Abstract
The discoidin domain receptors, DDR1 and DDR2, have been linked with numerous human cancers. We sought to determine expression level and distribution of DDRs in human breast cancer, and investigate prognostic determinates to determine whether levels of DDRs could predict survival. Tumor samples from 122 breast cancer patients were analyzed for relative expression of DDRs. An additional 24 matched tumor and normal tissues were tested for differential expression of DDR1 and DDR2. DDR2 was found to be significantly increased by 6-fold (P = 0.0005) and DDR1 decreased (P = 0.0001) in tumor vs. normal breast tissue. DDR1 expression was not predictive for patient survival; however, DDR2 expression was significantly associated with disease-free (HR = 0.55, 95 % CI = 0.24–0.78, P = 0.026) and overall survival (HR = 0.46, 95 % CI = 0.35–0.84, P = 0.019). Multivariate analysis revealed DDR2 is an independent favorable predictor for prognosis independent of tumor stage, histology, and patient age. The present research provided the first evidence that increased DDR2 mRNA expression in primary human breast cancer might be a powerful, independent predictor of recurrence and outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The discoidin domain receptors (DDRs) family which is composed of DDR1 and DDR2 are receptor tyrosine kinases (RTKs) belonging to the same enzyme family as EGFR. Compared to other RTKs, DDRs are quite unique because they bind different types of collagen as their ligands rather than growth factor-like peptides [1, 2]. Evidence from in vitro and in vivo studies suggests that DDRs can regulate cell proliferation and matrix metalloproteinase (MMP)-mediated ECM remodeling [3–6]. The DDRs are associated with a growing number of human diseases, including fibrotic diseases of the lung, kidney, and liver; atherosclerosis; as well as osteoarthritis [3, 5–8]. DDRs have also been shown to exhibit altered expression patterns in several kinds of human cancers, including esophageal, ovarian, brain, and lung tumors [9]. To our knowledge, no correlations of DDRs with relapse and prognosis has not been addressed in breast cancer yet.
Here, we sought to extensively explore the association of DDRs with human breast cancer by determining their expression levels in a larger cohort of primary breast tumors. We further demonstrated a significant increase in DDR2 expression and a decreased expression level of DDR1 in breast tumor tissue compared with matched normal tissues from the same patients; importantly our results proved that DDR2 expression was a good prognostic marker for early-stage breast cancer patients.
Materials and methods
Study cohort and tissue samples
A total of 122 primary breast tumor samples and an independent set of 24 matched tumor and adjacent normal breast tissues were harvested from breast cancer patients treated by surgical resection without adjuvant chemotherapy at Xijing Hospital, Fourth Military Medical University. This study was approved by the ethics committee of the Fourth Military Medical University.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA from all the samples were purified as recommended by the manufacturer using Trizol reagent (Invitrogen). cDNA synthesis was done using approximately 5 mg RNA per 20 mL with a cDNA reverse transcription kit (Fermentas). Real-time PCR was done on an ABI 7500 system (Applied Biosystems), using SYBR Green I (TAKARA). qPCR analysis was performed as described elsewhere [10].
Immunohistochemistry
Immunohistochemistry was performed using the avidin–biotin–peroxidase method [10]. Slides were incubated with antibody of DDR1 (1:50, Santa Cruz) or DDR2 (1:50 R&D). Biotinylated goat anti-rabbit and goat anti-mouse IgG (1:1,000, Sigma) was incubated with the sections for 1 h at room temperature and detected with a streptavidin–peroxidase complex. Negative and positive controls were conducted in each run of immunohistochemistry.
Statistical analysis
Matched t tests were used to determine differential expression of DDRs. The Spearman’s correlation, Kruskal–Wallis, and Wilcoxon test were used to assess association within and between molecular indices and the pathological or clinical factors. The Cox proportional hazards model was used to test the association of survival and DDR2 expression, where DDR2 expression was treated as a continuous variable. The Cox proportional hazards model was employed for the multivariate analysis. Differences with a P value of 0.05 or less were considered to be statistically significant.
Results
Differential expression patterns of DDR1 and DDR2 in human breast cancer tissues
We firstly examined the expression of DDRs by immunostaining of tumor tissue in situ. The observation showed that diffuse-positive staining for DDR2 was definitely seen in breast cancer specimens and detected primarily in the membrane and cytoplasm of tumor epithelial cells. Conversely, strongly positive staining of DDR1 was barely noticeable in tumor cells (Fig. 1).
Given the suggested role of DDRs in breast cancer progression, we next determined their mRNA levels in 122 breast tumor patient samples. The characteristics of the 122 breast cancer patients involved in the study cohort are shown in Table 1. Relative DDR1 and DDR2 mRNA levels were given as differences in RQ values as compared to mRNA levels for the housekeeping genes β-actin (Fig. 2). Expression levels of DDR2 were significantly higher in breast carcinoma (ductal carcinoma and lobular carcinoma) than in benign tumor (cystic hyperplasia and fibroadenoma) (Mann–Whitney U, P < 0.001, P < 0.01, P < 0.05, and P < 0.01, respectively). The difference between mucinous carcinoma and cystic hyperplasia or fibroadenoma were neither statistically significant (P = 0.6723 and P = 4,285), nor were there any differences between the two subtypes of benign tumor (P = 0.8124). Interestingly, cystic hyperplasia and fibroadenoma displayed significantly higher DDR1 expression than the other histological subtypes (Mann–Whitney U, P < 0.01, P < 0.01, P < 0.01 and P < 0.001, respectively). Furthermore, we analyzed a cohort of 24 samples from breast cancer patients, which included matched normal tissue for each tumor sample. DDR2 was shown to be increased by 2.6-fold (P < 0.001), and DDR1 decreased by an equivalent amount (2.1-fold, P < 0.001) in tumor tissue compared with matched normal samples (Fig. 3).
Expression pattern of DDRs mRNA levels in a cohort of breast cancer tissues. DDRs mRNA levels were measured by real-time quantitative RT-PCR (RQ-PCR) in 73 ductal carcinoma (IDC), 21 lobular carcinoma (ILC), 5 ductal carcinoma in suit (DCIS), 5 mucinous carcinoma (MC), 4 cystic hyperplasia (CH), 4 fibroadenoma (FA), and 4 invasive micropapillary carcinoma (IMPC)
Accumulated plot of DDRs qPCR data demonstrating the relative expression of DDR1 and DDR2 in normal and cancerous tissues from breast cancer patients. DDR2 was significantly upregulated by sixfold (P < 0.001), and DDR1 was significantly downregulated by 3.74-fold (P < 0.001) in tumor tissues when compared to normal tissues from breast cancer patients
The average RQ of DDR2 mRNA in breast carcinoma samples was 5.7, whereas the relative DDR2 mRNA expression detected in benign tumor was 0.95. Also, relative DDR2 mRNA expression in 24 cases of adjacent normal tissues was 1.103. The difference among the 3 groups of specimens was statistically significant (P < 0.001). In parallel, we found that the average RQ of DDR1 mRNA in breast carcinoma samples was 1.29, whereas the relative DDR1 mRNA level detected in benign tumor was 4.83. Besides, relative expression of DDR1 mRNA in 24 cases of adjacent normal tissues was 11.26, and the difference among the 3 groups of specimens was also statistically significant (P < 0.001). These results indicated that DDR1 expression was downregulated in breast carcinoma while there is a striking increase in DDR2 level, whereas they showed a diametrically opposed expression pattern in benign tumor of breast.
Association between expression of DDRs and clinicopathologic characteristics of breast cancer
On the basis of the qPCR results, no statistically significant correlations were observed between DDR1 mRNA expression and age at diagnosis, depth of mammary gland invasion, lymph node metastasis, metastases to distant tissues, or the TNM staging. However, statistically significant correlations between the high level of DDR2 mRNA expression were found with high degree of lymph node metastasis (P < 0.001), distant metastasis (P = 0. 005), and advanced TNM staging (P < 0. 001). Correlation coefficient was shown in Tables 2 and 3.
Moreover, our findings also suggest that the negative impact of altered expression of DDRs l on prognosis is independent of other prognostic markers (Table 2, 3). Thus, increased expression of DDR2 appears to define a previously unrecognized particularly aggressive subset of breast cancers.
Association between expression of DDRs and disease-free survival of breast cancer patients
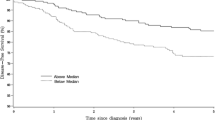
The Kaplan–Meier analysis was used to evaluate the disease-free survival of patients with breast cancer and DDR2 mRNA expression. Results showed that patients with negative DDR2 expression in breast tumor tissues had better disease-free survival than those with elevated DDR2 expression (Fig. 4a, log-rank test: P = 0. 003). Breast cancer patients with elevated DDR2 expression had a higher risk to relapse than in those with reduced DDR2 expression. In contrast to DDR1 whose expression level was not prognostic (Table 4), low expression of DDR2 was significantly associated with greater disease-free survival in this cohort of patients (HR = 0.55, 95 % CI = 0.24–0.78, P = 0.026) (Fig. 4).
Association between expression of DDR2 and overall survival of breast cancer patients
A statistically significant association between poor overall survival and elevated DDR2 mRNA level was found in patients with breast cancer. The Kaplan–Meier analysis for postoperative overall survival showed that breast cancer patients with reduced DDR2 expression had longer overall survival than patients with increased expression of DDR2 (Fig. 5a, log-rank test: P = 0.002). In contrast to DDR1 whose expression level was not prognostic (Table 4), low expression of DDR2 was associated with significantly favorable overall survival in this cohort of patients (HR = 0.46, 95 % CI = 0.35–0.84, P = 0.019) (Fig. 5).
Discussion
In the present study, we firstly observed a novel expression pattern of DDR1 and DDR2 in breast cancer tissues. We further discovered that DDR2 expression is significantly increased compared with its reduction in nonneoplastic breast tissues. Additionally, the expression levels of DDR2 were significantly correlated with advanced TNM stage and lymph node metastasis, suggesting that DDR2 expression might be of clinical relevance in the aggressiveness of breast carcinoma. Moreover, the impact of DDR2 expression on clinical outcome was assessed by the univariate and multivariate analyses; the results of which clearly demonstrated that DDR2 expression was a statistically significant risk factor affecting disease-free survival and overall survival of patients with breast tumor and were independent favorable prognostic marker for early-stage breast cancer patients.
Although DDR1 was first discovered in breast cancer cells and has been previously discussed in mammogenesis [11, 12], a direct link between breast tumorigenesis and DDRs especially the DDR2 has not yet been established. This work therefore represents the first study to extensively explore and quantify the expression levels of these unique RTKs in a large clinical cohort of breast cancer patients. Indeed, in our study, the DDRs expression pattern was more profound. The results detailing differences in DDRs expression level are of interest in that the preferential expression in tumor epithelial cells is DDR2 rather than DDR1, which is inconsistent with the preceding reports. It has long been considered that DDR1 and DDR2 were exclusively expressed in tumor cells and tumor stoma, respectively [13]. However, our current study clearly demonstrated that in breast cancer, DDR2 was predominantly localized in the tumor cells rather than in the stromal cells. Several explanations exist for the contradictory phenomenon. Breast cancer encompasses a broad range of clinical subtypes, and the scope and makeup of the two cohorts of eligible patients differed. In addition, we were not sure if the differences were owing to the methodology. In contrast to northern blot which does not allow precise quantification, our qPCR and analysis methods were robust and results repeatable. More interestingly, Maeyama and colleagues have reported that a striking increase in the DDR2 expression and a reciprocal decrease in the DDR1 expression were found in the mesenchymally transformed cells [14]. We therefore hypothesize that in the different periods of tumor progression, DDRs expression may appear to be flexible and distinct, which remains a future direction of study.
Although DDR1 appeared to be protective in our cohort, there were ultimately too few cases with quality expression data to make a firm conclusion. Intriguingly, DDR1 was used to be considered to associate with cancer malignant progression [15], however in fact, this kind of RTK was also reported to be a direct p53 transcriptional target, and essential for the pro-survival functions of p53 [16]. A possible explanation for this disparity is that the p53-dependent manner of DDR1 induction may be in the cells exposed to cellular stresses, which upregulate p53.
In conclusion, our data suggest that DDR2 might play an important role in the regulation of aggressiveness in human breast cancer. This is the first study to show a strong association of the collagen binding RTK, DDR2 with human breast cancer. We have conclusively shown that DDR2 is significantly more expressed in tumor as opposed to normal tissues from breast cancer patients. The upregulation of DDR2 might be closely associated with poor clinical outcome of patients with breast carcinomas, which demonstrated that it can be a strong prognostic indicator.
References
Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1(1):13–23.
Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, et al. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1(1):25–34.
Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107(6):727–35. doi:10.1172/JCI10720.
Hou G, Vogel WF, Bendeck MP. Tyrosine kinase activity of discoidin domain receptor 1 is necessary for smooth muscle cell migration and matrix metalloproteinase expression. Circ Res. 2002;90(11):1147–9.
Olaso E, Labrador JP, Wang L, Ikeda K, Eng FJ, Klein R, et al. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J Biol Chem. 2002;277(5):3606–13. doi:10.1074/jbc.M107571200M107571200.
Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, et al. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest. 2001;108(9):1369–78. doi:10.1172/JCI12373.
Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Manes S, et al. The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep. 2001;2(5):446–52. doi:10.1093/embo-reports/kve0942/5/446.
Ferri N, Carragher NO, Raines EW. Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol. 2004;164(5):1575–85.
Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: an update on discoidin domain receptor function. Cell Signal. 2006;18(8):1108–16.
Su J, Yu J, Ren T, Zhang W, Zhang Y, Liu X, et al. Discoidin domain receptor 2 is associated with the increased expression of matrix metalloproteinase-13 in synovial fibroblasts of rheumatoid arthritis. Mol Cell Biochem. 2009;330(1–2):141–52. doi:10.1007/s11010-009-0127-0.
Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21(8):2906–17. doi:10.1128/MCB.21.8.2906-2917.2001.
Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proc Natl Acad Sci U S A. 1993;90(12):5677–81.
Barker KT, Martindale JE, Mitchell PJ, Kamalati T, Page MJ, Phippard DJ, et al. Expression patterns of the novel receptor-like tyrosine kinase, DDR, in human breast tumours. Oncogene. 1995;10(3):569–75.
Maeyama M, Koga H, Selvendiran K, Yanagimoto C, Hanada S, Taniguchi E, et al. Switching in discoid domain receptor expressions in SLUG-induced epithelial-mesenchymal transition. Cancer. 2008;113(10):2823–31. doi:10.1002/cncr.23900.
Ford CE, Lau SK, Zhu CQ, Andersson T, Tsao MS, Vogel WF. Expression and mutation analysis of the discoidin domain receptors 1 and 2 in non-small cell lung carcinoma. Br J Cancer. 2007;96(5):808–14. doi:10.1038/sj.bjc.6603614.
Ongusaha PP, Kim JI, Fang L, Wong TW, Yancopoulos GD, Aaronson SA, et al. p53 induction and activation of DDR1 kinase counteract p53-mediated apoptosis and influence p53 regulation through a positive feedback loop. EMBO J. 2003;22(6):1289–301. doi:10.1093/emboj/cdg129.
Acknowledgments
This work was supported by the 973 Program 2010CB529705 and 2009CB521704 (Libo Yao), and the grants from the National Natural Science Foundation of China (NSFC) 81101470 (Tingting Ren).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ren, T., Zhang, J., Zhang, J. et al. Increased expression of discoidin domain receptor 2 (DDR2): a novel independent prognostic marker of worse outcome in breast cancer patients. Med Oncol 30, 397 (2013). https://doi.org/10.1007/s12032-012-0397-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-012-0397-3