) (Table 1).
Relationship between CIP2A expression and various clinicopathological factors
After logistic regression analysis, the expression of CIP2A had no significant association with patient age (P = 0.479). CIP2A expression was significantly related to the tumor size, histological grade, lymph node metastasis, triple-negative breast cancer, TNM stage, and distant metastasis (P = 0.035, 0.001, 0.028, 0.001, 0.001, and 0.001, respectively) (Table 2). We also performed Spearman correlation analysis to investigate the correlation between CIP2A expression and clinicopathological features. Finally, histological grade, lymph node metastasis, triple-negative breast cancer, TNM stage, and distant metastasis were observed to have significant correlation with CIP2A expression (P = 0.035, 0.001, 0.028, 0.001, 0.001, and 0.001, respectively) (Table 3).
Prognostic analysis
We further studied the relationship between CIP2A expression and chemotherapeutic sensitivity in 164 neoadjuvant chemotherapy breast cancers. CIP2A expression was expressed in 14.29, 27.63, 46.15, and 57.14 % in complete response, partial response, stable disease, and progressive disease patients (P = 0.003) (Table 4).
Furthermore, CIP2A along with histological grade, lymph node metastasis, triple-negative breast cancer, and TNM stage were shown to be the prognostic factors of breast cancer in Cox model regression analysis (P = 0.001, 0.001, 0.006, 0.010, and 0.011, respectively); (Figs. 2, 3); (Table 5).
Discussion
A recently characterized PP2A inhibitor protein, namely cancerous inhibitor of PP2A (CIP2A), has been found to be overexpressed at a high frequency in most of the human cancer types [9, 10]. However, our understanding of gene expression programs regulated by CIP2A is almost absent. Moreover, clinical relevance of the CIP2A-regulated transcriptome has not been addressed thus far [11]. Huang et al. reported that CIP2A protein was abundantly expressed in bladder cancer cell lines but not in non-tumor epithelial cell lines. Furthermore, CIP2A was specifically expressed in transitional cell carcinoma (TCC) of the bladder tumor tissues but not in adjacent non-tumor bladder tissue. They raised that the role of CIP2A in bladder cancer progression and its usefulness for the surveillance of recurrence or progression of human bladder cancer [12]. In another study, Xue et al. investigated its expression pattern, clinical significance, and biological function in urothelial cell carcinoma (UCC) of the bladder. The study indicated that CIP2A expression was significantly associated with tumor stage, histological grade, and lymph node status. CIP2A is an independent predictor of poor prognosis of bladder UCC patients, and inhibition of its expression might be of therapeutic significance [13]. In hepatocellular carcinoma, inhibition of CIP2A determines the effects of erlotinib on apoptosis. Overexpression of CIP2A was reported to up-regulate phospho-Akt and protected Hep3B cells from erlotinib-induced apoptosis. CIP2A may be useful as a therapeutic biomarker for predicting clinical response to erlotinib in HCC treatment [14].
Recently, a study found that CIP2A was a major molecular determinant of the sensitivity of TNBC cells to bortezomib-induced apoptosis through CIP2A-dependent p-Akt down-regulation. It may be used as a target of bortezomib in human triple-negative breast cancer cells [15]. CIP2A signature was shown to cluster with basal-type and human epidermal growth factor receptor (HER) 2-positive (HER2+) breast cancer signatures. Accordingly, CIP2A protein expression was significantly associated with basal-like (P = 0.0014) and HER2+ breast cancers. CIP2A expression also associated with MYC gene amplification. However, there is still no clinical study addressing the clinical implications of CIP2A expression in breast cancer patients.
In our study, 35.00 % of the enrolled cases positively expressed CIP2A protein. Spearman correlation analysis showed that CIP2A expression has line correlation with histological grade, lymph node metastasis, distant metastasis, triple-negative breast cancer, and TNM stage. After multivariate analysis, tumor size, histological grade, lymph node metastasis, triple-negative breast cancer, distant metastasis, and TNM stage were related to CIP2A expression. We also investigated the relationship between CIP2A expression and chemotherapeutic sensitivity in breast cancer. It was found that CIP2A protein expression was related to the chemotherapy resistance in breast cancer. Finally, histological grade, lymph node metastasis, triple-negative breast cancer, TNM stage, and distant metastasis were detected as the independent prognostic factors. In conclusions, CIP2A expression may be a potential biomarker for chemotherapeutic sensitivity and prognosis of breast cancer.
References
Dowling EC, Klabunde C, Patnick J, Ballard-Barbash R. For the International Cancer Screening Network (ICSN): breast and cervical cancer screening programme implementation in 16 countries. J Med Screen. 2010;17:139–46.
Gaffan J, Dacre J, Jones A. Educating undergraduate medical students about oncology: a literature review. J Clin Oncol. 2006;24(12):1932–9.
Xu D, Xu H, Ren Y, et al. Cancer stem cell-related gene periostin: a novel prognostic marker for breast cancer. PLoS ONE. 2012;7(10):e46670.
Liu Caigang, Chen Bo, Zhu Jun, et al. Clinical implications for nestin protein expression in breast cancer. Cancer Sci. 2010;101(3):815–9.
Kikuno R, Nagase T, Ishikawa K, et al. Prediction of the coding sequences of unidentified human genes. XIV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 1999;6(3):197–205.
Junttila MR, Puustinen P, Niemelä M, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130(1):51–62.
Böckelman C, Koskensalo S, Hagström J, et al. CIP2A overexpression is associated with c-Myc expression in colorectal cancer. Cancer Biol Ther. 2012;13(5):289–95.
Vaarala MH, Väisänen MR, Ristimäki A. CIP2A expression is increased in prostate cancer. J Exp Clin Cancer Res. 2010;29:136.
Xu P, Xu XL, Huang Q, Zhang ZH, Zhang YB. CIP2A with survivin protein expressions in human non-small-cell lung cancer correlates with prognosis. Med Oncol. 2012;29(3):1643–7.
Côme C, Laine A, Chanrion M, et al. CIP2A is associated with human breast cancer aggressivity. Clin Cancer Res. 2009;15(16):5092–100.
Niemelä M, Kauko O, Sihto H, et al. CIP2A signature reveals the MYC dependency of CIP2A-regulated phenotypes and its clinical association with breast cancer subtypes. Oncogene. 2012;31(39):4266–78.
Huang LP, Savoly D, Sidi AA, et al. CIP2A protein expression in high-grade, high-stage bladder cancer. Cancer Med. 2012;1(1):76–81.
Xue Y, Wu G, Wang X, et al. CIP2A is a predictor of survival and a novel therapeutic target in bladder urothelial cell carcinoma. Med Oncol. 2013;30(1):406.
Yu HC, Chen HJ, Chang YL, et al. Inhibition of CIP2A determines erlotinib-induced apoptosis in hepatocellular carcinoma. Biochem Pharmacol. 2013;85(3):356–66.
Tseng LM, Liu CY, Chang KC, et al. CIP2A is a target of bortezomib in human triple negative breast cancer cells. Breast Cancer Res. 2012;14(2):R68.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, G., Liu, G., Dong, J. et al. Clinical implications of CIP2A protein expression in breast cancer. Med Oncol 30, 524 (2013). https://doi.org/10.1007/s12032-013-0524-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0524-9