Abstract
When telomeres are shortened to a critical length, they will initiate chromosomal instability (CIN) and may finally cause tumorigenesis. The purpose of the present study was to evaluate the shortened telomere as a potential biomarker for tumorigenesis in gastric carcinoma. The telomeres in matched cancer and adjacent noncancer mucosa samples from 86 gastric carcinoma patients were measured by real-time polymerase chain reaction (PCR). According to the International Union Against Cancer (UICC), tumor stages were classified into four groups: stage I (n = 23), stage II (n = 20), stage III (n = 23), and stage IV (n = 20). Telomere length decreased with aging in both adjacent noncancer mucosa and cancer tissue (r = −0.261 (P = 0.008) and r = −0.27 (P = 0.012), respectively). The telomere length of UICC stage I tumors was significantly shorter than the average telomere length in adjacent noncancer mucosa (P = 0.023). Telomere length increased gradually with increasing UICC stage (P = 0.032). The telomere length of UICC stage IV tumors was significantly longer, when compared to that in noncancer mucosa (P = 0.019) and stage I tumors (P = 0.002). In summary, telomere length undergoes shortening in early stage gastric carcinoma and lengthening in advanced gastric carcinoma. Additionally, telomere shortening may initiate the tumorigenesis of gastric carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric carcinoma (GC) represents the second most common cause of death from cancer in humans [1]. GC still remains difficult to cure because it is often diagnosed at an advanced stage [2]. The canceration molecular mechanism of GC is poorly understood [3, 4], thus restricting the development of molecular tumor markers, effective molecular screening procedures, and molecular-targeted therapies.
Gastric carcinoma rarely develops in health gastric mucosa but is closely linked to precursor conditions in the background mucosa: chronic gastritis, atrophy, intestinal metaplasia, and dysplasia [5]. The precancerous carcinoma process during gastrocarcinogenesis remains under debate. At the molecular level, the stepwise progression from an early dysplastic lesion to a malignant tumor is associated with increases in chromosomal instability (CIN), loss of p53 checkpoint function, and telomere shortening [6]. Experimental data demonstrated that telomere length is shortened in the gastric mucosa in chronic gastritis caused by Helicobacter pylori infection [7] and in peripheral leukocytes of high-risk populations for GC. These studies indicated that telomere shortening functions as an important co-factor in initiating CIN and inducing carcinogenesis of GC.
Previous studies have improved our understanding of the molecular mechanisms of gastric carcinogenesis. However, CIN and telomere length and their consequences in tumor tissue and nonneoplastic gastric mucosa of GC in different stages are still hotly debated issues. Few studies have addressed telomere length alteration in gastric cancer tissue using histopathologic tumor parameters. In the present study, we analyzed telomere length by real-time quantitative polymerase chain reaction (qPCR) in adjacent noncancer mucosa and cancer tissue of gastric carcinoma patients treated with gastrectomy. Our present study attempts to validate whether telomere length can be a reliable marker for progression of gastric carcinoma.
Patients and methods
Patient and tumor specimens
The study consisted of 86 patients who were diagnosed with histologically confirmed primary gastric adenocarcinoma. All incident cases were recruited from the Department of Gastroenterological Surgery, the First Affiliated Hospital, Medical College, Zhejiang University, Hangzhou, China. In total, 58 men (age range, 32–80 years; median age, 62 years) and 28 women (age range, 21–81 years; median age, 56 years) participated in the study. The tumor stages were classified according to the International Union Against Cancer [8] (UICC; Table 1). From the resected specimen of each patient, samples from both the cancer tissue and the adjacent noncancer mucosa (greater than 5 cm from cancer) were obtained, immediately shock-frozen in liquid nitrogen, and stored at −80°C until use.
DNA extraction
Genomic DNA was extracted from about a 25-mg tissue section from each sample using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Before and after each section, a 7-μm cryostat section from each tissue sample was stained with hematoxylin and eosin for histopathologic analysis. Histology-guided sample selection was performed to exclude any contamination of the adjacent mucosa samples with carcinoma cells and to identify tumor content greater than 80% in the cancer samples. Extracted DNA was quantified spectrophotometrically at 260- and 280-nm wavelengths for DNA concentration and uniform DNA concentration (20 ng/μl).
Telomere length measurement
The standard method for measuring telomere length is Southern blot analysis [9], which was used to measure the telomere length in eight different cell lines: 293T (human embryonic kidney cell line); K562 (human chronic myelogenous leukemia cell line); MRC (human fetal lung fibroblast cell line); BJ42.5, BJ26, and BJ79 (human fibroblast cell lines); HeLa (human cervical carcinoma cell line); and HuH-7 (human hepatocellular carcinoma cell line. We also measured the relative telomere-to-single copy gene (T/S) ratios by qPCR, and then we acquired the correlation curve and correlation coefficient between the relative T/S ratios measured by qPCR and terminal restriction fragment (TRF) patterns measured by Southern blotting.
The telomere length of samples was assessed by qPCR as previously described [10]. The qPCR was divided into two: telomere PCRs and β-globin (HBG) PCRs. They were always performed in separate 96 wells with each sample run in triplicate. Telomere and β-globin primer sequences written 5′ to 3′ were as follows: CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT (tel1), GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT (tel2), GCTTCTGACACAACTGTGTTCACTAGC (HBG1), and CACCAACTTCATCCACGTTCACC (HBG2). The two PCR master mixes were the same except for the primers, 2× iTaq SYBR green Supermix with ROX (Bio-Rad Laboratories, Hercules, CA, USA), the final concentrations of tel1 and tel2 (100 and 900 nM, respectively), and the final concentrations of HBG1 and HBG2 (300 and 700 nM, respectively). A 5 ng (2.5 μl) aliquot of template DNA was added to each reaction containing 22.5 μl of the PCR master mix. A standard curve of a diluted reference DNA (293T; range, 2.5–40 ng/μl) and a negative control (water) was included in each run to allow relative quantification of the samples.
Polymerase chain reactions were performed on a real-time PCR machine (ABI 7500; Applied Biosystems, Foster City, CA, USA). The thermal cycling profile for the telomere amplification was 95°C or 10 min, followed by 30 cycles of 95°C for 15 s and 56°C for 1 min. For HBG amplification, the thermal cycling profile was 95°C for 10 min, followed by 35 cycles of 95°C for 15 s and 54°C for 1 min. Standard curves were generated using ABI Prism 7500 SDS software 1.7. R 2 for each standard curve was ≥0.98. Standard deviations (for Ct values) were accepted at <0.2. All analyses were performed blinded to patient characteristics.
In the qPCR, the relative ratio of telomere (T) repeat copy number to a single copy gene (S)copy number (T/S ratio) was certain, and it was directly proportional to the average telomere length. The relative telomere length was believed to reflect the actual differences in telomere length in individuals. The T/S ratio was determined using the formula T/S = 2−∆Ct, where ∆Ct = average Cttelomere − average Ctβ-globin. The relative T/S ratio was 2−(∆Ct1 − ∆Ct2) = 2−∆∆Ct, where ∆Ct1 = the T/S value of the sample and ∆Ct2 = the T/S value of the reference sample (293T) [10]. From the formula, we can calculate each sample’s relative telomere length, which could be converted into TRF using the correlation curve we had obtained.
Statistical analyses
Statistical analyses were performed using the SPSS16.0 software package. Data were expressed as means (\( \bar{X} \)) ± standard deviation (S). Differences in telomere length between matched tissue samples were determined by the Wilcoxon test for matched pairs. Associations between telomere length and age were determined by linear regression. Differences among various groups of patients discriminated by histopathologic parameters were analyzed by the Kruskal–Wallis test and the Mann–Whitney two-sample test. ANOVA was used to determine the differences in telomere length as a continuous variable by tumor node metastasis (TNM) stage, tumor invasion, and tumor grade. All tests were performed at a significance level of P ≤ 0.05.
Results
Telomere lengths of adjacent noncancer mucosa and cancer tissue decrease with aging
In men (n = 58), the telomere lengths of adjacent noncancer mucosa and cancer tissue were 8.08 ± 0.56 kb and 8.10 ± 0.68 kb, respectively; in women (n = 28), they were 8.31 ± 0.78 kb and 8.39 ± 1.02 kb, respectively. No significant difference between men and women was observed in the telomere length of adjacent noncancer mucosa or cancer tissue (P = 0.189; Table 1).
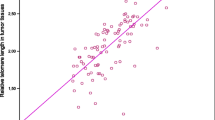
We next analyzed the association between telomere length and age. The telomere lengths of adjacent noncancer mucosa and cancer tissue all decreased with age (r = −0.261/P = 0.008 and r = −0.27/P = 0.012, respectively; Fig. 1), with a telomere length reduction of about 17 bp per year in adjacent noncancer mucosa and about 13 bp per year in cancer tissue.
Telomere length shortens in the early stage of gastric cancer and lengthens with tumor progression
Overall, a statistically significant correlation was found between the telomere length in cancer tissue and TNM stage (P = 0.032). We also compared the telomere length of cancer tissue at different stages with the average telomere length of adjacent noncancer mucosa. The average telomere length of stage I tumors (7.84 ± 0.58 kb; n = 23) in cancer tissue was significantly shorter than the average telomere length in adjacent noncancer mucosa (8.14 ± 0.64; n = 86; P = 0.023). Telomeres of stage I tumors were significantly shorter than those of both stage III tumors (8.32 ± 0.75 kb; n = 23; P = 0.035) and stage IV tumors (8.59 ± 1.01 kb; n = 20; P = 0.002). Telomeres of stage II tumors (8.05 ± 0.71 kb; n = 20) were significantly shorter than those of stage IV tumors (P = 0.028). The average telomere length of stage IV tumors in cancer tissue was significantly longer than that in noncancer mucosa (P = 0.019; Fig. 2). Telomeres of early stage tumors (UICC stage I; n = 23) were significantly shorter than telomeres of advanced tumors (UICC stage II through IV; 8.32 ± 0.84 kb; n = 63; P = 0.028).
With increasing tumor invasion depth (pT), the telomere length in cancer tissue increased gradually (P = 0.043; Fig. 3). Comparing the telomeres of adjacent noncancer mucosa with those of cancer tissues at different pTs, we found that pT4 cancer tissues possessed obviously longer telomeres than those of adjacent noncancer mucosa. Telomeres of positive lymphatic invasion cancer tissue were significantly longer (8.32 ± 0.85 kb; n = 55) than telomeres of negative lymphatic invasion cancer tissue (7.93 ± 0.58 kb; n = 31; P = 0.032; Fig. 4). There was no significant difference between them with adjacent noncancer mucosa. There was no correlation between telomere length and tumor grade.
Discussion
Telomeres are protective structures at the ends of eukaryotic chromosomes and progressively shorten during each cell cycle [11]. Our results are consistent with earlier studies [12] demonstrating that an inverse relationship exists between telomere length and age relative to the reduction of 40 bp per year in normal gastric mucosa [13, 14]. Our result yielded a smaller reduction in the telomere length: 13 bp/year in noncancer mucosa and 17 bp/year in cancer tissue. Differences in the telomere reduction may be because of the reactivation of telomerase, which compensates for replicative telomere losses [15, 16]. Our findings also showed that telomere length was shorter in men than in women for both noncancer mucosa and cancer tissue, although the differences were not significant. This result is in-line with previous observations [17]. The effect of gender on telomere length may be attributed to the different types and levels of sex hormones [18].
In the present study, we revealed a significant correlation between telomere length and clinicopathological parameters. We showed a trend toward longer telomeres in cancer tissues with increasing depth of tumor invasion, and telomeres of positive lymphatic invasion were significantly longer than telomeres of negative lymphatic invasion. From stage I to IV tumors, telomeres were increased gradually and telomeres in stage I tumors were significantly shorter than those in noncancer mucosa, while those in stage IV tumors were significantly longer. Our findings support the hypothesis that critically shortened telomeres can initiate CIN that, in turn, may foster tumorigenesis [19]. The most important function of telomeres is maintaining genomic stability. When telomeres are shortened to a critical length, the result is telomere dysfunction with no further cell division and the cell undergoes either replicative senescence or apoptosis [20]. If the cell bypasses these two processes, it will continue to proliferate through activation of telomerase, and genomic instability will be initiated and accumulate. Finally, it will promote cancer development [21].
In this context, telomeres are longer in advanced-stage tumors, as previously reported for colorectal carcinomas [22], suggesting that complete telomere stabilization may be achieved late in tumorigenesis after extensive cell proliferation and telomere shortening have already occurred [22]. When dysfunctional telomeres initiate tumorigenesis, telomerase reactivation was required secondarily for tumor progression. According to earlier observations, approximately 85% of human cancers express telomerase [23]. Thus, telomere maintenance or even elongation appears to be essential for the tumor to maintain its proliferative capacity and to continue further tumor invasion and progression [20].
Our data show for the first time that telomeres are shorter in early stage gastric carcinoma and longer in the advanced stage. Further investigations are needed to determine the underlying mechanism of telomere shortening in gastric carcinoma occurrence and progression. In conclusion, telomere shortening appears to initiate the tumorigenesis of gastric carcinoma, and telomere length maintenance or elongation through telomerase reactivation is also important for gastric carcinoma invasion and progression.
References
Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374(9688):477–90.
Catalano V, et al. Gastric cancer. Crit Rev Oncol Hematol. 2009;71(2):127–64.
Milne AN, Carneiro F, O’Morain C, Offerhaus GJ. Nature meets nurture: molecular genetics of gastric cancer. Hum Genet. 2009;126(5):615–28.
Tahara E. Molecular mechanism of stomach carcinogenesis. J Cancer Res Clin Oncol. 1993;119(5):265–72.
Bornschein J, Kandulski A, Selgrad M, Malfertheiner P. From gastric inflammation to gastric cancer. Dig Dis. 2010;28(4–5):609–14.
Chin L, et al. p53 deficiency rescues the advance effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97(4):527–38.
Aida J, et al. Telomere length variations in 6 mucosal cell types of gastric tissue observed using a novel quantitative fluorescence in situ hybridization method. Hum Pathol. 2007;38(8):1192–200.
Sobin LH, Wittekind CH. TNM classification of malignant tumors. 6th ed. New York, NY, USA: Wiley; 2002.
Canela A, Klatt P, Blasco MA. Telomere length analysis. Methods Mol Biol. 2007;371:45–72.
Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47.
Blackbum EH. Structure and function of telomeres. Nature. 1991;350:569–73.
Djojosubroto MW, Choi YS, Lee HW, Rudolph KL. Telomeres and telomerase in aging, regeneration, and cancer. Mol Cells. 2003;15(2):164–75.
Furugori E, et al. Telomere shortening in gastric carcinoma with aging despite telomerase activation. J Cancer Res Clin Oncol. 2000;126(8):481–5.
Nakamura K, et al. Correlation of telomere lengths in normal and cancers tissue in the large bowel. Cancer Lett. 2000;158:179–84.
Gumus-Akay G, et al. Telomerase activity could be used as a marker for neoplastic transformation in gastric adenocarcinoma: but it does not have a prognosyic significance. Genet Mol Res. 2007;6(1):41–9.
Nakamura Y, Tahara E, Tahara H, Yasui W, Ide T. Quantitative reevaluation of telomerase activity in cancerous and noncancerous gastrointestinal tissues. Mol Carcinog. 1999;16(4):312–20.
Liu XN, et al. Constitutive telomere length and gastric cancer risk: case–control analysis in Chinese Han population. Cancer Sci. 2009;100(7):1300–5.
Blackburn EH. Telomeres and telomerase: their mechanism of action and the effects of action and the effects of altering their functions. FEBS Lett. 2005;579:859–62.
Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225(4):951–60.
Artandi SE, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–5.
Cosme-Blanco W, Chang S. Dual roles of telomere dysfunction in initiation and suppression of tumorigenesis. Exp Cell Res. 2008;314:1973–9.
Gertler R, et al. Telomere length and human telomerase reverse transcriptase expression as markers for progression and prognosis of colorectal carcinoma. J Clin Oncol. 2004;22:1807–14.
Kim NW, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011.
Acknowledgments
The study was partially supported by the National Natural Science Foundation of China (No. 81071964), by the First Affiliated Hospital, College of Medicine, Zhejiang University (B1248), and by the The Scientific Research Plan Projects of Zhejiang Provincial Education Department (No. Y200804129).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mu, Y., Zhang, Q., Mei, L. et al. Telomere shortening occurs early during gastrocarcinogenesis. Med Oncol 29, 893–898 (2012). https://doi.org/10.1007/s12032-011-9866-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-9866-3