Abstract
The objective of this study was to investigate the significance of an alternative dosing schedule for sunitinib in metastatic renal cell carcinoma (mRCC) patients. This study included 154 patients treated with sunitinib as first-line systemic therapy for mRCC, consisting of 62, 47, and 45 receiving sunitinib based on a traditional schedule (TS, 4 weeks on and 2 weeks off) alone (TS group), alternative schedule (AS, 2 weeks on and 1 week off) alone (AS group), and TS followed by AS after the development of dose-limiting toxicities (TS-to-AS group), respectively. There were no significant differences in the major clinicopathological characteristics among these three groups. The progression-free survival in the TS group was significantly shorter than in the other two groups, while no significant differences in the overall survival were noted among the three groups. Adverse events (AEs) ≥ grade 3 in the TS and TS-to-AS groups occurred more frequently than in the AS group. Furthermore, there were significant differences in the incidences of AEs, including diarrhea, fatigue, and hypertension, among the three groups, favoring the AS compared with the other two groups. Despite the lack of a significant difference in the incidence of dose reduction among the three groups, the incidences of the interruption and discontinuation of sunitinib in the AS group were significantly lower than in the other two groups. These findings suggest that the introduction of AS for sunitinib during first-line treatment for mRCC patients may promote favorable clinical outcomes regarding the prognosis as well as tolerability compared with treatment on TS alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sunitinib, an orally available multitargeted tyrosine kinase inhibitor, was shown to exhibit powerful antiangiogenic as well as antitumor activities in preclinical experimental studies [1, 2]. In a clinical setting as well, a pivotal phase III randomized clinical trial (RCT) of treatment-naïve patients with metastatic renal cell carcinoma (mRCC) demonstrated that sunitinib showed a significantly superior efficacy to interferon-α with a median progression-free survival (PFS) of 11 versus 5 months, respectively, and led to median overall survival (OS) > 2 years for the first time [3, 4]. Based on several favorable clinical outcomes in real-world clinical practice [5, 6] in addition to RCT findings [3, 4], sunitinib has been regarded as one of the standards of care for first-line therapy against mRCC, and become the most commonly introduced agent in this setting [7].
The currently recommended traditional schedule (TS) for sunitinib is 50 mg daily for 4 weeks, followed by 2 weeks off (4 weeks on and 2 weeks off), which was determined based on the data from a pharmacokinetic/pharmacodynamic study to maintain the optimal plasma level of this agent [8]. However, if treated with sunitinib on TS, a comparatively large proportion of patients have been reported to require dose reduction, interruption, or discontinuation due to adverse events (AEs), such as thrombocytopenia, diarrhea, hypertension, hand-foot syndrome, and fatigue [9], whereas an increased exposure to sunitinib was shown to be associated with a longer OS and prolonged time to progression in a previous meta-analysis involving patients with several types of malignant tumor, including mRCC [10].
These considerations have encouraged a number of investigators to try to identify an alternative schedule (AS) for sunitinib to improve its tolerability without compromising drug exposure and subsequent disease control [11]. Although several dosing schedules have been assessed as AS for sunitinib, the 2 weeks on and 1 week off schedule is currently regarded as the most useful AS which has been consistently shown to be characterized by lower toxicity compared with TS [11,12,13,14,15,16,17]; however, it has not been well-documented whether the use of AS improves the prognosis of mRCC patients receiving sunitinib. Furthermore, limited data remain available with respect to the introduction of sunitinib on AS as an initial dosing schedule for mRCC patients rather than that converted from TS after encountering dose-limiting toxicity. Considering these findings, this study retrospectively included a total of 154 consecutive mRCC patients who were treated with sunitinib as first-line systemic therapy, and the clinical outcomes in these patients were compared by dividing them into 62, 47, and 45 receiving sunitinib on TS alone (TS group), AS alone (AS group), and TS followed by AS after the development of dose-limiting toxicities (TS-to-AS group), respectively, in order to comprehensively clarify the significance of the introduction of AS for sunitinib during first-line therapy for mRCC patients.
Patients and methods
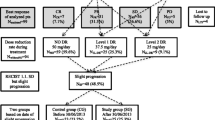
The Research Ethics Committee of our institution approved the design of this study, and the need to obtain informed consent to be involved in the present study from all patients was waived because of its retrospective design. This study included a total of 154 consecutive Japanese mRCC patients who received sunitinib as first-line systemic therapy between January 2010 and June 2017 at our institution. Of the 154 patients, 13 who did not undergo surgical therapy for the resection of the primary tumor underwent biopsies of either the primary or metastatic lesion to assess the histopathological findings; therefore, all 154 were pathologically diagnosed with primary RCC.
In this series, sunitinib was initially administered based on either the TS or AS, as previously reported [4, 5, 13, 14], and the selection of initial dosing schedules for sunitinib was basically determined considering the preference of the physician without strict criteria. In general, 50 mg of sunitinib was orally administered once daily on both TS and AS, consisting of 4 weeks on followed by 2 weeks off and 2 weeks on followed by 1 week off, respectively. Treatment with sunitinib using either dosing schedule was continued until the development of disease progression or intolerable AEs. In patients developing severe AEs related to sunitinib on TS, the treating physician determined whether to switch to AS or attempt dose reduction according to the subjective as well as objective toxicities in each patient. In cases requiring dose reduction due to AEs, the daily dose could be reduced to 37.5 mg, and then further to 25 mg daily and it was also permitted to modify the starting dose considering patient factors, including the age, body weight, and physiological functions.
As baseline assessments prior to the start of treatment with sunitinib, the clinicopathological examinations and performance status (PS) were evaluated using the 7th edition of the UICC TNM classification and the scale of Karnofsky PS, respectively, while both the Memorial Sloan-Kettering Cancer Center (MSKCC) and International Renal Cell Carcinoma Database Consortium (IMDC) systems were employed for risk assessment in each patient [18, 19]. Before the initial administration of sunitinib, radiological examinations, consisting of computed tomography (CT) of the brain, chest, and abdomen and/or radionuclide bone scintigraphy, were performed for all of the included patients. As a rule, the tumor size was measured by CT before and every 6–12 weeks after the introduction of sunitinib. The Response Evaluation Criteria in Solid Tumors v.1.1 and National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 were used to assess the responses and AEs, respectively, during treatment with sunitinib.
The chi-square test was used to analyze differences in several factors among the three groups. PFS and OS rates were assessed by the Kaplan–Meier method, and differences were examined by the log-rank test. All statistical analyses were conducted using Statview 5.0 software (Abacus Concepts, Inc., Berkley, CA, USA), and P < 0.05 was considered significant.
Results
Of the 154 included patients, 107 (69.5%) and 47 (30.5%) initially received sunitinib on TS and AS, respectively. However, 45 of the 107 patients treated on TS had the dosing schedule for sunitinib changed to AS after encountering dose-limiting toxicities, while the administration of sunitinib on TS was continued in the remaining 62 throughout first-line therapy. Accordingly, we classified the 154 patients into 62, 47, and 45 receiving sunitinib on TS alone (TS group), AS alone (AS group), and TS followed by AS (TS-to-AS group), respectively. Major clinicopathological parameters in the 154 patients according to the dosing schedule are shown in Table 1. There were no significant differences in these parameters among TS, AS, and TS-to-AS groups.
Table 2 presents a comparison of oncological outcomes among the three groups. No significant difference in the response rate, clinical benefit rate, or proportion of patients going on to receive second-line therapy was noted among the three groups. In this series, the median durations of PFS in the TS, AS, and TS-to-AS groups were 6.3, 13.8, and 12.2 months, respectively, while those of OS in the TS, AS, and TS-to-AS groups were 30.8, 39.2, and 39.1 months, respectively. As shown in Fig. 1, despite the lack of a significant difference in the OS among the three groups, the PFS in the TS group was significantly poorer compared with those in the AS and TS-to-AS groups.
a Progression-free survival of the 154 patients with metastatic renal cell carcinoma (mRCC) receiving sunitinib as first-line systemic therapy according to the following three dosing schedules: traditional schedule (TS, 4 weeks on and 2 weeks off) alone (TS group), alternative schedule (AS, 2 weeks on and 1 week off) alone (AS group), and TS followed by AS after the development of dose-limiting toxicities (TS-to-AS group). b Overall survival of the 154 patients with mRCC receiving sunitinib as first-line systemic therapy according to the following three dosing schedules: TS group, AS group, and TS-to-AS group
Table 3 shows a comparison of the findings regarding commonly observed AEs related to treatment with sunitinib among the TS, AS, and TS-to-AS groups. All patients in each group experienced AEs; thus, no significant differences in the overall incidence of AEs were noted among the three groups. However, the proportion of patients developing AEs ≥ grade 3 in the AS group was significantly smaller than in the other two groups. Furthermore, there were significant differences in the incidences of AEs among the three groups, including diarrhea, fatigue, and hypertension, all of which favored the AS rather than the other two groups, while thrombocytopenia corresponding to ≥ grade 3 occurred more frequently in the TS and TS-to-AS groups than AS group. Despite the absence of significant differences in the proportions of patients requiring dose reduction among the three groups, the incidences of the interruption as well as discontinuation of sunitinib in the AS group were significantly lower than those of the other two groups.
Discussion
Since accumulating findings of several studies conducted as both RCTs and in routine clinical settings showed the powerful therapeutic activity of sunitinib for patients with treatment-naïve mRCC [3,4,5,6], this agent is currently regarded as one of the standards of care for first-line systemic therapy for mRCC patients [7]. Despite its satisfactory efficacy, sunitinib has been shown to cause significant AEs in a comparatively large proportion of mRCC patients, resulting in marked interference with its efficacy for disease control [9]. In fact, a higher level of exposure to sunitinib was reported to be likely to lead to favorable prognostic outcomes in patients with mRCC [10], whereas dose reduction or interruption due to AEs associated with sunitinib was required in approximately 50% of patients in a phase III RCT [3]. Taken together, a number of studies assessing the AS of sunitinib have been carried out to optimize its dosing schedule, and AS, particularly 2 weeks on and 1 week off, has been widely introduced for treating mRCC patients in routine clinical practice [11,12,13,14,15,16,17]. However, the impact of AS on the improvement of the prognosis of mRCC patients remains controversial, and it also remains unclear whether AS should be introduced as an initial dosing schedule or transitioned from TS after the occurrence of severe AEs; accordingly, this study included a total of 154 patients with mRCC receiving sunitinib as first-line systemic therapy, and conducted a comparative assessment of their clinical outcomes according to the three dosing schedules used in this series.
In recent years, AS has been commonly introduced during treatment of mRCC patients with sunitinib at our institution based on our experience of the frequency as well as severity of AEs,
associated with the use of this agent [6]. Although 62 of the 154 patients included in this series received sunitinib on TS, the remaining 92 were treated with sunitinib on AS; that is, 47 patients were started on AS, and 45 were eventually switched from TS to AS. Furthermore, there were no significant differences in the major clinicopathological parameters among the TS, AS, and TS-to-AS groups; thus, the clinical outcomes in these 3 groups were comprehensively compared in order to investigate the significance of AS for sunitinib against mRCC.
In this study, despite the absence of significant differences in the OS among the three groups, the PFS in the TS was significantly poorer compared with those in the AS and TS-to-AS groups. To date, inconsistent findings with respect to the effect of AS on the prognosis of mRCC patients have been reported [11]. For example, Atkins et al. showed that both the OS and PFS in mRCC patients receiving sunitinib on TS were significantly poorer than those on AS, and that the administration of sunitinib on TS was identified as one of the independent factors associated with the decreased OS and PFS [12], while Lee et al. conducted a phase II trial comparing TS and AS for mRCC patients, and found that failure-free survival, but not PFS or OS, in the AS group was significantly superior to that in the TS group [13]. Such conflicting findings on the prognostic significance of AS in mRCC patients among previous studies could be mainly explained by heterogeneities in backgrounds of the included patients as well as study designs [11,12,13, 16, 17].
It is of interest to characterize the effects of AS for sunitinib on the profile of AEs in mRCC patients. In the present series, sunitinib-induced toxicity with respect to the incidence as well as severity in the AS group was shown to be significantly reduced compared with those in the other two groups, which was particularly marked for AEs accompanying problematic symptoms, including diarrhea, fatigue, and hypertension. The lower toxicity of the AS than TS is consistently supported by previous studies. For example, Lee et al. reported that neutropenia and fatigue less frequently developed in an AS than a TS group in a phase II trial including mRCC patients treated with sunitinib [13]. Furthermore, there have been several reports on the significant improvement of AEs by switching from TS to AS for sunitinib in mRCC patients [11, 12, 14, 15]. We also previously reported that the transition to AS resulted in relief from severe AEs, leading to the achievement of a favorable quality of life of patients with mRCC [15]. However, once introduced as TS irrespective of switching to AS, treatment with sunitinib is likely to induce severe AEs; therefore, it might be preferable to start administering sunitinib for mRCC patients on AS from the viewpoint of the AE profile associated with this agent.
Here, we would like to emphasize several limitations of this study. Firstly, this was performed as a retrospective study including a comparatively small number of patients. Secondly, the decisions to select either TS or AS at the introduction of sunitinib and to switch from TS to AS rather than reduce the dosage at the development of severe AEs were subjectively made by the treating physician without the use of strictly determined criteria, which may have influenced the outcomes of this study. Thirdly, despite heterogeneous practice patterns of previously reported ASs for sunitinib [11], only a schedule of 2 weeks on and 1 week off was used in this series. Therefore, even if the current view is that the 2 weeks on and 1 week off schedule is the most suitable AS based on the outcomes of previous studies [11,12,13,14,15,16,17], including pharmacokinetic studies [20], we may not be able to rule out the existence of more efficacious ASs for sunitinib than 2 weeks on and 1 week off. Finally, this study consisted of solely Japanese patients, who were shown to be less tolerant of sunitinib than Western populations [6, 21]; thus, it might be difficult to apply the findings of the present study to overall mRCC cohorts treated with sunitinib.
In conclusion, our retrospective assessment revealed that mRCC patients who were started on or transitioned to AS from TS for sunitinib achieved a significantly better PFS, but not OS, than those on TS, and that the initiation of sunitinib on AS resulted in a significantly superior tolerability to that on TS, irrespective of switching to AS after encountering severe AEs. Collectively, these findings suggest that despite the need to conduct a prospective study, AS, such as 2 weeks on and 1-week off, may reduce the toxicity of sunitinib and consequently promote an equivalent or even superior prognosis compared with TS in mRCC patients receiving this agent.
References
Gan HK, Seruga B, Knox JJ. Sunitinib in solid tumors. Expert Opin Investig Drugs. 2009;18:821–34.
Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37.
Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24.
Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–90.
Gore ME, Szczylik C, Porta C, et al. Final results from the large sunitinib global expanded-access trial in metastatic renal cell carcinoma. Br J Cancer. 2015;113:12–9.
Miyake H, Miyazaki A, Harada K, Fujisawa M. Assessment of efficacy, safety and quality of life of 110 patients treated with sunitinib as first-line therapy for metastatic renal cell carcinoma: experience in real-world clinical practice in Japan. Med Oncol. 2014;31:978.
Rousseau B, Kempf E, Desamericq G, et al. First-line antiangiogenics for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Crit Rev Oncol Hematol. 2016;107:44–53.
Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35.
Wood L. Sunitinib malate for the treatment of renal cell carcinoma. Expert Opin Pharmacother. 2012;13:1323–36.
Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ. Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol. 2010;66:357–71.
Bracarda S, Negrier S, Casper J, et al. How clinical practice is changing the rules: the sunitinib 2/1 schedule in metastatic renal cell carcinoma. Expert Rev Anticancer Ther. 2017;17:227–33.
Atkinson BJ, Kalra S, Wang X, et al. Clinical outcomes for patients with metastatic renal cell carcinoma treated with alternative sunitinib schedules. J Urol. 2014;191:611–8.
Lee JL, Kim MK, Park I, et al. RandomizEd phase II trial of Sunitinib four weeks on and two weeks off versus Two weeks on and One week off in metastatic clear-cell type REnal cell carcinoma: RESTORE trial. Ann Oncol. 2015;26:2300–5.
Najjar YG, Mittal K, Elson P, et al. A 2 weeks on and 1 week off schedule of sunitinib is associated with decreased toxicity in metastatic renal cell carcinoma. Eur J Cancer. 2014;50:1084–9.
Miyake H, Harada K, Miyazaki A, Fujisawa M. Improved health-related quality of life of patients with metastatic renal cell carcinoma treated with a 2 weeks on and 1 week off schedule of sunitinib. Med Oncol. 2015;32:78.
Bracarda S, Iacovelli R, Boni L, et al. Sunitinib administered on 2/1 schedule in patients with metastatic renal cell carcinoma: the RAINBOW analysis. Ann Oncol. 2015;26:2107–13.
Ezz El Din M. Sunitinib 4/2 Versus 2/1 schedule for patients with metastatic renal cell carcinoma: tertiary care hospital experience. Clin Genitourin Cancer. 2017;15:e455–62.
Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–96.
Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9.
Di Paolo A, Bracarda S, Arrigoni E, et al. Sunitinib in metastatic renal cell carcinoma: the pharmacological basis of the alternative 2/1 schedule. Front Pharmacol. 2017;8:523.
Uemura H, Shinohara N, Yuasa T, et al. A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol. 2010;40:194–202.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H. Miyake has received lecture fees from Pfizer. Y. Matsushita, H. Watanabe, K. Tamura, T. Suzuki, D. Motoyama, T. Ito, T. Sugiyama, and A. Otsuka declare that they have no conflict of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived for individual participants included in the study given the retrospective nature of this work.
Rights and permissions
About this article
Cite this article
Miyake, H., Matsushita, Y., Watanabe, H. et al. Significance of introduction of alternative dosing schedule for sunitinib during first-line treatment of patients with metastatic renal cell carcinoma. Med Oncol 35, 133 (2018). https://doi.org/10.1007/s12032-018-1195-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1195-3