Abstract
Telomere shortening occurs in many organs and tissues and is accelerated by oxidative injury and rapid cell turnover. Short telomeres initiate chromosomal instability and may eventually contribute to tumorigenesis. To evaluate telomere length as potential biomarker for gastric cancer (GC) risk, we measured average telomere length using quantitative real-time PCR in GC tissues and in non-neoplastic mucosa from patients with GC and without GC. We obtained of 217 GC patients matched biopsies from the GC and adjacent tissues as well as gastric biopsies of 102 subjects without GC. Relative telomere length was measured in genomic DNA by real-time PCR. Relative telomere length decreased gradually in Helicobacter pylori (H. pylori) negative and positive gastric mucosa of GC free subjects compared with adjacent mucosa and cancer tissue from GC patients (4.03 ± 0.3 vs. 2.82 ± 0.19 vs. 0.82 ± 0.07 vs. 0.29 ± 0.09, P < 0.0001). In non-neoplastic mucosa of GC patients, shorter telomeres were found significantly more often than in that of GC free subjects (age, sex, and H. pylori adjusted odds ratio = 7.81, 95 % confidence interval = 4.71–12.9, P < 0.0001). Telomere shortening in non-neoplastic mucosa was associated with chronic inflammation (P = 0.0018) and intestinal metaplasia (P < 0.0001). No significant associations were found between relative telomere length and clinicopathological features of GC and overall survival. Telomere shortening in gastric mucosa reflects a field effect in an early stage of carcinogenesis and is associated with an increased risk of GC. Telomere length in GC is not associated with clinicopathological features or prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the most common malignancies worldwide, accounting for approximately 70,000 new cases and 650,000 deaths per year [1, 2]. Many patients have advanced disease at diagnosis, and treatment outcome for these patients is poor [3]. Although Helicobacter pylori (H. pylori) infection is a strong predisposing factor for GC [4], the existence of an H. pylori infection alone is insufficiently accurate for predicting cancer risk in areas with a high H. pylori infection rate, especially Asian countries. Identification of molecular markers which more precisely predict GC risk may improve our capacity for early detection, which will improve prognosis.
Telomeres consist of numerous repetitions of the nucleotide sequence TTAGGG and an associated terminal protein complex that assist in the preservation of chromosomal integrity [5]. Telomeres shorten during each replicative cycle and short telomeres reflect a long replicative lifespan or accelerated replicative activity due to, e.g., oxidative stress [6, 7]. Shortened telomeres are associated with cellular senescence and decreased tissue renewal capacity [8, 9]. Telomerase is an enzyme complex present in stem cells and in most cancer cells, composed of the reversed transcriptase TERT and the template RNA TERC, which allows the cells to replenish telomeric repeat DNA. In telomerase knockout mouse models, which possess critically shorter telomeres, a higher incidence of cancer occurs [10, 11]. Short telomeres have also been observed in human epithelial cancers, in association with the formation of complex non-reciprocal translocations and increased chromosomal instability [12–16].
In the stomach, telomeres were found to be shorter in H. pylori infected than in non-infected gastric mucosa [17, 18]. Short telomeres were also observed in GC [19, 20]. This suggests that telomere shortening might be associated with the earliest stages of neoplastic transformation, which may be useful for identifying populations at risk of developing GC. However, differences in telomere length between individual tumors may also reflect differences in proliferative activity and may be useful for estimating outcome of GC.
The gold standard for measuring telomere length was Southern blotting, which has been used on samples of gastric mucosa [19]. Subsequently, a real-time PCR–based telomere assay was established, which provides reproducible results on minute amounts of DNA [21].
To evaluate telomere length as a potential biomarker for GC, we applied a real-time PCR–based telomere length assay to gastric mucosa of patients without and those with GC as well as GC biopsy samples of the latter. We assessed associations between telomere length and the presence of GC and potential associations between telomere length in GC tissues and clinicopathological features, including overall survival (OS).
Materials and methods
Study population and sample DNA extraction
We enrolled 319 subjects attending the Endoscopy Center of Fujita Health University Hospital from September 2004 to February 2008. The Ethics Committee of Fujita Health University School of Medicine approved the protocol, and written informed consent was obtained from all subjects. The series of 319 patients included 217 patients with gastric cancer (GC) who were being treated in our hospital and 102 non-cancer subjects. Non-cancer subjects visited our hospital to undergo upper gastroscopy for their health checks, for secondary complete checkups for stomach cancer following barium x-ray examination, or for complaints of abdominal discomfort. None of the non-cancer subjects had any evidence of GC after upper gastroscopy. Twenty-one subjects were diagnosed with ulcer disease, including 12 subjects with a gastric ulcer and 9 with a duodenal ulcer. For the remaining non-cancer subjects, gastroscopy did not result in any findings. The non-cancer group did not include patients who had severe systemic diseases, current or previous history of a malignancy in a different organ, received non-steroidal anti-inflammatory drugs after H. pylori eradication therapy, or a history of gastric surgery. This cohort was partly recruited from our other study investigating the association between telomere length and severity of gastritis and non-steroidal anti-inflammatory drug use [18]. All GC were diagnosed histologically and were classified according to the Lauren classification [22]. Of the GC patients, 174 underwent gastrectomy and 37 chemotherapy; treatment information was not available for 6 patients. Ten surgically resected GC cases showed mixed histologic features; however, all cases showed predominance of intestinal type, and therefore we diagnosed these cases as intestinal type histology. Detailed information about anatomic location, lymph node or other metastases, and peritoneal dissemination was also obtained using the criteria of the Japanese classification of gastric carcinoma [23]. Epstein-Barr (EB) virus status was determined using EBER1-in situ hybridization. Ten GC patients were diagnosed with EB virus positive GC. Overall survival, defined as the time from gastrectomy or the start of the initial administration of chemotherapy to the date of cancer-related death, was determined for 210 patients.
Of all patients, biopsy specimens were taken from endoscopically non-pathological mucosa of the antrum and upper corpus along the greater curvature. Of GC patients, biopsy specimens from cancerous tissue were also taken. The specimens taken from the antrum and cancerous tissue were cut into two pieces, and one was immediately frozen and stored at −80 °C until use. Genomic DNA was extracted directly from these frozen specimens using a standard extraction and precipitation method. The other half was fixed in 10 % buffered formalin and embedded in paraffin for microscopic examination. Histological examination of hematoxylin and eosin (H&E)-stained histological slides confirmed that all biopsies from non-neoplastic mucosa contained more than 70 % epithelial cells and that all biopsies from cancerous tissue contained more than 70 % cancer cells. H. pylori infection status was assessed by histological analysis of biopsy specimens from the greater curvature of the gastric antrum and from the upper corpus by immunohistochemistry, using the polyclonal rabbit anti-Helicobacter pylori antibody (FLEX Anti-Helicobacter Pylori, Code GA523; Dako, Tokyo, Japan). H&E-stained histological slides from non-neoplastic mucosa were also evaluated for the presence of chronic inflammation and intestinal metaplasia (IM). The degree of mononuclear cell infiltration and IM were assessed according to the updated Sydney system [24], each factor being scored from 0 (normal) to 3 (marked). We defined a biopsy with a mononuclear cell infiltration ≥2 as inflammatory mucosa. For IM, the presence of IM (metaplasia score ≥ 1) was considered as IM positive.
Relative average telomere length measurement
Relative telomere length was measured by comparing the abundance of telomeric template relative to a single-copy gene (T/S) in individual samples to a reference pool of DNA from healthy blood samples by quantitative real-time PCR as described previously [25], with some modifications. A 2-μL aliquot with 10 ng of DNA was used in 20 μL of either a telomere or a single-copy gene (h-globin) PCR reaction. The telomere reaction mixture consisted of 1× iTaq SYBR Green Supermix (Bio-Rad), 100 nmol/L of Tel-1b primer (CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT), 900 nmol/L of Tel-2b primer (GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT), and 1 % DMSO. The reaction proceeded for 1 cycle at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 54 °C for 2 min. The h-globin reaction consisted of 1× iTaq SYBR Green Supermix (Bio-Rad), 400 nmol/L hbg1 primer (GCTTCTGACACAACTGTGTTCACTAGC), and 400 nmol/L hbg2 primer (CACCAACTTCATCCACGTTCACC). The h-globin reaction proceeded for 1 cycle at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 58 °C for 1 min. For quantitative real-time PCR, the ABI Prism 7900HT Real-Time PCR System (Applied Biosystems) was used.
The telomere and the single-copy gene (h-globin) were analyzed on the same plate to reduce inter-assay variability. For each standard curve, pooled leukocyte DNA, derived from healthy individuals as a reference sample, was diluted serially in water by 5-fold per dilution to produce five concentrations of DNA ranging from 24 to 0.0384 ng/L. The T/S ratio (−dCt) for each sample was calculated by subtracting the average h-globin Ct value from the average telomere Ct value. The relative T/S ratio (−ddCt) was determined by subtracting the T/S ratio of the 24 ng standard curve point from the T/S ratio of each unknown sample [25]. All measurements were performed in duplicate and averaged. The coefficients of variation within duplicates of the telomere and single-gene assay were 1.2 and 2.7 %, respectively.
Statistical analysis
Differences in telomere length between the two groups were assessed using Student’s t test. To compare telomere length between more than two groups, we used one-way ANOVA. The association between telomere length in non-neoplastic gastric mucosa and the frequency of occurrence of GC was evaluated by logistic regression analysis. The association between telomere length and age was assessed using Spearman’s correlation coefficient. The association between telomere length and OS in GC patients was assessed using the Kaplan-Meier method and the log-rank test. We used the Bonferroni correction for multiple testing. A smaller P value threshold, defined as 0.05 divided by the multiple testing frequency, was considered to be statistically significant.
Results
Subject characteristics are summarized in Table 1. Age and the H. pylori infection rate were significantly higher in the GC group than in the non-cancer group, whereas the male/female ratio was not significantly different. As the GC group was significantly older than the non-cancer group, we randomly selected from the group of GC subjects a subgroup of the same number as the non-GC groups with matched average age (Table 1).
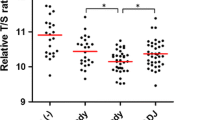
We first compared relative telomere length between four different types of biopsies: H. pylori negative and H. pylori positive gastric mucosa from non-cancer subjects (n = 30 and 72, respectively) and paired adjacent and cancerous tissues from 217 GC patients. GC samples presented the shortest telomeres and tissue samples adjacent to GC were second shortest (0.29 ± 0.09 vs. 0.82 ± 0.07). Conversely, the longest telomeres were observed in H. pylori negative gastric mucosa and the second longest in H. pylori positive gastric mucosa (4.03 ± 0.36 vs. 2.82 ± 0.19). These differences were significant by one-way ANOVA (P < 0.0001, Fig. 1).
We then assessed by logistic regression analysis whether in non-neoplastic gastric mucosa from GC patients telomeres were more often shortened than in gastric biopsies from non-GC subjects. Short telomeres occurred more often in non-tumoral gastric biopsies from GC patients than in biopsies from non-GC subjects (age, sex, and H. pylori adjusted odds ratio (OR) = 7.81, 95 % confidence interval (CI) = 4.71–12.9, P < 0.0001, Table 2). This result was maintained in an age-matched subgroup of GC (all P < 0.0001, Table 3).
Telomeres were significantly shorter in both intestinal (OR = 5.84, 95 % CI = 3.51–9.70, P < 0.0001) and diffuse GC (OR = 14.29, 95 % CI = 6.06–33.69, P < 0.0001) (Table 2). Because GC risk is thought to be associated with the degree of inflammation of gastric mucosa and the presence of IM [4], we investigated possible associations between relative telomere length and chronic inflammation and IM in non-neoplastic gastric mucosa. Telomeres were significantly shorter in cases with chronic inflammation (P = 0.0018) and IM (P < 0.0001) compared with those without (Fig. 2).
Association between relative telomere length and chronic inflammation (a) and intestinal metaplasia (IM) (b). Non-inflammation and non-IM, gastric mucosa without chronic inflammation and IM; Inflammation and IM, gastric mucosa with chronic inflammation and IM. Statistical analysis was performed by Student’s t test
We also investigated whether relative telomere length was associated with gender; H. pylori and EB virus status; tumor location; lymph node, peritoneal, liver, and other forms of distant metastasis; and stage. No significant associations were found between relative telomere length and any of these features (Tables 3 and 4). We also investigated possible associations between detailed T and N stage of 174 patients who underwent surgical resection of GC, using the Japanese classification of gastric carcinoma [23], but no significant associations were found (Table S1).
Telomere shortening has been observed with advancing age in several tissue types [8, 10]. We did not observe significant associations between age and telomere length in any of our samples, neither in gastric mucosa from subjects without cancer nor in GC samples nor in mucosa adjacent to GC (Fig. S1).
Finally, we investigated whether relative telomere length is associated with OS of GC patients. To this end, we divided subjects into four quartile categories of telomere length and found no significant differences in OS between the four categories by log-rank test (P = 0.71, Fig. 3).
Discussion
We observed the shortest telomeres in GC. In non-neoplastic gastric mucosa, telomeres were shorter in H. pylori infected biopsies, which was in line with several earlier studies [17, 18]. Moreover, by logistic regression analysis, we found in non-neoplastic gastric mucosa of GC patients telomere shortening more often than in gastric mucosal biopsies from non-GC subjects, which was confirmed in the randomly selected age-matched GC group. This suggests that telomere shortening is involved in early stages of gastric carcinogenesis. Shorter telomeres were also observed in patients with moderate or severe chronic gastritis and IM, who are at increased risk of developing GC [4]. The phenomenon that in gastric mucosa shorter telomeres are associated with a higher frequency of GC can be explained by the concept of a “field effect,” molecular alterations associated with exposure to oncogenic influences which precede neoplastic transformation. H. pylori infection is a strong predisposing factor for the development of GC [4], and evidence shows that H. pylori eradication [26, 27] and endoscopic examination [28] reduce GC risk and mortality but additional markers for estimating GC risk might reduce risks and cost associated with repeated gastroscopy. Telomere length in non-neoplastic gastric mucosa may constitute a promising biomarker for GC risk. Relative to the analysis by Southern blotting, the real-time PCR–based telomere length assay is more reproducible and can be performed on tiny amounts of DNA, which are important advantages in a clinical setting.
We also hypothesized that differences in telomere length between tumors might be associated with outcome. A previous study reported shorter telomeres in early stage GC, but a relative gradual increase along with stage [20]. We did not find telomere length in GC to be significantly associated with clinicopathological features, including stage. In addition, telomere length was not associated with OS of GC patients. In view of our finding that telomeres shorten in the early stages of gastric carcinogenesis, we propose that telomere length might be used as a molecular biomarker for GC risk. It has been suggested that telomere length might be used to assess prognosis or response to H. pylori eradication treatment in terms of a decreased risk of metachronous GC after endoscopic resection [26], which is supported by the observation that telomere length is restored after eradication [29]. Our results do not provide evidence supporting these suggestions. Whether telomere length can predict GC risk in patients after H. pylori eradication needs to be clarified by well-designed future studies.
References
Ferlay J, Bray F, Parkin DM, Pisani P (2001) Gobocan 2000: cancer prevalence and mortality worldwide (IARC cancer bases no. 5). IARC Press, Lyon
Lau M, Le A, El-Serag HB (2006) Noncardia gastric adenocarcinoma remains an important and deadly cancer in the United States: secular trends in prevalence and survival. Am J Gastroenterol 101:2485–2492
Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. (2013) Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer 16:1–27
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345:784–789
de Lange T (2005) The protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, et al. (2003) Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst 95:1211–1218
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345:458–460
Vulliamy T, Marrone A, Dokal I, Mason PJ (2002) Association between aplastic anaemia and mutations in telomerase RNA. Lancet 359:2168–2070
Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6:611–622
Chang S (2005) Modeling aging and cancer in the telomerase knockout mouse. Mutat Res 576:39–53
Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, et al. (2000) Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406:641–645
Bailey SM, Murnane JP (2006) Telomeres, chromosome instability and cancer. Nucleic Acids Res 34:2408–2017
Cheung AL, Deng W (2008) Telomere dysfunction, genome instability and cancer. Front Biosci 13:2075–2090
Murnane JP (2006) Telomeres and chromosome instability. DNA Repair (Amst) 5:1082–1092
Parsonnet J (1993) Helicobacter pylori and gastric cancer. Gastroenterol Clin N Am 22:89–104
Aida J, Izumiyama-Shimomura N, Nakamura K, Ishii A, Ishikawa N, Honma N, et al. (2007) Telomere length variations in 6 mucosal cell types of gastric tissue observed using a novel quantitative fluorescence in situ hybridization method. Hum Pathol 38:1192–1200
Tahara T, Shibata T, Kawamura T, Ishizuka T, Okubo M, Nagasaka M, Nakagawa Y, Arisawa T, Ohmiya N, Hirata I (2016) Telomere length in non-neoplastic gastric mucosa and its relationship to H. pylori infection, degree of gastritis, and NSAID use. Clin Exp Med. 16:65–71
Maruyama Y, Hanai H, Fujita M, Kaneko E (1997) Telomere length and telomerase activity in carcinogenesis of the stomach. Jpn J Clin Oncol 27:216–220
Mu Y, Zhang Q, Mei L, Liu X, Yang W, Yu J (2012) Telomere shortening occurs early during gastrocarcinogenesis. Med Oncol 29:893–898
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Dixon MF, Genta RM, Yardley JH, Correa P (1996) Classification and grading of gastritis: the updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 20:1161–1181
McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I (2007) Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomark Prev 16:815–819
Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, et al. (1997) Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomark Prev 6:639–642
Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. (2004) China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291:187–194
Hosokawa O, Tsuda S, Kidani E, Watanabe K, Tanigawa Y, Shirasaki S, et al. (1998) Diagnosis of gastric cancer up to three years after negative upper gastrointestinal endoscopy. Endoscopy 30:721–773
Aslan R, Bektas A, Bedir A, Alacam H, Aslan MS, Nar R, et al. (2013) Helicobacter pylori eradication increases telomere length in gastric mucosa. Hepato-Gastroenterology 60:601–604
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 54 kb)
Rights and permissions
About this article
Cite this article
Tahara, T., Shibata, T., Kawamura, T. et al. Telomere length shortening in gastric mucosa is a field effect associated with increased risk of gastric cancer. Virchows Arch 469, 19–24 (2016). https://doi.org/10.1007/s00428-016-1948-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-1948-3