Abstract
Cell-cell adhesion, which is essential for the maintenance of epithelial plasticity, is mediated by a class of proteins called cadherins. Immunohistochemical study of E-cadherin has been reported to be important in predicting response to chemotherapy in breast cancer subjects. An inverse relationship has been documented between expression of E-cadherin and invasiveness of tumors. The aim of the study is to assess E-cadherin expression in patients of carcinoma breast and to study correlation of E-cadherin expression with clinical response to neoadjuvant chemotherapy. A prospective single-center study was conducted with a sample size of 33 patients of carcinoma breast who were planned for neoadjuvant chemotherapy. E-cadherin expression in core needle biopsy specimens was assessed using immunohistochemistry. The response of the patients to chemotherapy was assessed clinically and pathologically. E-cadherin expression has no association with tumor size and grade, lymph node status, and estrogen receptor, progesterone receptor, or Her-2/neu receptor positivity. Stronger expression of E-cadherin intensity and proportion was associated with good clinical response as well as higher pathological complete response after neoadjuvant chemotherapy. E-cadherin expression correlates with response to neoadjuvant chemotherapy in patients of carcinoma breast, indicating its role as prognostic marker in predicting response to neoadjuvant chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignancy among woman and its incidence is increasing worldwide and in the Indian subcontinent [1]. Various options are available for the treatment of breast cancer which include surgery, chemotherapy, radiotherapy, hormonal therapy, and targeted therapy. Chemotherapy is ubiquitously used in breast cancer both in neoadjuvant and adjuvant settings. Neoadjuvant chemotherapy is necessary for local and systemic tumor control and it improves the resectability of the tumor by downsizing it [2]. However, about 20% of patients subjected to neoadjuvant chemotherapy do not show response [3, 4]. To predict the response of the tumor for neoadjuvant chemotherapy, several factors have been proposed such as ER (estrogen receptor), PR (progesterone receptor), Her-2/neu receptor, and Ki-67 index. But, independently none of the above factors could accurately predict chemotherapy response. Hence, there is ongoing research for analyzing other factors predicting chemotherapy response in patients of breast carcinoma.
E-cadherin is one such protein being studied for its role in predicting response to neoadjuvant chemotherapy. Cadherins are Ca2+-dependent trans-membrane proteins that are connected to other proteins like actin through other cytoplasmic proteins. E-cadherin loss has been implicated in the epithelial-mesenchymal transition in several malignancies [5]. Selective loss of E-cadherin protein can lead to epithelial de-differentiation and invasiveness in human carcinomas [6, 7]. An inverse relationship has been documented between expression of E-cadherin and invasiveness of tumors [7].
Among the histological types of breast carcinoma, loss of E-cadherin was associated with majority of the invasive lobular carcinomas when compared with invasive ductal carcinomas and is frequently used for differentiating the histological varieties [8]. The results from various studies were contradictory about the association of E-cadherin with tumor size; nodal status; ER, PR, and Her-2/neu receptor positivity; and overall survival. But most of the studies concluded that E-cadherin has limited role as a prognostic factor but it is useful as a phenotype marker [8,9,10,11,12,13,14]. However, there have been promising results from studies comparing E-cadherin with response to chemotherapy in triple-negative breast cancers [15, 16]. These studies are also complimented with studies correlating other cadherins with response to chemotherapy [17]. This has been the basis of our interest to conduct this study with the aim of comparing E-cadherin expression with response to neoadjuvant chemotherapy in breast carcinoma.
Patients and Methods
Institutional ethics committee approval for the study was taken prior to the commencement as it involved human participants. All patients were enrolled in the study after taking written informed consent.
Study Design and Population
A prospective single-center study was conducted in our institution which is a tertiary care and academic center located in India from November 2016 to March 2018. Thirty-three samples of paraffin blocks from breast cancer core biopsy and mastectomy specimens have been included in this study on accrual. All female patients who presented to the surgical department of our hospital during the study period satisfying the inclusion and exclusion criteria and who consented were enrolled in the study. All patients belonged to a single cohort as per the protocol and were followed up prospectively until the end of neoadjuvant chemotherapy and surgery. The average follow-up period was 10 weeks.
Inclusion Criteria
-
Female patients with diagnosis of invasive carcinoma of breast (both ductal and lobular) on core needle biopsy who were planned for neoadjuvant chemotherapy.
Exclusion Criteria
-
Patients with metastasis at presentation.
-
Patients with recurrent disease.
-
Patients who were lost to follow-up during neoadjuvant chemotherapy.
-
Patients in who were unfit for chemotherapy.
Procedure
Female patients who presented to the surgical department with history of symptoms and signs of breast malignancy were evaluated by triple assessment and the size of the lump was measured clinically and radiologically. Patients with a diagnosis of breast carcinoma on core needle biopsy for whom neoadjuvant chemotherapy was planned were invited to enroll in the study. Indications of chemotherapy were:
-
All patients with locally advanced breast carcinoma
-
Patients with early breast carcinoma to downsize the tumor to make it amenable for breast conservation surgery.
All patients in the study were administered 3 cycles of CAF (cyclophosphamide, adriamycin, and 5-flurouracil) as 3-weekly regimen. Size of the lump was monitored every 3 weeks and final size was documented at the end of neoadjuvant chemotherapy. The response was assessed as per the RECIST (Response Evaluation Criteria in Solid Tumors) criteria 1.1 and patients graded into clinical complete responder, partial responder, and non-responder (stable or progressive disease) [18]. E-cadherin expression is compared with neoadjuvant chemotherapy response, age, tumor size, lymph node status, tumor grade, and ER, PR and Her-2/neu receptor status. After the surgery, postoperative specimen was subjected to histopathological examination and pathological complete response if present was documented.
Immunohistochemistry for E-cadherin
The immunohistochemical analysis was performed on formalin-fixed, paraffin-embedded tissue sections. The tissue sections were initially deparaffined in xylene and rehydrated using graded alcohols. The sections were then put in microwave along with buffer solution to retrieve the antigens using heat-induced epitope retrieval technique. Endogenous peroxidase activity was reduced by using hydrogen peroxide solution. Then, the sections were incubated with primary antibody towards E-cadherin for 90 min. We used mouse monoclonal antibody E-cadherin (clone: EP6, Biogenex, USA). The slides were washed and reincubated with secondary antibody and then followed by streptavidin-biotin-peroxidase complex. After washing, brown color reaction was developed using diaminobenzidine as the chromogen, counter stained with haematoxylin, and then observed. All steps were carried out at room temperature. Normal breast tissue sections were used as controls on which all steps were carried out except for the addition of primary antibody. Staining was considered to be positive when the pattern was membranous or membranous and cytoplasmic.
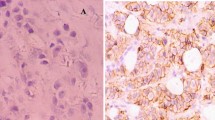
The intensity of E-cadherin was graded as 0 (no color), 1+ (light yellow), 2+ (claybank), 3+ (sepia), and 4+ (dark brown). The proportion of cells stained was graded as 0 (absent), 1+ (≤ 30% of cells stained), 2+ (31–60% of cells stained), and 3+ (> 60% of cells stained) (Fig. 1a, b, and c).
Microscopic images showing various intensities and proportions of E-cadherin staining× 400. a IHC staining of E-cadherin, intensity 4+ and proportion 3+, × 400. b IHC staining showing E-cadherin, intensity 3+ and proportion 3+, × 400. c IHC staining of E-cadherin, intensity 1+ and proportion 1+, × 400
Statistical Analysis
The data acquired was analyzed using Statistical Package for Social Sciences (SPSS) version 21.0 using Kruskal-Wallis’s test and Fisher’s exact test.
Results
Out of 33 female patients, 32 patients had invasive ductal carcinoma on histology. Only 1 patient had invasive lobular carcinoma on histology which stained negative for E-cadherin. There was no sample regression due to loss of follow-up.
Mean age of patients was 54.82 years with range of 25 to 80 years. Out of 33 patients, 3.03% patients were below 25 years of age, 39.39% of patients were in the age range of 26–50 years of age, 45.45% of patients were in the age range of 51–75 years, and 12.21% of patients were above the age of 75 years (Table 1). Six out of 33 patients were triple negative constituting 18.1% of subjects. Out of 33 subjects, 24.2% patients belonged to stage IIB, 46.4% patients belonged to stage IIIA, 20.2% patients belonged to stage IIIB, and 9.2% patients belonged to stage IIIC.
Out of 33 patients, 3.0% patients showed no reactivity of E-cadherin, 12.1% were 1+ positive, 9.1% were 2+ positive, 36.4% were 3+ positive, and 39.4% were 4+ positive for E-cadherin expression (Table 2).
Out of 33 patients, 3.0% patients were negative, 9.1% showed E-cadherin proportion of 1+, 15.2% showed E-cadherin proportion of 2+, and 72.7% showed E-cadherin proportion of 3+ (Table 2).
There was no significant association between tumor size, lymph node positivity, tumor grade, ER positivity, PR positivity, Her-2/neu receptor positivity, and E-cadherin intensity (Table 3). There was significant association between clinical response to neoadjuvant chemotherapy and E-cadherin intensity with p value of < 0.001 (Table 3). There was significant association between E-cadherin intensity and pathological complete response with p value of 0.001 (Table 3).
There was no significant association between tumor size, lymph node positivity, tumor grade, ER positivity, PR positivity, Her-2/neu receptor positivity, and E-cadherin proportion (Table 4). There was significant association between clinical response to neoadjuvant chemotherapy and E-cadherin intensity with p value of 0.003 (Table 4). There was significant association between E-cadherin intensity and pathological complete response with p value of 0.008 (Table 4).
Discussion
E-cadherin is a glycoprotein belonging to the class of calcium-dependent cell adhesion molecules [19]. It is coded by CDH1 gene located at locus 16q22.1 and is a tumor suppressor gene. It plays a very important role in cell-cell adhesion and tissue morphogenesis [20]. The protein is also connected to the intracellular cytoskeleton and also plays a role in various signaling pathways [21]. Loss of E-cadherin is associated with cancer progression in various malignancies [22]. This has prompted research regarding the role of E-cadherin as a prognostic marker in carcinoma breast.
The association of E-cadherin with various prognostic variables is contradictory in various studies. In a study by Singhai R et al., E-cadherin was not associated with any variables like tumor size, lymph node status, and ER, PR, and Her-2/neu receptor status [8]. In a study by Parker C et al., there was significant association between E-cadherin and tumor grade and ER positivity [21]. In a study by Fulga V et al., there was positive association between E-cadherin expression and lymph node metastasis and ER positivity [23]. In a study by Younis LK et al., there was significant correlation between E-cadherin expression and lymph node negative status [9]. In a study by Qureshi HS et al., there was no correlation between E-cadherin and established prognostic variables [24]. In a study by Chintamani et al., there has been positive correlation between E-cadherin expression and ER positivity status and inverse relation with p53 gene mutation [25]. In the present study, there was no significant association between E-cadherin expression and studied prognostic variables similar to the previous studies. However, the association between E-cadherin intensity and ER positivity was close but not significant.
Epithelial to mesenchymal transformation will result in the formation of fibroblastoid cells which are less sensitive to chemotherapy [26]. Epithelial to mesenchymal transformation will also result in upregulation of N-cadherin which is also postulated to have a role in cell invasion [27]. In various studies, it has been proved that lobular carcinomas respond less to chemotherapy and this is attributed to loss E-cadherin [28, 29]. Loss of E-cadherin was associated with decreased pathological complete response after neoadjuvant chemotherapy and poor prognosis after adjuvant therapy in patients of triple-negative breast cancer [15, 16, 30]. In the present study, the significant association proved between E-cadherin intensity and proportion and response to chemotherapy can be correlated with previous studies on patients with lobular breast carcinoma and triple-negative breast cancers. The prognostic value of other cadherins like T-cadherin was also studied and negative expression was correlated with better response to chemotherapy [17].
The limitation of the present study was small sample size and single-center population. Other prognostic predictors like Ki-67 and p53 were not studied simultaneously. However, this study stimulates further research in this regard due to exiting results.
Conclusion
Immunohistochemically detected E-cadherin effectively predicts response to chemotherapy in breast cancer subjects as seen in this study of rather smaller sample of 33 patients. E-cadherin negative cases should be advised early surgery as the role of neoadjuvant chemotherapy is limited in this setting. A multicenter study with a larger sample size and multiple prognostic indicators would help to conclude the above results.
Data Availability
Available on request from researchers.
References
Rangarajan B, Shet T, Wadasadawala T, Nair NS, Sairam RM, Hingmire SS, Bajpai J (2016) Breast cancer: an overview of published Indian data. South Asian J Cancer 5(3):86–92. https://doi.org/10.4103/2278-330X.187561
Breslin TM (2011) Neoadjuvant chemotherapy for breast cancer: a surgeon’s checklist. Breast Cancer Res Treat 127(1):129–131. https://doi.org/10.1007/s10549-011-1376-7
Chen S, Huang L, Chen CM, Shao ZM (2015) Progesterone receptor loss identifies luminal-type local advanced breast cancer with poor survival in patients who fail to achieve a pathological complete response to neoadjuvant chemotherapy. Oncotarget 6:18174–18182. https://doi.org/10.18632/oncotarget.4225
Park K, Choi MK, Jung HH, Do IG, Lee KH, Ahn T, Kil WH, Kim SW, Lee JE, Nam SJ, Kim DH, Ahn JS, Im YH et al (2015) Molecular characterization of patients with pathologic complete response or early failure after neoadjuvant chemotherapy for locally advanced breast cancer using next generation sequencing and nCounter assay. Oncotarget 6:24499–24510. https://doi.org/10.18632/oncotarget.4119
Baranwal S, Alahari SK (2009) Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun 384(1):6–11. https://doi.org/10.1016/j.bbrc.2009.04.051
Behrens J, Weidner KM, Frixen UH et al (1991) The role of E-cadherin and scatter factor in tumor invasion and cell motility. EXS. 59:109–126. https://doi.org/10.1007/978-3-0348-7494-6_8
Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Löchner D, Birchmeier W (1991) E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 113(1):173–185. https://doi.org/10.1083/jcb.113.1.173
Singhai R, Patil VW, Jaiswal SR, Patil SD, Tayade MB, Patil AV (2011) E-cadherin as a diagnostic biomarker in breast cancer. N Am J Med Sci 3(5):227–233. https://doi.org/10.4297/najms.2011.3227
Younis LK, El Sakka H, Haque I (2007) The prognostic value of E-cadherin expression in breast cancer. Int J Health Sci (Qassim) 1(1):43–51
Liu J, Sun X, Qin S et al (2016) CDH1 promoter methylation correlates with decreased gene expression and poor prognosis in patients with breast cancer. Oncol Lett 11(4):2635–2643. https://doi.org/10.3892/ol.2016.4274
Nass SJ, Herman JG, Gabrielson E, Iversen PW, Parl FF, Davidson NE, Graff JR (2000) Aberrant methylation of the estrogen receptor and E-cadherin 5′ CpG islands increases with malignant progression in human breast cancer. Cancer Res 60(16):4346–4348
Ricciardi GR, Adamo B, Ieni A, Licata L, Cardia R, Ferraro G, Franchina T, Tuccari G, Adamo V (2015) Androgen receptor (AR), E-cadherin, and Ki-67 as emerging targets and novel prognostic markers in triple-negative breast cancer (TNBC) patients. PLoS One 10(6):e0128368. https://doi.org/10.1371/journal.pone.0128368
Kashiwagi S, Yashiro M, Takashima T, Nomura S, Noda S, Kawajiri H, Ishikawa T, Wakasa K, Hirakawa K (2010) Significance of E-cadherin expression in triple-negative breast cancer. Br J Cancer 103(2):249–255. https://doi.org/10.1038/sj.bjc.6605735
Querzoli P, Coradini D, Pedriali M, Boracchi P, Ambrogi F, Raimondi E, la Sorda R, Lattanzio R, Rinaldi R, Lunardi M, Frasson C, Modesti F, Ferretti S, Piantelli M, Iacobelli S, Biganzoli E, Nenci I, Alberti S (2010) An immunohistochemically positive E-cadherin status is not always predictive for a good prognosis in human breast cancer. Br J Cancer 103(12):1835–1839. https://doi.org/10.1038/sj.bjc.6605991
Kashiwagi S, Yashiro M, Takashima T, Aomatsu N, Kawajiri H, Onoda N, Nakata B, Ishikawa T, Hirakawa K (2011) Is E-cadherin a useful surrogate marker to predict chemo-sensitivity of chemotherapy for triple-negative breast cancer? J Clin Oncol 29(15):1077
Kashiwagi S, Yashiro M, Takashima T, Aomatsu N, Ikeda K, Ogawa Y, Ishikawa T, Hirakawa K (2011) Advantages of adjuvant chemotherapy for patients with triple-negative breast cancer at stage II: usefulness of prognostic markers E-cadherin and Ki67. Breast Cancer Res 13(6):R122. https://doi.org/10.1186/bcr3068
Kong D, Wang MH, Yang J, Li L (2017) Association of T-cadherin levels with the response to neoadjuvant chemotherapy in locally advanced breast cancer. Oncotarget 8(8):13747–13753. https://doi.org/10.18632/oncotarget.14630
Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, Seymour L (2016) RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer 62:132–137. https://doi.org/10.1016/j.ejca.2016.03.081
Pećina-Slaus N (2003) Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int 3(1):17. https://doi.org/10.1186/1475-2867-3-17
Gumbiner BM (2005) Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 6(8):622–634. https://doi.org/10.1038/nrm1699
Parker C, Rampaul RS, Pinder SE, Bell JA, Wencyk PM, Blamey RW, Nicholson RI, Robertson JFR, Ellis IO (2001) E-cadherin as a prognostic indicator in primary breast cancer. Br J Cancer 85(12):1958–1963. https://doi.org/10.1054/bjoc.2001.2178
Kourtidis A, Lu R, Pence LJ, Anastasiadis PZ (2017) A central role for cadherin signaling in cancer. Exp Cell Res 358(1):78–85. https://doi.org/10.1016/j.yexcr.2017.04.006
Fulga V, Rudico L, Balica AR, Cimpean AM, Saptefrati L, Margan MM, Raica M (2015) Differential expression of e-cadherin in primary breast cancer and corresponding lymph node metastases. Anticancer Res 35(2):759–765
Qureshi HS, Linden MD, Divine G, Raju UB (2006) E-cadherin status in breast cancer correlates with histologic type but does not correlate with established prognostic parameters. Am J Clin Pathol 125(3):377–385. https://doi.org/10.1309/wmx7-drwt-fvqp-2lqt
Chintamani RB, Bansal A, Bhatnagar D, Saxena S (2010) Expression of E-cadherin in breast carcinomas and its association with other biological markers - a prospective study. Indian J Surg Oncol 1(1):40–46. https://doi.org/10.1007/s13193-010-0010-1
Altundag K, Altundag O, Akyurek S, Karakaya E, Turen S (2006) Inactivation of E-cadherin and less sensitivity of lobular breast carcinoma cells to chemotherapy. Breast. 15(3):300. https://doi.org/10.1016/j.breast.2005.10.007
Cavallaro U, Schaffhauser B, Christofori G (2002) Cadherins and the tumour progression: is it all in a switch? Cancer Lett 176(2):123–128. https://doi.org/10.1016/s0304-3835(01)00759-5
Cocquyt VF, Blondeel PN, Depypere HT, Praet MM, Schelfhout VR, Silva OE, Hurley J, Serreyn RF, Daems KK, van Belle SJP (2003) Different responses to preoperative chemotherapy for invasive lobular and invasive ductal breast carcinoma. Eur J Surg Oncol 29(4):361–367. https://doi.org/10.1053/ejso.2002.1404
Nagao T, Kinoshita T, Hojo T, Tsuda H, Tamura K, Fujiwara Y (2012) The differences in the histological types of breast cancer and the response to neoadjuvant chemotherapy: the relationship between the outcome and the clinicopathological characteristics. Breast. 21(3):289–295. https://doi.org/10.1016/j.breast.2011.12.011
Kraus JA, Beriwal S, Dabbs DJ, Ahrendt GM, McGuire KP, Johnson RR, Badve P, Puhalla SL, Bhargava R (2012) Predictors of pathologic complete response after standard neoadjuvant chemotherapy in triple-negative breast carcinoma. Appl Immunohistochem Mol Morphol 20(4):334–339. https://doi.org/10.1097/PAI.0b013e31823f4663
Acknowledgments
The authors would like to thank Dr. Himanshu Agrawal for his inputs and advice to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki 1964. Approval was granted by the Ethics Committee of PGIMER/RMLH with approval number TP (MD/MS) (68/2016)/IEC/PGIMER/RMLH.
Consent to Participate
Consent was taken for participation was taken from all participants.
Consent for Publication
Consent for publication has been taken for publication of results and data from all participants.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Korpal, M., Yelamanchi, R., Durga, C.K. et al. Immunohistochemical E-cadherin Expression and Response to Chemotherapy in Breast Cancer Subjects. Indian J Surg 83 (Suppl 2), 421–426 (2021). https://doi.org/10.1007/s12262-020-02576-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-020-02576-2