Abstract
Sunitinib is a multikinase inhibitor used as first- and second-line treatment of metastatic renal cell carcinoma. However, there are few reports on the necessary doses of sunitinib to get better clinical outcome in general practice with Japanese patients. We examined the relationship between the efficacy and the necessary doses of sunitinib therapy in a multi-institutional retrospective study. A study population of 94 metastatic renal cell carcinoma patients was eligible for this investigation. The most frequent grade 3/4 laboratory adverse events were decreased platelet (31.9 %) and white blood cell (21.3 %) counts. Treatment was discontinued in 18 patients (31.0 %) initially receiving a 50-mg/day dose within only one course, and median 1-month relative dose intensity was 74.3 %. Median progression-free survival time was 2.3 months in patients treated for only one course and 10.8 months in patients treated for more than one course (P < 0.001). Multivariate analysis showed that only one course of treatment and 60 % and less of 1-month relative dose intensity were significantly associated with inferior progression-free survival (P < 0.001 and P = 0.027, respectively). Moreover, modified Memorial Sloan-Kettering Cancer Center poor risk was significantly associated with progression-free survival time. It is difficult for Japanese patients to continue an initial dose of sunitinib therapy without drug withdrawal. Continuing therapy for more than one course and maintaining more than 60 % of 1-month relative dose intensity were very important in the prolongation of progression-free survival time regardless of the initial treatment doses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) is the most common tumor arising in the kidney [1]. The treatment for metastatic RCC (mRCC) has dramatically changed over the last 5 years. This has been driven by two groups of targeted agents: vascular endothelial growth factor (VEGF)-targeted therapies and mammalian target of rapamycin (mTOR) inhibitors [2–5].
Sunitinib is an oral, multitargeted receptor tyrosine kinase (RTK) inhibitor of VEGF receptors, platelet-derived growth factor receptors, and other RTKs with direct antitumor and antiangiogenic activity [6–10]. Single-agent sunitinib showed unprecedented antitumor activity in two phase II trials of patients with mRCC, demonstrating an objective response rate of about 30 % [11, 12]. Furthermore, sunitinib showed superior first-line efficacy over interferon-alpha (IFN-α), with significantly longer progression-free survival (PFS) [5, 13]. Moreover, the proportion of grade 3 or 4 adverse event (AE) profiles ranged from 1 to 13 % in the pivotal phase III trial, and sunitinib was tolerated [5, 13]. In a phase II study in Japan, sunitinib was consistently effective and tolerable, although there was a trend toward greater antitumor efficacy and higher incidence of hematological adverse events in comparison with the Western phase III trial [14, 15]. Thus, sunitinib has been approved worldwide, including Japan, for first- and second-line treatment for advanced RCC.
A commonly asked question is whether patients with RCC in clinical trials are representative of the general population with this disease. Many patients with RCC do not meet the trial inclusion criteria, particularly those with poorer prognosis. Gore et al. [16] reported the results of sunitinib use derived from a global, expanded-access study of patients with metastatic RCC to evaluate the efficacy and safety profile in a “real-world” setting, and the efficacy and the AE profile of their study were similar to those of the phase III study. Although the expanded-access study included Asian patients, the efficacy and AE profile of sunitinib as used in general practice in Japanese patients only were not addressed.
In the present study, we analyzed the clinical results of the general practice of sunitinib therapy in Japanese patients with mRCC in a multi-institutional study. We also examined patient clinical features to determine which might predict a superior prognosis of sunitinib therapy.
Materials and methods
Patients
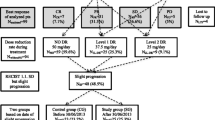
We retrospectively analyzed a database comprising 106 patients treated from April 2008 to July 2011 with sunitinib as both first- and second-line therapy for mRCC at Osaka University Graduate School of Medicine and its affiliated hospitals listed in the acknowledgements. Patients treated with neoadjuvant (n = 3) and presurgical therapies (n = 5) were excluded. Four patients were excluded because they were not evaluated PFS time. Thus, 94 patients were eligible for this study. The patients were evaluated at the time of sunitinib administration according to Modified Memorial Sloan-Kettering Cancer Center (MSKCC) risk groups [14, 16]. For patients undergoing second-line therapy, risk factors were Eastern Cooperative Oncology Group performance status (ECOG PS) ≥2 and low hemoglobin and high calcium levels. For patients undergoing first-line therapy, additional risk factors were increased lactate dehydrogenase level and time from diagnosis to use of sunitinib of <1 year [16–18]. The patients’ initially diagnosed tumors were staged according to the AJCC (2002) cancer staging classification [19]. In 58 patients, sunitinib was administered at a starting dose of 50 mg orally, once daily, in repeated 6-week cycles according to a 4/2 schedule (4 weeks on treatment followed by 2 weeks off). Dose reductions to 37.5 mg/day and then to 25 mg/day were permitted on the basis of individual tolerability. Sunitinib was discontinued due to disease progression, unacceptable toxicity, or decision of the physicians. We previously reported that 1-month relative dose intensity (1M-RDI) ≥50 % predicted favorable PFS in sorafenib therapy, and we examined 1M-RDI of sunitinib therapy as previously reported [20].
The study was approved by an institutional review board of Osaka University, which provided the necessary institutional data-sharing agreements before initiation of the study.
Follow-up regimen
Patient follow-up generally consisted of a history, physical examination, routine blood work, abdominopelvic computed tomography (CT), and chest radiography. Elective bone scan and chest CT were performed when clinically indicated by several urologists. Tumor response was evaluated by the treating urologist every 1–3 months according to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines [21]. Objective response (OR) was defined as the number of complete response (CR) and partial response (PR), and cases of clinical benefit were defined as the number of patients with OR and stable disease (SD) for more than 3 months. The AEs related to sunitinib therapy were recorded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0. PFS time was measured from the date of initiation of sunitinib therapy until documented disease progression, death from disease progression, or the date of the patient’s last follow-up visit. The PFS rates were calculated by the Kaplan–Meier method.
Statistical analysis
The primary aim was to examine the clinical outcome of the general practice of sunitinib therapy in Japanese patients and to determine which of the aforementioned clinical features could become a predictive marker of PFS for mRCC patients. Associations between duration of therapy and clinicopathological features were evaluated with Fisher’s exact test. We used the Cox regression model to calculate the hazard ratio (HR) for univariate and multivariate analysis. Prognostic factors related to PFS were analyzed with Cox regression analysis for multivariate analysis. Statistical significance was set as P < 0.05. Statistical analysis was performed with the Statistical Package for the Social Sciences software, version 16.0 (SPSS, Inc., Chicago, IL).
Results
Patient characteristics
The clinical and pathological characteristics of the 94 patients with mRCC in this study are shown in Table 1. Median age was 66 years (range, 32 to 84 years), and the majority of the patients were men (77.7 %). Most patients had an ECOG PS score of 0 or 1 (83 patients) and were in the intermediate modified MSKCC risk group (47.9 %). Eighty (85.1 %) patients had undergone radical nephrectomy, and their primary histology was almost clear cell carcinoma (69.1 %). Sunitinib was used as first-line therapy in 35 (37.2 %) patients. Forty-eight (49.0 %) patients had been treated previously with IFN-α, and 14 (14.3 %) and 35 (35.7 %) patients had been treated with interleukin-2 and sorafenib, respectively. The number of metastatic sites was single in 41 patients, and the most common site of metastasis was lung. The initially administered doses of sunitinib were 50 mg/day in 58 patients, and median 1M-RDI was 64.3 % (range 12.5–100). Evaluable lesions were present in 66 patients, and radiologically confirmed PR and SD for 3 months as the best ORs were observed in 9 (13.6 %) and 23 (34.8 %) patients, respectively, and the OR rate and disease control rate were 13.6 and 48.5 %, respectively (Table 2). Median follow-up time was 7.5 months (range 0.5–31.7 months).
Adverse events
The grade 3/4 AEs of the 94 patients with mRCC in this study are shown in Table 3. The most common grade 3/4 AEs were hypertension (9.6 %), hand–foot skin reaction (6.4 %), and liver dysfunction (5.3 %). The rate of grade 3/4 AEs was smaller than that of the phase II study in Japanese patients [14]. No grade 5 treatment-related AEs were reported.
The most frequently occurring grade 3/4 laboratory AEs were decreases in platelet count (31.9 %), white blood cells (21.3 %), and red blood cells (5.3 %), as well as increased lipase level (3.2 %). The rate of grade 3/4 laboratory AEs was similar to that of the phase II study in Japanese patients but much larger than that reported from other countries [11, 16]. In the follow-up period time, 37 (39.4 %) and 44 (46.8 %) patients were needed to reduce the dose of sunitinib due to hematologic toxicities and constitutional symptoms, respectively. Between two groups (hematologic toxicities and constitutional symptoms), there was no significant difference in clinical features. The patients initially treated by 50 mg/day were significantly tended to occur either hematologic toxicities or constitutional symptoms (P < 0.001; data not shown).
The result of medication in the general practice of sunitinib therapy
In the first course of sunitinib therapy, continued administration of sunitinib appeared to be intolerable due to AEs in 25 patients (26.6 %) and to disease progression in 3 patients (3.1 %), and sunitinib therapy was interrupted within only one course in these patients (intolerant group). The median period of sunitinib administration was 17 days (range 9–38 days) in the intolerant group. Treatment was discontinued in 18 patients (31.0 %) receiving an initial dose of 50 mg/day patients, in 3 patients (15%) receiving 37.5 mg/day, and in 7 patients (43.8%) receiving 25 mg/day (Table 1). Moreover, only 2 patients (3.3%) who were initially treated with 50 mg/day could continue receiving this oral dose, in spite of attempts to control therapy in order to treat at the maximum dose. Median 1M-RDI of initial sunitinib doses 50, 37.5, and 25 mg/day were 74.3, 53.6, and 44.3 %, respectively (Fig. 1), and there were statistically significant differences between three groups (P < 0.001). In regard to CR, only 1 (8.3 %) of the patients treated for only one course received clinical benefit, whereas 31 (58.6 %) patients treated for more than one course received clinical benefit (Table 2). Woman was tended to discontinue sunitinib therapy within one course (P = 0.015; Table 1).
Univariate and multivariate analysis of predictive factors for PFS and OS
Median PFS time was 6.3 months (Table 2). Median PFS time was 2.3 months (95 % confidence interval (CI), 0.3–4.3) in the patients medicated for only one course and 8.3 months (95 % CI, 2.8–13.8) in the patients medicated for more than one course (P < 0.001; Fig. 2a; Table 2). There was significant difference at 1M-RDI cutoff value associated with an increase in PFS time when the 1M-RDI cutoff value was >60 %, though there was no significant difference between initial doses of sunitinib therapy (Fig. 2b; Tables 4, 5). As well, prior nephrectomy, the number of metastatic sites, prior therapy, age, the kind of toxicities, and sex did not confer significant differences in PFS (data not shown).
Multivariate analysis showed duration of medication and 1M-RDI to be significantly associated with PFS (P < 0.001 and P = 0.027). Other factors were not significantly associated with PFS by multivariate analysis (Table 5).
Discussion
Sunitinib is a multikinase inhibitor with proven efficacy as a first- and second-line treatment in mRCC [13, 14, 22]. We retrospectively examined a multicenter database of Japanese patients receiving sunitinib therapy for mRCC to determine efficacy and AEs of the therapy. We also investigated the correlation between clinical features and clinical outcome. In the present study, it was difficult to treat most Japanese patients with maintaining an initial dose of sunitinib, and duration of therapy for more than one course and maintaining 1 M-RDI > 60 % were found to be statistically significant predictive factors of favorable PFS, regardless of initial doses of sunitinib therapy. Moreover, modified MSKCC poor risk was found to be statistically significant predictive factor of unfavorable PFS.
In previous reports, clinical outcome in a Japanese phase II study was better than those of Western phase II and III studies. Uemura et al. [14] reported data from a phase II study of sunitinib in Japan showing median PFS in 51 patients of 11.5 months, and 47.1 % of patients attained OR. However, the clinical outcome results in our study were inferior to those of the other studies. One reason for the poor result in our study might be the high rate of discontinuance of sunitinib therapy within only one course. In fact, PFS time of the patients treated for more than one course was better than that of the worldwide expanded-access trial and similar to that of the Japanese phase II study [14, 16]. Our univariate and multivariate analysis indicated that it was necessary to treat with sunitinib for at least more than one course and to maintain 1 M-RDI > 60 % to prolong PFS time, regardless of initial doses of sunitinib.
Of interest was the fact that the incidence of AEs, especially those of grade 3/4 hematologic toxicities such as decreases in white blood cells and platelets, was higher than that reported in phase II and phase III studies in Western countries [7, 12, 13]. Similarly, in general clinical practice, the rate of grade 3/4 hematologic toxicities in our study was much higher than that of the worldwide expanded-access trial [16]. Uemura et al. [14] reported that the AUC values of sunitinib and its active metabolite (SU12662) were similar between Japanese and Caucasian subjects, and the difference in AE profiles might be due to differences in the pharmacological properties of sunitinib. Thrombocytopenia might be the main reason to discontinue treatment within only one course because physicians might be afraid that a grade 3/4 AE of hemorrhage might occur concurrently in general practice, so the rate of discontinuation of treatment in the present study was higher than that of other reports.
We previously reported that a full dose of sorafenib (800 mg/day) induced more severe adverse events for Japanese patients than Westerners, and a 1M-RDI of not less than 50 % was necessary to prolong PFS time in second-line therapy of mRCC [20]. For sunitinib as well, it was interesting to us whether 50 mg/day was a suitable initial dose as a general practice in Japanese patients. We found that a high rate of patients treated with 50 mg/day sunitinib discontinued therapy and only 2 patients (3.3 %) who were initially treated with the 50 mg/day dose could continue therapy at this dose. Although 1M-RDI of the patients treated with initial 50 mg/day dose was higher than that of the other doses, it is necessary to medicate with care of severe adverse events peculiar to Japanese in order to maintain 1M-RDI > 60 %.
In the present study, 11 patients (12.2 %) with ECOG PS 2/3 were included, and the median PFS time of the patients with ECOG PS 0/1 was 8.3 months, whereas that of the patients with PS 2/3 was 5.1 months (P = 0.007). Although there was no bias between duration of medication and ECOG PS, other molecular targeted therapy or mTOR inhibitors could be used for patients with poor ECOG PS.
Recently, the difference between Caucasians and other Asians including Japanese has become more important when treating with the new molecular targeted therapies in terms of the efficacy and especially the AE profile. Most recently, the open-label phase III AXIS trial included 76 % Caucasian and 24 % other races including Asians [23], although Asian people were not included in the phase III sunitinib study. In fact, the efficacy of the present study was similar to that of the worldwide expanded-access trial, but the AE rate and rate of discontinuation of treatment within only one course were much higher than those of the expanded-access trial [16]. The results of the present study are very important in showing the efficacy, AE profile, and appropriate dosage of sunitinib used in the general practice of therapy against mRCC in Japanese patients.
In conclusion, it was difficult for Japanese patients to continue an initial dose of sunitinib therapy without drug withdrawal, and continuation of sunitinib therapy for more than one course and maintaining 1M-RDI > 60 % were very important to prolong PFS time regardless of the initial treatment doses. A larger study will be necessary to determine appropriate doses and the schedule of sunitinib for first-line therapy only.
References
Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353(23):2477–90.
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–81.
Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24(35):5601–8.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–56.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–90.
Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2(5):471–8.
Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25–35.
Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–37.
O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101(9):3597–605.
O’Farrell AM, Foran JM, Fiedler W, Serve H, Paquette RL, Cooper MA, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003;9(15):5465–76.
Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24.
Motzer RJ, Michaelson MD, Rosenberg J, Bukowski RM, Curti BD, George DJ, et al. Sunitinib efficacy against advanced renal cell carcinoma. J Urol. 2007;178(5):1883–7.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24.
Uemura H, Shinohara N, Yuasa T, Tomita Y, Fujimoto H, Niwakawa M, et al. A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol. 2010;40(3):194–202.
Tomita Y, Shinohara N, Yuasa T, Fujimoto H, Niwakawa M, Mugiya S, et al. Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol. 2010;40(12):1166–72.
Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10(8):757–63.
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–96.
Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22(3):454–63.
Greene FL. AJCC cancer staging manual. Berlin: Springer; 2002.
Kawashima A, Takayama H, Arai Y, Tanigawa G, Nin M, Kajikawa J, et al. One-month relative dose intensity of not less than 50 % predicts favourable progression-free survival in sorafenib therapy for advanced renal cell carcinoma in Japanese patients. Eur J Cancer. 2011;47(10):1521–6.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295(21):2516–24.
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–9.
Acknowledgments
The authors wish to thank the investigators, their staff, and the affiliated institutions for the important contribution to this study in The Osaka Renal Cell Carcinoma Clinical Study Collaboration: Dr. Susumu Miyoshi (Osaka Rosai Hospital); Dr. Seiji Yamaguchi (Osaka General Medical Center Hospital); Dr. Toshitsugu Oka (National Hospital Organization Osaka National Hospital); Dr. Norio Meguro (Toyonaka Municipal Hospital); Dr. Hironori Nomura, Dr. Kiyomi Matsumiya (Osaka Police Hospital); Dr. Satoko Fukuda, Dr. Tsuneo Hara (Ikeda Municipal Hospital); Dr. Kenji Nishimura (Hyogo Prefectural Nishinomiya Hospital); Dr. Nobukazu Murosaki, Dr. Masato Honda (Kinki Central Hospital of the Mutual Aid Association of Public School Teachers); Dr. Daizo Oka, Dr. Nobumasa Fujimoto (Osaka Koseinenkin Hospital); Dr. Toshiaki Yoshioka (Sumitomo Hospital); Dr. Yasuyuki Kojima (Inoue Hospital); Dr. Shigeru Saiki (Otemae Hospital); Dr. Miyaji Kyakuno (Osaka Seamen’s Insurance Hospital); Dr. Minoru Koga, Dr. Hideki Sugao (Minoh Municipal Hospital); and Dr. Takahiro Yoshida, Dr. Mototaka Sato, Dr. Koji Hatano, Dr. Akira Nagahara (Osaka University Graduate School Of Medicine).
Conflict of interest statement
There are no financial disclosures to report from any author.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Kawashima, A., Tsujimura, A., Takayama, H. et al. Importance of continuing therapy and maintaining one-month relative dose intensity in sunitinib therapy for metastatic renal cell carcinoma. Med Oncol 29, 3298–3305 (2012). https://doi.org/10.1007/s12032-012-0236-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-012-0236-6