Abstract
Breast cancer is considered as one of the multifactorial diseases. The aim of the current study is to investigate the association between P-cadherin and molecular subtypes of breast cancer, especially the basal-like subtype. Two hundred and thirteen breast–invasive ductal carcinomas were involved in this study. The expressions of P-cadherin were detected via immunohistochemistry. The 213 cases were divided into luminal A, luminal B, HER2 overexpression subtype, and normal breast-like and basal-like subtypes according to the standard of molecular breast cancer subtypes. In addition, the expressions of CK5/6 and CK14 were detected to distinguish between the normal breast-like and the basal-like subtypes. P-cadherin expression was found in 91 cases of 213 breast–invasive ductal carcinomas, with a positive rate of 42.7 %. P-cadherin correlated negatively with estrogen receptor (ER) (p = 0.001) and progesterone receptor (p = 0.001), whereas it positively correlated with histologic grade (p = 0.003), NPI (p = 0.005), p53 (p = 0.038), and Ki67 (p = 0.022). P-cadherin expression showed a strong correlation with recurrence and distant metastasis (p = 0.009), and invasion of the vascular and soft tissues (p = 0.004). Moreover, P-cadherin expression existed in the basal-like and non-basal-like subtypes. During prognosis, P-cadherin expression was associated with decreased disease-free survival in patients (p = 0.009) and overall survival (OS) (p = 0.005). In addition, multivariate analysis showed that tumor grade (p = 0.021), ER (p = 0.015), clinical stage (p = 0.001), and P-cadherin (p = 0.033) were significant predictors of OS. The current data suggest that P-cadherin may be used to distinguish the basal-like subtype and to predict the outcome in view of the relationship with DFS and OS. Furthermore, P-cadherin expression may be useful in making treatment decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer has been considered as one of the most common cancers and is one of the most leading causes of disease worldwide. In China, especially in the metropolis, the incidence rate of female breast cancer is in the first or second place of all cancers [1]. Breast cancer has been traditionally classified only according to its morphology. The hormone receptor status and human epidermal growth factor receptor-2 (HER-2) expression are important prognostic parameters. However, breast cancers have different clinical outcomes in routine practice despite the homogenous morphologic characters and hormone receptors [2]. Moreover, breast cancer is a heterogeneous disease, encompassing a number of distinct biological entities that are associated with specific morphological and immunohistochemical features and clinical behaviors [3–5].
A study related to the characteristics of breast cancer gene expression was completed by Perou et al. [6]. The results suggested that breast cancer should be divided into four subtypes: luminal, basal-like, HER2 expression, and normal breast-like subtypes. However, different molecular subtypes vary in their prognosis and sensitivity to chemotherapy. On the other hand, Sorlie et al. [7] expanded the number of test specimens and verified the results of Perou in a study that included 78 cases of breast cancer. They also verified the existence of two types of luminal, basal-like, HER2 expression, and normal breast-like subtypes in breast cancer, in a study of 115 patients.
The molecular classification was confirmed by other scholars in the independent data system [9] and has been gradually recognized by all. However, the concept of molecular subtype was not suitable for application in clinical pathology because it was proposed at gene level. Differences exist in giving molecular subtypes to new cases of breast cancer despite the gratifying results mentioned above, in which the primary reason is the lack of a unified criterion of molecular subtypes. Thus, accurate determination of the molecular subtypes based on these existing traditional pathological parameters is still a contention. Immunohistochemistry (IHC) was applied for breast cancer molecular subtypes to facilitate the prognosis of treatment because it had been widely used in clinical pathology and is relatively mature.
Common clinicopathological parameters of breast cancer have been generally considered to be related with certain molecular subtype. ER and HER2 are generally considered to be the features of basal cell-like and normal breast-like subtypes [6–8, 10–18]. However, the identification of basal-like breast cancer is still contentious. Sousa et al. [17] reputed that the combination of CK5 with P-cadherin, vimentin, or CK14 has been proven to be a reliable option for distinguishing the basal phenotype, whereas others think that the five-biomarker method (ER-/PR-/HER2-/CK5-/EGFR-) was accurate [10, 12, 18]. Therefore, finding a new prognostic indicator becomes a highlight of research field for all cases, especially for subtypes.
Metastasis was seen as the most serious sign of poor prognosis in the development of breast cancer. In the beginning, tumor cells should step into the circulation from the primary sites. Scholars believe that this phenomenon is due to the change of cell and cell adhesion properties so that cadherins may play an important role in tumor invasion. Classical and desmosomal cadherins mediate cell–cell adherin and classical cadherins, such as P-, E-, and N-cadherin, which are the best characterized subtypes [19]. In addition, HER2 expression and basal-like subtypes have the worst prognosis in the five mentioned subtypes [6], and basal-like cancer is the most aggressive breast tumor type. On the other hand, the abnormal expression of P-cadherin has been found in a small subtype of breast cancer. Microarray technology helps determine P-cadherin, which was basically discovered in those named basal-like subtype breast cancers (BLBC). BLBC tumors have poor prognosis and do not have effective therapy [20]. Therefore, detecting specific markers that are only expressed in basal cells opens a new era in the diagnosis, prognosis assessment, and treatment of breast cancer. Moreover, the study of P-cadherin in BLBC becomes an interesting topic.
Materials and methods
Tumor samples
Formalin-fixed, paraffin-embedded tissues of 213 non-specific breast–invasive ductal carcinoma (IDC) samples were obtained from Baodi Clinical Institute of Tianjin Medical University and Cancer Hospital of Tianjin Medical University. All hematoxylin and eosin-stained sections were reviewed by two senior pathologists, pertaining to various kinds of clinicopathological parameters, including histologic grade, lymph node metastasis status, HR and HER2 status, and so on. Histologic typing and grading were performed according to WHO 2003 version [21]. Thirty-three of the 213 IDC were classified as grade I, 127 as grade II, and 53 as grade III.
Nottingham prognostic index (NPI) was calculated based on three factors: the size of the cancer, whether or not the cancer has spread to the lymph nodes under the arm (and if so, how many nodes are affected), and the grade of cancer. The formula is: NPI = (0.2 × tumor diameter in cm) + lymph node stage + tumor grade [22].
Five cancer subtypes have been classified based on their ER and HER2 expression [10]. A tumor would be classified as luminal subtype in the condition of ER-positive. Luminal subtype would be further classified as A and B, depending on whether the HER2 overexpression exists or not. In addition, the subtype would be named “HER2 overexpression subtype” when a tumor is ER-negative, and HER2 is overexpressed. On the other hand, if a tumor is ER-negative and has no HER2 amplification, it would be classified as basal-like and normal breast-like subtypes, which would be further identified according to the two basal markers (CK5/6 and CK14) [23]. However, if either one or both basal markers are positive, the basal-like subtype would be distinguished, otherwise, it is identified as normal breast-like subtype.
Overall survival (OS) was defined as the time (in months) from the date of the primary surgical treatment to the time of death from breast cancer. Disease-free survival (DFS) was defined as the interval (in months) from the date of the primary surgical treatment to the first loco regional recurrence or distant metastasis. All 213 cases were contacted through letter or telephone for a median follow-up of 62 months.
Immunohistochemistry (IHC)
The expression of P-cadherin was analyzed using the mouse anti-human monoclonal P-cadherin antibody (Thermo Scientific, Lab Vision, USA) by IHC with a working dilution of 1:50. After de-waxing and hydration, four micron sections were retrieved using a pH = 6 citrate buffer. The slides were then cooled for 20 min under room temperature. Hydrogen peroxide (3 %) was used to eliminate endogenous peroxidase. The sections were then incubated with P-cadherin monoclonal antibody for more than 12 h at a temperature of 4 °C. Finally, DAB plus (maxin-bio, China) and hematoxylin counterstain were used. Moreover, negative controls were performed using PBS instead of P-cadherin. Normal breast tissue was used as positive control for P-cadherin [24]. P-cadherin color is located in the membrane with occasional cytoplasm. Either membranous or cytoplasmic immunoreactivity was considered positive when more than 10 % of the neoplastic cells expressed this marker [25]. Furthermore, Ki67 and p53 expressions were both observed using the method mentioned above. ER, PR, and HER2 status were found from the archival. Basal markers (CK5/6 and CK14) were positive if >10 % tumor cells were colored. The positive controls of P-cadherin, [24]CK5/6, and CK14 [12] were normal breast tissues. A reagent company provided positive control slides to maintain the expression of Ki67 and p53.
Statistical analysis
Pearson χ2 or Fisher’s exact test was used to assess P-cadherin expression correlation with each clinicopathologic parameters. Rank data used Spearman test, and Spearman test was used to analyze rank data. Kaplan–Meier analysis was performed using log-rank test for the comparison of linear treads with OS and DFS. Univariate Cox proportional hazard ratio model was used for calculating the hazard ratio (HR) of each factor. All tests were two-sided. A p value <0.05 was considered as a reflection of a significant association. SPSS 13.0 statistical package was used to perform the analyses.
Results
P-cadherin expression in breast–invasive ductal carcinoma (IDC)
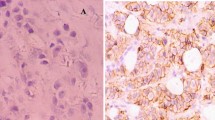
Ninety-one cases (42.7 %) of breast carcinomas in the current experimental series were observed to be P-cadherin-positive. P-cadherin expression was restricted to the membrane with occasional cytoplastic staining. Representative P-cadherin immunostaining is shown in Fig. 1. In normal breast tissue, P-cadherin expression was also found in the myoepithelial cells.
Correlation between P-cadherin expression and clinicopathological variables
A negative association between P-cadherin expression and ER (χ2 = 11.660, p = 0.001), PR (χ2 = 10.997, p = 0.001) was noted (Table 1). In addition, an inverse association between P-cadherin and histologic grade (χ2 = 11.698, p = 0.003), NPI (χ2 = 10.457, p = 0.005), p53 (χ2 = 4.326, p = 0.038), Ki67 (χ2 = 5.229, p = 0.022), HER2 (χ2 = 3.942, p = 0.047), LN stage (χ2 = 6.972, p = 0.031), and recurrence or distant metastasis (χ2 = 6.888, p = 0.009), vascular, and soft tissue invasion (χ2 = 8.232, p = 0.004) was also noted. However, no correlation was found between P-cadherin and age, tumor size, and clinical stage (p>0.05).
P-cadherin expression in molecular subtypes
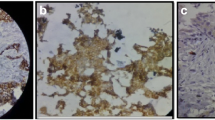
Two hundred and thirteen cases of breast cancer cells were identified as 72 luminal A type, 27 luminal B type, 35 HER2 overexpression type, 36 basal-like type, and 43 normal breast-like type according to the HR, CK5/6, and CK14 status. The expression of P-cadherin in various molecular subtypes is shown in Fig. 2. Significant difference existed between the subtypes (χ2 = 25.945, p = 0.000). Difference also existed between the BLBC and non-BLBC subtypes (χ2 = 12.641, p = 0.000).
Patient’s outcome
The mean follow-up time was 2–74 months. DFS and OS were significantly different between the classes of P-cadherin expression, as calculated through Kaplan–Meier. P-cadherin-positive cases had shorter DFS and OS than P-cadherin-negative cases. The log-rank and p values were 6.759, 0.009 and 7.873, 0.005, respectively (Figs. 3, 4).
The univariate analysis showed the tumor grade and size, clinical stage, lymph node metastatic status, HR, HER2, Ki67, NPI, and P-cadherin (HR = 2.202; 95 % confidence interval (CI), 1.269–3.819; p = 0.005) as significant predictors of OS. Multivariate analysis was performed using a Cox regression model, which included the tumor grade and size, clinical stage, lymph node metastatic status, HR, HER2, Ki67, NPI, and P-cadherin. The analysis showed that the tumor grade, ER, and clinical stage were significant predictors of OS (Table 2).
Discussion
The unity of various cellular processes is needed in the course of tissue and organ formation, cell polarization, aggregation, segregation, migration, and so on during embryogenesis. Adhesion proteins take part in the processes mentioned above [26]. The cadherins are a family of transmembrane glycoproteins that mediate cell–cell adhesion. Classical cadherins consist of E-, N-, and P-cadherin. The first two cadherins were discussed largely in various tumor-related literature, whereas P-cadherin gained the concern of many scholars in recent years, especially its relation with breast cancer.
Breast cancer is the most common disease in women in some extent and endangers the health of women seriously. In routine practice, pathology report card includes tumor grade and size, lymph node metastasis status, HR and HER2 expression status, and so on. However, doctors cannot obtain enough prognostic and treatment response news from traditional information. This phenomenon indicated that breast cancer is a heterogeneous group of tumors, which has diverse biologic behavior, outcome, and treatment response. Therefore, extensive research was carried out against this problem. Perou and Sorlie et al. [6, 7] proposed the concept of molecular subtypes through a large number of experimental data, which opened a new era in breast cancer research.
Many scholars have observed the P-cadherin expression in various tumors. However, its role in the process of carcinogenesis is not clear because it is difficult to achieve uniform results on account of different reactions in various models. For example, in malignant melanoma, P-cadherin functions as a tumor suppressor gene, inhibiting invasion and metastasis [27]. However, in other studies, P-cadherin displays as an enhancer in cell invasion and tumor aggressiveness, particularly in breast tumor [24]. Thus, some putative value of P-cadherin in diagnosis, prognosis, and treatment needs further experiments for confirmation. Abnormal P-cadherin expression is detected in approximately 30 % of breast cancer cell lines; however, the positive rate of coverage varies [26]. Earlier studies showed that P-cadherin-positive rate was below 20 %, but with the appearance of P-cadherin monoclonal antibody, the positive rate of IDC becomes 30–50 %. In the current study, P-cadherin (Clone 56 monoclonal antibody) was used to detect 213 cases of IDC; the positive rate was 42.7 %, which is consistent with literature reports. However, contrary to the findings above, Madhavan et al. [28] have found that P-cadherin-positive rate was 71 %. The cause of these contradicting results may be derived from the use of polyclonal antibody, which produces cross response with other cadherins.
The current study showed that the expression of P-cadherin was inversely related to hormonal receptor, which indicated good prognosis. In addition, P-cadherin was positively correlated with high histologic grade, p53, Ki67, and HER2, which indicated poor prognosis. This phenomenon can infer that the expression of P-cadherin indicates poor prognosis. This result is consistent with many studies of scholars, such as that of Paredes et al. [24, 29–33]. Consequently, the value of NPI is proportional to P-cadherin expression, which also supports the conclusion mentioned above. P-cadherin expression was observed to be positively related with lymph node metastasis, recurrence, distant metastasis, and vascular- and soft tissue invasion group. This phenomenon infers that P-cadherin is involved in tumor cell invasion. In the current research, a negative co-relation exists between the expressions of P-cadherin and FOXA1. Previous records and archives indicated that FOXA1 is a transcription factor required in the transcription process of ER mediation because it regulates ER. In clinical practice, patients of mammary cancer with positive FOXA1 expression usually have better prognosis [34]. Researches in vitro indicate that the absence of ERa signal is related to abnormal expression of P-cadherin. However, the mechanism of ERa signal suppression resulting in such abnormal expression remains unknown. The use of anti-estrogenic drugs in MCF-7 cell line is found in the research of Albergaria A, which shows that the alteration of CDH3 promoter configuration regulates P-cadherin expression in a positively related manner. Moreover, anti-estrogenic drugs could disable ER signals and suppress ERa and thus produce an aggressive phenotype [31]. In light of the aforesaid relationships among FOXA1, ERa, and P-cadherin combined with the negative relationship between FOXA1 and P-cadherin observed in the current study, the abnormal expression of P-cadherin might be derived from the absence of FOXA1 expression.
The results vary with the relationship between the P-cadherin and DFS or OS. An association between P-cadherin expression and shorter DFS/OS was found in the data of Paredes et al. [24], but failed to appear in the data of Kovacs [29]. The current study showed that P-cadherin expression was negatively correlated with longer DFS and OS, which is consistent with the findings of Paredes et al. [24]. Combined with the relationship with P-cadherin and clinicopathological parameters extend the conclusion that P-cadherin is an indicator of poor prognosis.
The basal-like and HER2 overexpression subtypes are the worst prognosis of all breast cancers. Basal cell-like subtype attracted attention of many researchers due to its unique pattern of gene expression and poor prognosis [35, 36]. It has distinct biological characteristic and clinical outcome, and further clinical research demonstrated that it should be considered as a special subtype [35]. Although the morphology of BLBC had been observed [37], no uniform standard can be accepted widely. However, in the routine pathological work, BLBC is needed to be distinguished as its poor outcome. In this group, the results show that frequent P-cadherin expression is found in BLBC, whereas a different analysis shows that P-cadherin expression was associated with BLBC. Thus, it can be concluded that P-cadherin can be applied to identify BLBC in ER-/HER2- group.
Arnes et al. [38] suggested that P-cadherin combined with p63 and CK5 can distinguish BLBC, whereas others [12] maintain that only basal CKs could distinguish BLBC without the expression of other markers. The inconsistency mentioned above illustrates that bulk specimens are still needed for a more accurate conclusion.
P-cadherin and other markers would be of assistance for oncologists in more accurately predicting clinical outcome. In addition, P-cadherin would be a novel target in the treatment of breast cancer and a predicting marker for identifying the basal-like subtype.
References
Liu H, Xun P, Chen K-x, et al. The trend of clinical characteristics and prognosis of women’s breast cancer 1981–2000. Natl Med J China. 2007;87(34):2405–7.
Alizadeh AA, Ross DT, Perou CM, et al. Towards a novel classification of human malignancies based on gene expression patterns. J Pathol. 2001;195:41–52.
Reis-Filho JS, Simpson PT, Gale T, et al. The molecular genetics of breast cancer: the contribution of comparative genomic hybridization. Pathol Res Pract. 2005;201:713–25.
Lacroix M, Toillon RA, Leclercq G. Stable ‘portrait’ of breast tumors during progression: data from biology, pathology and genetics. Endocr Relat Cancer. 2004;11:497–522.
Simpson PT, Reis-Filho JS, Gale T, et al. Molecular evolution of breast cancer. J Pathol. 2005;205:248–54.
Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74.
Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23.
van’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6.
Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–74.
Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11:5678–85.
Rakha EA, El-Sayed ME, Green AR, et al. Breast carcinoma with basal differentiation: a proposal for pathology definition based on basal cytokeratin expression. Histopathology. 2007;50:434–8.
Calza S, Hall P, Auer G, et al. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006;8:R34.
Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–60.
Jumppanen M, Gruvberger-Saal S, Kauraniemi P, et al. Basal-like phenotype is not associated with patient survival in estrogen-receptor-negative breast cancers. Breast Cancer Res. 2007;9:R16.
Tang P, Wei B, David G, et al. Breast cancer molecular subtypes and clinical application. Chin J Pathol. 2009;38:13–7.
Sousa B, Paredes J, Milanezi F, et al. P-cadherin, vimentin and CK14 for identification of basal-like phenotype in breast carcinomas: an immunohistochemical study. Histol Histopathol. 2010;25(8):963–74.
Turashvili G, McKinney SE, Goktepe O, et al. P-cadherin expression as a prognostic biomarker in a 3,992 case tissue microarray series of breast cancer. Mod Pathol. 2011;24(1):64–81.
Knudsen KA, Wheelock MJ. Cadherins and the mammary gland. J Cell Biochem. 2005;95:488–96.
Rakha EA, Putti TC. Abd El-Rehim DM, et al. Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. J Pathol. 2006;208:495–506.
Tavassoli FA, Devilee P, editors. The WHO classification of tumors of the breast and female genital organs (M). Lyon: IARC Press; 2003. p. 10–112.
Miller DV, Leontovich AA, Lingle WL, et al. Utilizing Nottingham prognostic index in microarray gene expression profiling of breast carcinomas. Mod Pathol. 2004;17:756–64.
Rakha EA, Ellis IO. Triple-negative/basal-like breast cancer: review. Pathology. 2009;41:40–7.
Paredes J, Albergaria A, Oliveira JT, et al. P-cadherin overexpression is an indicator of clinical outcome in invasive breast carcinomas and is associated with CDH3 promoter hypomethylation. Clin Cancer Res. 2005;11:5869–77.
Matos I, Dufloth R, Alvarenga M, et al. p63, cytokeratin 5, and P-cadherin: three molecular markers to distinguish basal phenotype in breast carcinomas. Virchows Arch. 2005;447:688–94.
Paredes J, Correia AL, Ribeiro AS, et al. P-cadherin expression in breast cancer: a review. Breast Cancer Res. 2007;9:214.
Van Marck V, Stove C, Van Den Bossche K, et al. P-cadherin promotes cell–cell adhesion and counteracts invasion in human melanoma. Cancer Res. 2005;65:8774–83.
Madhavan M, Srinivas P, Abraham E, et al. Cadherins as predictive markers of nodal metastasis in breast cancer. Mod Pathol. 2001;14:423–7.
Kovacs A, Dhillon J, Walker RA. Expression of P-cadherin, but not E-cadherin or N-cadherin, relates to pathological and functional differentiation of breast carcinomas. Mol Pathol. 2003;56:318–22.
Paredes J, Stove C, Stove V, et al. P-cadherin is up-regulated by the antiestrogen ICI 182,780 and promotes invasion of human breast cancer cells. Cancer Res. 2004;64:8309–17.
Albergaria A, Ribeiro AS, Pinho S, et al. ICI 182,780 induces P-cadherin overexpression in breast cancer cells through chromatin remodelling at the promoter level: a role for C/EBPbeta in CDH3 gene activation. Hum Mol Genet. 2010;19(13):2554–66.
Ribeiro AS, Albergaria A, Sousa B, et al. Extracellular cleavage and shedding of P-cadherin: a mechanism underlying the invasive behaviour of breast cancer cells. Oncogene. 2010;29(3):392–402.
Sarrió D, Palacios J, Hergueta-Redondo M, et al. Functional characterization of E- and P-cadherin in invasive breast cancer cells. BMC Cancer. 2009;3(9):74.
Liu N, Niu Y, Wang SL, et al. Diagnostic and prognostic significance of FOXA1 expression in molecular subtype of breast invasive ductal carcinoma. Zhonghua Yi Xue Za Zhi. 2010;90(20):1403–7.
Niu Y. Basal-like carcinoma: one subtype of breast cancer recognized recently. Chin J Pathol. 2007;36:849–52.
Yang GZ, Gao LX, Ding HY. Basal-like subtype of breast cancer. Chin J Diag Pathol. 2007;14:241–3.
Gao LX, Yang GZ, Ding HY, et al. Morphological features of basal-like subtype invasive carcinoma of breast. Chin J Pathol. 2008;37:83–7.
Arnes JB, Brunet JS, Stefansson I, et al. Placental cadherin and the basal epithelial phenotype of BRCA1-related breast cancer. Clin Cancer Res. 2005;11:4003–11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, N., Yu, Q., Liu, T.J. et al. P-cadherin expression and basal-like subtype in breast cancers. Med Oncol 29, 2606–2612 (2012). https://doi.org/10.1007/s12032-012-0218-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-012-0218-8