Abstract
Prediction of oncological outcomes facilitates individualized risk-adapted management for clinical stage I testicular nonseminomatous germ cell tumors (CS I NSGCTs). We investigated risk factors for relapse following orchidectomy, with particular focus on patients with active surveillance. Patients with CS I NSGCTs treated by retroperitoneal lymph node dissection (RPLND), chemotherapy, or surveillance between January 1997 and December 2009 were identified. Demographic and post-operative records were collected. Disease-specific survival and progression-free survival (PFS) rates were estimated using Kaplan–Meier analysis. Cox regression analysis was used to confirm variables that influenced disease relapse. A median follow-up period of 82 months was achieved in 89 patients, of whom 9 (8 in surveillance and 1 in chemotherapy group) had relapses. Cumulative 5-year PFS rates were 74.1, 92.3, and 100 % for the surveillance, chemotherapy, and RPLND groups, respectively (p = 0.01). The relapse rate was significantly higher in patients presented with lymphatic/vascular invasion (LVI) than in those without LVI (26.6 vs. 6.8 %, p = 0.02). In the surveillance group, a higher relapse rate was associated with history of cryptorchidism (50 vs. 13.3 %, p = 0.02) and an age older than 13 years (33.3 vs. 5.9 %, p = 0.04). On multivariate analysis, patient age (OR 1.16; p = 0.05), history of cryptorchidism (OR 0.09; p = 0.01), and LVI (OR 12.10; p = 0.01) were significantly associated with relapse during surveillance. The disease-free period is short in the patients with surveillance. LVI, patient age, and history of cryptorchidism may be used as predictors for relapse during surveillance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 50 % of patients with testicular nonseminomatous germ cell tumors (NSGCTs) have clinical stage I (CS I) disease [1]. Orchiectomy is the initial treatment and ensures an accurate histological diagnosis. However, the optimal treatment following surgery remains controversial. Primary chemotherapy, retroperitoneal lymph node dissection (RPLND), and active surveillance are established treatment options for CS I NSGCTs [2–4]. When compared with surveillance group, RPLND and chemotherapy groups have lower relapse rates, with risk of antegrade ejaculation loss and bowel obstruction following RPLND [5, 6] as well as secondary malignant neoplasms, cardiovascular disease, and late toxicities following chemotherapy [7, 8]. Active surveillance is an acceptable management for all patients with CS I NSGCT but with a relapse rate of about 30 % [9, 10]. Therefore, patients with a high risk of tumor progression should be informed that they may have a great need for RPLND and/or chemotherapy.
As has been reported previously, some pathohistological and molecular markers were investigated to identify occult metastasis so as to facilitate individualized risk-adapted management. However, their positive predictive values for relapse in high-risk groups remain less than 70 % [3, 8, 11, 12]. Therefore, we aimed to investigate predictors for long-term outcomes of surveillance, RPLND, and primary chemotherapy following orchiectomy in patients with CS I NSGCT and to explore the high-risk factors for relapse in surveillance group.
Materials and methods
Patients and methods
This study was approved by the Institutional Review Board at Sun Yat-Sen University Cancer Center. Clinical data of patients with CS I NSGCT treated between January 1997 and December 2009 were reviewed. Clinical and pathological stages were reconfirmed according to the 2009 UICC TNM classification and staging system. We excluded stage IS patients because active surveillance is not indicated for those patients. We also excluded stage I yolk sac tumors at pediatric age because evidences supported that radical orchidectomy is effective for those cases.
Demographics and clinical variables, including pre- and post-orchiectomy serum levels of tumor markers (alpha fetoprotein, AFP; β-human chorionic gonadotropin, HCG; lactate dehydrogenase, LDH), were recorded. Relapse was considered when imaging revealed new lesions, or when serum tumor markers were abnormally increased.
Treatment procedures
Initially, all patients underwent radical orchidectomy. Lymphatic or vascular invasion (LVI) and a predominant component (>50 %) of embryonal carcinoma (EC) on histological examination were considered risk factors for occult metastasis, and therefore, RPLND or chemotherapy was recommended. Pediatric patients (under 13 years old) without identified metastatic diseases were recommended for surveillance. Patients with persistently elevated serum levels of tumor markers after orchidectomy were considered at risk of metastatic disease; therefore, chemotherapy or RPLND (for those not willing to undergo chemotherapy) was recommended [1, 13].
Primary modified RPLND was performed by an open approach or by a laparoscopic approach since 2007, with similar surgical boundaries. For right-sided testicular tumors, the paracaval, precaval, interaortocaval, upper pre-aortic, para-aortic, and right iliac nodes were resected. For left-sided tumors, the precaval, para-aortic, upper interaortocaval, upper pre-aortic, and left iliac areas were resected. Dissection below the origin of the inferior mesenteric artery was not performed unless a palpable mass was present in this area [14]. Nerve-sparing techniques were performed if clinically indicated [15]. The ipsilateral spermatic vein was removed in all cases.
Patients in the chemotherapy group were offered BEP regimen with bleomycin (20 mg/days/m2, on the first, eighth, and fifteenth days), etoposide (100 mg/days/m2, from the first to the fifth days), and cisplatin (30 mg/days/m2, from the first to the fifth days) every 21 days for two cycles. Pre- and post-chemotherapy, bone marrow function, respiratory function, hepatic and renal function were evaluated.
Follow-up
The active surveillance group underwent outpatient review every 3 months in the first year, every 4 months in the second year, every 6 months in the third year, and every year thereafter. Physical examination, detection of serum tumor markers, blood routine tests, and hepatic and renal function tests were performed at each outpatient visit. Chest radiographs, abdominal and pelvic CT scans were performed every 6 months during the first 2 years, and annually thereafter. Patients were treated by RPLND or salvage chemotherapy when relapse was detected during active surveillance.
The RPLND and chemotherapy groups were followed up every 3 months in the first year, every 6 months in the second year, and annually thereafter. Patient ejaculation status was assessed every 3 months after surgery.
Statistical methods
The demographic characteristics of all patients were analyzed using descriptive statistics. Continuous variables were compared using the independent samples t test. The occurrence of relapse was compared between different groups using the χ2 test. Kaplan–Meier analysis was used to estimate overall survival (OS), disease-specific survival (DSS), and progression-free survival (PFS). The Cox regression model was used to quantify independent predictors of disease progression. All statistical tests were two-sided, and statistical significance was set at p ≤ 0.05. Analyses were performed with SPSS v17.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 195 patients with nonseminomatous tumors were treated at Sun Yat-Sen University Cancer Center during the study period, among whom 89 (46.5 %) had CS I NSGCT. Demographic characteristics of patients are summarized in Table 1. Of the 89 patients, 38 were under surveillance, 30 underwent RPLND, and 21 underwent chemotherapy after orchidectomy. All 89 patients were followed up for at least 1 year.
Patients in the chemotherapy group completed at least two cycles of BEP regimen. In the RPLND group, 18 patients underwent open RPLND, and 12 underwent laparoscopic RPLND, three of them had positive retroperitoneal nodes and were offered two to three cycles of adjuvant BEP chemotherapy. All patients in the RPLND group who were potent before surgery achieved antegrade ejaculation during follow-up.
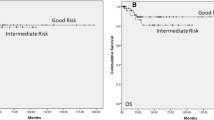
During a median (range) follow-up of 82 (18–195) months, 8 patients in the surveillance group and 1 in the chemotherapy group developed tumor relapse. Metastatic lesions were found in 5 patients during the first year, 3 in the second year, and 1 in the third year of follow-up. All the patients were cured by salvage treatment (Table 2). Cumulative 5-year PFS rates were 74.1, 92.3, and 100 % in the surveillance, chemotherapy, and RPLND groups, respectively (p = 0.013; Fig. 1a). The 5-year DSS and OS rates were 100 %.
Cumulative 5-year progression-free survival (PFS) curves of patients with clinical stage I nonseminomatous testicular germ cell tumor (CS I NSGCT) treated by chemotherapy, retroperitoneal lymph node dissection (RPLND), or surveillance: a 5-year PFS curves of the three groups of patients, b 5-year PFS curves of the three groups excluding patients with predominant EC and/or LVI from the surveillance group
The rate of relapse was significantly higher in patients with LVI than in those without LVI (26.6 vs. 6.8 %, p = 0.02). Predominant EC component presented in 15 patients, among whom 5 developed metastasis, which was significant higher than those without the EC component (33.3 vs. 8.1 %, p = 0.001). The relapse rate was significantly higher in patients with both LVI and predominant EC component than in those with none of them (42.9 vs. 9.4 %, p = 0.04). Considering potential side effects of treatment, 3 patients with predominant EC component, 2 with LVI, and 3 with both LVI and predominant EC component were initiatively enrolled into the surveillance group. When patients with predominant EC and/or LVI were excluded, the relapse rates remained significantly higher in the surveillance group than in the chemotherapy and RPLND groups (17.2 vs. 4.8 % and 0 %, p = 0.04; Fig. 1b).
In the surveillance group, patients with history of cryptorchidism had a higher relapse rate and a lower 5-year PFS rate than those without cryptorchidism (50 vs. 13.3 %, p = 0.02; 33.3 vs. 86.7 %, p = 0.002; Fig. 2a); 17 of 38 patients in the surveillance group were younger than 13 years old, and the median age in the surveillance group was 18.4 years, which was significantly younger than that in the RPLND and chemotherapy groups (p < 0.01). In the surveillance group, the patients older than 13 years had a higher relapse rate (33.3 vs. 5.9 %, p = 0.04) and a lower 5-year PFS rate than those no more than 13 years old (94.1 vs. 65.3 %, p = 0.04; Fig. 2b).
Multivariable regression analysis revealed that treatment options (OR 0.22; p = 0.04), history of cryptorchidism (OR 0.07; p = 0.001), and LVI (OR 5.02; p = 0.02) were independent predictors of relapse. In the surveillance group, multivariate analyses revealed that age (OR 1.16; p = 0.05), history of cryptorchidism (OR 0.09; p = 0.01), and LVI (OR 12.10; p = 0.01) were significantly associated with relapse (Table 3).
Discussion
Testicular cancer accounts for approximately 1–2 % of male malignancies. Previous studies have reported that RPLND, primary chemotherapy, and surveillance are all associated with long-term survival of patients with CS I NSGCT. In spite of improvements in imaging techniques, there is a 25–35 % rate of under-staging in CS I disease [2, 11]. Relapse after surveillance typically necessitates cisplatin-based chemotherapy or RPLND combined with chemotherapy, which may aggravate patient psychological, physical, and economic statuses [16].
Both LVI and predominant EC component have been suggested as risk factors for relapse. Several risk stratification schemes [12, 13] have been used to classify patients with none, either, or both risk factors into low-, intermediate-, or high-risk groups, respectively. The metastasis rate of low-risk patients is less than 20 % [2]. Active treatment is essential for those at high risk of relapse. However, we found that the relapse rate remained high for low-risk patients who only underwent surveillance. Other predictors of outcome should be investigated to make more reasonable decision of surveillance.
Our results suggested that elder age and history of cryptorchidism were risk factors for relapse during surveillance. Ye et al. [17] reported that radical inguinal orchidectomy is only effective for pediatric stage I yolk sac tumors. We found relapse rate still lower in pediatric patients even excluded stage I yolk sac tumors from them. As the incidence of testicular cancer is increasing among post-pubescent men, the reduced protective effect of later puberty may be responsible for the increased incidence in young adults [18]. Fossa et al. [19] found that an elder age at diagnosis, especially an age of over 40 years, is significantly associated with increased mortality. Young patients and their parents may also be concerned more about treatment-related morbidity, such as fertility and late side effects caused by chemotherapy. So it is more suitable to have younger patients enrolled in surveillance.
The occurrence of testicular cancers in patients who underwent orchidopexy for cryptorchidism appeared to be 5–10 times more common than in those with no history of cryptorchidism [20]. Cryptorchidism is a variable and diverse phenotypic trait and is caused by either endocrine or genetic abnormalities. Studies revealed that the exposure of male fetuses to estrogens led to a failure of the testes to descend [21–23]. Estrogens have also been proved to induce human testicular germ cell cancer through a membrane-mediated activation of extracellular regulated kinase and protein kinase [24]. Androgen receptor genes CAG and GGC repeat lengths were associated with slow and incomplete testicular descent [25, 26]. Interestingly, Giwercman et al. [27] found that CAG repeat lengths played a role in the development of testicular germ cell cancer, and it was significantly higher among patients with metastatic nonseminomas.
One patient in our surveillance group experienced relapse 35 months after orchidectomy. Patient’s compliance with scheduled follow-ups is a perquisite for successful surveillance and early detection of relapse. More than 90 % of relapses occur within the first 2 years following orchidectomy, although late relapses are seen in 1–5 % of patients [7, 10]. It has been shown that low socioeconomic class and low educational levels are risk factors for the development and mortality of testicular tumor [28, 29]. The lack of regular follow-up means that patients on surveillance may have recurrence at advanced stages, which are less likely to be cured by salvage therapy.
Our results highlight some predictors of oncological outcome in patients with CS I NSGCT treated at tertiary referral centers. Most relapse cases were in the surveillance group. Patients with risk predictors had a high possibility of relapse, and treatments for these patients should be more aggressive. However, as limited by the nature of a retrospective and single-centered study, the values of these predictors in treatment planning and patient counseling should be confirmed by further prospective studies.
Conclusions
Surveillance, RPLND, and adjuvant chemotherapy are reliable treatment strategies for patients with CS I NSGCT, with comparable OS rates. However, the disease-free period is shorter in the surveillance group. LVI is an independent risk factor for metastasis, and those with LVI are therefore recommended for RPLND or chemotherapy. Elder patients and those with history of cryptorchidism experience more relapse during surveillance.
References
Albers P, Albrecht W, Algaba F, et al. EAU guidelines on testicular cancer: 2011 update. Actas Urol Esp. 2012;36(3):127–45.
Choueiri TK, Stephenson AJ, et al. Management of clinical stage I nonseminomatous germ cell testicular cancer. Urol Clin N Am. 2007;34(2):137–48.
Nguyen CT, Fu AZ, Gilligan TD, et al. Defining the optimal treatment for clinical stage I nonseminomatous germ cell testicular cancer using decision analysis. J Clin Oncol. 2010;28(1):119–25.
Sonneveld DJ, Koops HS, Sleijfer DT, et al. Surgery versus surveillance in stage I non-seminoma testicular cancer. Semin Surg Oncol. 1999;17(4):230–9.
Nelson JB, Chen RN, Bishoff JT, et al. Laparoscopic retroperitoneal lymph node dissection for clinical stage I nonseminomatous germ cell testicular tumors. Urology. 1999;54(6):1064–7.
Rassweiler JJ, Scheitlin W, Heidenreich A, et al. Laparoscopic retroperitoneal lymph node dissection: does it still have a role in the management of clinical stage I nonseminomatous testis cancer? A European perspective. Eur Urol. 2008;54(5):1004–15.
Shahidi M, Norman AR, Dearnaley DP, et al. Late recurrence in 1263 men with testicular germ cell tumors. Multivariate analysis of risk factors and implications for management. Cancer. 2002;95(3):520–30.
Van den Belt-Dusebout AW, de Wit R, Gietema JA, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25(28):4370–8.
Schmoll HJ, Jordan K, Huddart R, et al. Testicular non-seminoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):89–96.
Motzer RJ, Agarwal N, Beard C, et al. NCCN clinical practice guidelines in oncology: testicular cancer. J Natl Compr Canc Netw. 2009;7(6):672–93.
Gels ME, Hoekstra HJ, Sleijfer DT, et al. Detection of recurrence in patients with clinical stage I nonseminomatous testicular germ cell tumors and consequences for further follow-up: a single-center 10-year experience. J Clin Oncol. 1995;13(5):1188–94.
Albers P, Siener R, Kliesch S, et al. Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the german testicular cancer study group trial. J Clin Oncol. 2003;21(8):1505–12.
Vergouwe Y, Steyerberg EW, Eijkemans MJ, et al. Predictors of occult metastasis in clinical stage I nonseminoma: a systematic review. J Clin Oncol. 2003;21(22):4092–9.
Liu ZW, Zhou FJ, Han H, et al. Efficacy of modified retroperitoneal lymph node dissection for testicular nonseminomatous germ cell tumors. Chin J Cancer. 2008;27(12):1302–6 (in Chinese).
Steiner H, Zangerl F, Stohr B, et al. Results of bilateral nerve sparing laparoscopic retroperitoneal lymph node dissection for testicular cancer. J Urol. 2008;180:1348–52.
Hendry WF, Norman A, Nicholls J, et al. Abdominal relapse in stage 1 nonseminomatous germ cell tumours of the testis managed by surveillance or with adjuvant chemotherapy. BJU Int. 2000;86(1):89–93.
Ye YL, Sun XZ, Zheng FF, et al. Clinical analysis of management of pediatric testicular germ cell tumors. Urology. 2012;79(4):892–7.
Weir HK, Kreiger N, Marrett LD. Age at puberty and risk of testicular germ cell cancer (Ontario, Canada). Cancer Causes Control. 1998;9(3):253–8.
Fossa SD, Cvancarova M, Chen L, et al. Adverse prognostic factors for testicular cancer-specific survival: a population-based study of 27,948 patients. J Clin Oncol. 2011;29(8):963–70.
Herrinton LJ, Zhao W, Husson G. Management of cryptorchism and risk of testicular cancer. Am J Epidemiol. 2003;157(7):602–5.
McLachlan JA, Newbold RR, Burow ME, et al. From malformations to molecular mechanisms in the male: three decades of research on endocrine disruptors. APMIS. 2001;109(4):263–72.
Emmen JMA, McLuskey A, Adham IM, et al. Involvement of insulin-like factor 3 (Insl3) in diethylstilbestrol-induced cryptorchidism. Endocrinology. 2000;141(2):846–9.
Nef S, Shipman T, Parada LF. A molecular basis for estrogen induced cryptorchidism. Dev Biol. 2000;224(2):354–61.
Bouskine A, Nebout M, Mograbi B, et al. Estrogens promote human testicular germ cell cancer through a membrane-mediated activation of extracellular regulated kinase and protein kinase A. Endocrinology. 2008;149(2):565–73.
Ferlin A, Garolla A, Bettella A, et al. (2005) Androgen receptor gene CAG and GGC repeat lengths in cryptorchidism. Eur J Endocrinol. 2005;152(3):419–25.
Garolla A, Ferlin A, Vinanzi C, et al. Molecular analysis of the androgen receptor gene in testicular cancer. Endocr Relat Cancer. 2005;12(3):645–55.
Giwercman A, Lundin KB, Eberhard J, et al. Linkage between androgen receptor gene CAG trinucleotide repeat length and testicular germ cell cancer histological type and clinical stage. Eur J Cancer. 2004;40(14):2152–8.
Mackillop WJ, Zhang-Salomons J, Groome PA, et al. Socioeconomic status and cancer survival in Ontario. J Clin Oncol. 1997;15(4):1680–9.
Hoffman KE, Chen MH, Punglia RS, et al. Influence of year of diagnosis, patient age, and sociodemographic status on recommending adjuvant radiation treatment for stage I testicular seminoma. J Clin Oncol. 2008;26(24):3937–42.
Conflict of interest
All authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, P., Liu, ZW., Li, XD. et al. Risk factors for relapse in patients with clinical stage I testicular nonseminomatous germ cell tumors. Med Oncol 30, 494 (2013). https://doi.org/10.1007/s12032-013-0494-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-013-0494-y