Abstract
The expression of EphA2 and three epithelial–mesenchymal transition-related proteins (E-cadherin, β-catenin and vimentin) was detected by immunohistochemistry in human gastric cancer and normal gastric mucosa. The expression of EphA2 and vimentin was significantly higher in gastric cancer tissues than in normal gastric mucosa tissues, and similar results were found for negative E-cadherin expression and ectopic β-catenin expression. Further analysis showed that the expression of EphA2 was closely correlated with the depth of tumor invasion, tumor–node–metastasis (TNM) stages and lymph node metastasis. Down-regulated expression of the epithelial protein E-cadherin, overexpression of the mesenchymal protein vimentin and ectopic expression of β-catenin were associated with the depth of tumor invasion, tumor differentiation, TNM stages and lymph node metastasis. The Spearman rank test indicated that the positive expression of EphA2 was negatively associated with E-cadherin expression and was positively correlated with β-catenin ectopic expression and vimentin expression. In addition, the Kaplan–Meier survival analysis showed that the overexpression of EphA2 and vimentin, ectopic expression of β-catenin and down-regulation of E-cadherin indicate a poor outcome. Moreover, multivariate Cox analysis showed that TNM stages, lymph node metastasis, EphA2 expression, E-cadherin expression and β-catenin ectopic expression were independent prognostic factors for postoperative gastric cancer. These findings indicate that the overexpression of EphA2 correlates with the loss of epithelial proteins and the appearance of mesenchymal proteins. Therefore, EphA2 may play a role in epithelial–mesenchymal transition in gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is one of the most common carcinomas in the world and has a poor prognosis [1]. In gastric cancer patients, invasion, metastasis and recurrence are the major causes of death [2]. However, the mechanism of the development, invasion and metastasis of gastric cancer is still not clear.
Originally, EphA2, also known as epithelial cell kinase (Eck), was found in adult human epithelial cells [3]. As a member of the family of receptor tyrosine kinases (RTK), EphA2 plays a critical role in embryonic patterning, neuronal targeting and vascular and tumor development, and it is an attractive target for cancer therapy [4]. The overexpression of EphA2 has been discovered in many cancers and is associated with primary tumor initiation, progression, angiogenesis and metastasis [5]. In our previous study [6], we found that EphA2 is highly expressed in human gastric cancer and is correlated with the depth of tumor invasion, TNM stages and lymph node metastasis. Meanwhile, the expression of E-cadherin negatively correlates with that of EphA2.
Epithelial–mesenchymal transition (EMT) was first recognized as a central feature of normal development and plays an important role in embryonic development, tumor invasion and metastasis [7]. In epithelial carcinomas, EMT endows tumor cells with the ability to leave the primary tumor and invade into the local tissue and blood vessels, setting the stage for metastatic spread [8].
As an epithelial marker and a cell adhesion molecule, E-cadherin acts as a tumor suppressor that inhibits invasion and metastasis, and the loss of E-cadherin expression is considered to be a common indicator of the onset of EMT [9].
Nuclear accumulation of β-catenin has been associated with EMT [10] and indicates high tumor invasion and metastasis. β-Catenin functions in a dual manner in epithelial cells, depending on its intracellular localization. In epithelial cells, β-catenin is an important component of adherens junctions, acting in cell–cell adhesion by linking E-cadherin at the cell membrane [11].
Vimentin is a mesenchymal marker that is most frequently associated with EMT. The upregulation of mesenchymal markers, particularly vimentin or Fibronectin, is accompanied by an increase in cell migration and invasion [12].
RTK/Ras signaling has been found to contribute to EMT. The repression of E-cadherin has emerged as one important step driving EMT, and this stage is currently linked with many new molecules [13]. EphA2 is a component of RTK/Ras signaling, and our early research has revealed the basic relationship between EphA2 and E-cadherin in human gastric cancer. However, whether EphA2 has a connection with EMT in gastric cancer remains unclear.
In the present study, the expression of EphA2 and three epithelial–mesenchymal transition markers (E-cadherin, β-catenin and vimentin) was detected in gastric cancer tissues, and their clinical and pathological importance and prognostic values were investigated.
Materials and methods
Patient selection and tissue preparation
Between January 2003 and June 2005, 158 gastric cancer tissue samples were acquired from the patients who experienced radical resection in the Department of General Surgery of Xiangya Hospital in Central South University, which included 105 men and 53 women, between 30 and 77 years of age (mean age of 52.7 years). Control samples (normal gastric mucosa) were collected from each patient in the operation at a distance of 6 cm from the tumor. Fresh specimens were first put into 10% neutral buffered formalin for 24 h and were then embedded in paraffin. None of the patients had radiotherapy or chemotherapy before surgery. The study was approved by the ethics committee of Central South University in accordance with the ethical standard of the Helsinki Declaration of 1975, as revised in 2000.
Immunohistochemical methods and analyses
Paraffin-embedded samples were sectioned at 4 μm thickness for collection. Immunohistochemical staining was carried out under the guide of the Histostain®-Plus kits (Zymed, Carlsbad, USA). The samples were then dewaxed in xylene and rehydrated in a graded ethanol series. The sections were immersed in 2.5% hydrogen peroxide for 30 min at room temperature to block the endogenous peroxidase activity. Afterward, the slides were placed in citrate buffer at 100°C for 10 min for antigen retrieval. The sections were incubated at 4°C overnight with the following antibodies: rabbit EphA2 polyclonal antibody (sc-924, dilution 1:200, Santa Cruz, CA, USA), rabbit E-cadherin polyclonal antibody (sc-7870, dilution 1:200, Santa Cruz, CA, USA), mouse vimentin monoclonal antibody (sc-6260, dilution 1:100, Santa Cruz, CA, USA) and mouse β-catenin monoclonal antibody (sc-7963, dilution 1:100, Santa Cruz, CA, USA). After washing with PBS, the slides were incubated with the secondary antibody for 15 min at 37°C. The slides were washed with PBS again, horseradish peroxidase-conjugated streptavidin was added, and the slides were then incubated for 15 min at 37°C. Immunoreactive proteins were visualized with diaminobenzidine and were then counterstained with hematoxylin. The primary antibody was substituted with PBS in negative control. In addition, EphA2-positive breast carcinoma, E-cadherin-positive normal breast cell, β-catenin-positive liver carcinoma and vimentin-positive breast carcinoma were used as positive controls.
For the EphA2 staining, the sections were graded according to the percentage of positive cells: 0 points = 0–5%, 2 points = 6–50% and 3 points = more than 50%. The staining intensity was rated as 1 point for weak intensity, 2 points for moderate intensity and 3 points for strong intensity. The scores of these two methods were summed; a sum of scores ≥3 points was considered to be positive, while a sum of scores <3 points was regarded as negative [14, 15].
For E-cadherin and β-catenin staining, the slides were rated by the staining intensity of the positive control core specimens: negative staining (−); cytoplasmic staining (+); heterogeneous staining (++); and normal membranous staining (+++) [16]. All specimens without membranous E-cadherin expression were regarded as negative [17]. β-Catenin membranous staining (>90%) and cytoplasmic and nuclear (>10%) staining were considered to be positive β-catenin expression. We classify negative expression of β-catenin and positive β-catenin expression in the cell membrane and cytoplasm as normal, and its expression in nucleus was defined as ectopic expression.
Vimentin is a special marker that is highly expressed in stromal and inflammatory cells, but is not usually present in epithelial cells [18]. Therefore, the expression of vimentin in tumor cells was considered to be positive in all slides.
Statistical analysis
The χ2-test was used to analyze the differences in the protein expression patterns between the normal mucosa group and the cancer group. The relationship between the expression of each marker and clinicopathologic variables was also analyzed with the χ2-test. The nonparametric Spearman rank correlation coefficient was applied to evaluate the relationship between EphA2, E-cadherin, β-catenin and vimentin expression. The survival rate was estimated using the Kaplan–Meier method, and the statistical significance of the differences was compared using the log-rank test. The overall survival time was calculated from the date of surgery to the date of death (resulting from any cause) or June 30, 2010. The log-rank test was used for the univariate analysis, and a Cox proportional hazards regression model was applied for the multivariate analysis. All statistical analyses were performed with SPSS software (version 16.0 for windows). Statistical significance was considered at P < 0.05.
Results
Expression of EphA2, E-cadherin, β-catenin and vimentin in gastric cancer and normal gastric mucosa
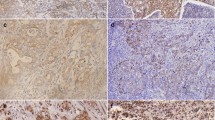
Among all of the 158 gastric cancer samples, positive EphA2 immunostaining was most obviously detected in the cytoplasm. In normal gastric mucosa, EphA2 positive staining scarcely emerged (Fig. 1a, b). The proportion of EphA2 positive expression was 60.8% (96/158) in gastric cancer and was 44.9% (71/158) in normal gastric mucosa. By way of the χ2-test, a conspicuous difference in EphA2 expression between the gastric cancer group and the normal gastric mucosa group (P < 0.01) was present (Table 1).
Immunostaining of EphA2, E-cadherin, β-catenin and vimentin in gastric cancer and normal gastric mucosa tissues. a Negative immunostaining of EphA2 in normal gastric mucosa. b Positive cytoplasmic staining of EphA2 in gastric cancer. c Positive membranous staining of E-cadherin in normal gastric mucosa. d Negative immunostaining of E-cadherin in gastric cancer. e Positive membranous staining of β-catenin in normal gastric mucosa. f Positive cytoplasmic and nuclear staining of β-catenin in gastric cancer. g Negative immunostaining of vimentin in normal gastric mucosa. h Positive cytoplasmic staining of vimentin in gastric cancer. (SP method; original magnification: ×400)
Normally, positive E-cadherin expression can be found in the membrane of epithelial cells. The percentage of E-cadherin positive expression was 30.4% (48/158) in gastric cancer and 56.3% (89/158) in normal gastric mucosa. Through the χ2-test, a significant difference in E-cadherin expression between the cancer group and the normal mucosa group (P < 0.001) was detected (Table 1). Representative immunohistochemical staining of E-cadherin is shown in Fig. 1c, d.
β-Catenin expression can be detected in the cell membrane, cytoplasm and nucleus. The expression of β-catenin in the cell membrane and cytoplasm was defined as normal; in contrast, its expression in the nucleus was regarded as ectopic expression. In gastric cancer tissues, 66.5% (105/158) displayed β-catenin positive expression. In 105 β-catenin-positive sections, 36 sections showed β-catenin ectopic expression, which was a rate of 22.8% (36/158) of all of the cancer tissues. In normal gastric mucosa tissues, 59.5% (94/158) contained β-catenin positive expression, and only 6.3% (10/158) of all the normal gastric mucosa tissues had β-catenin ectopic expression. The χ2-test indicates no significant difference in the β-catenin positive expression between the cancer group and the normal gastric mucosa group (P > 0.05). However, distinct disparity can be found in β-catenin ectopic expression between the two groups (P < 0.001) (Table 1). The immunohistochemical staining of β-catenin is shown in Fig. 1e, f.
Positive vimentin immunostaining was distributed in the epithelial cell cytoplasm. The ratio of vimentin positive expression was 17.1% (27/158) in gastric cancer and only 7.0% (11/158) in normal gastric mucosa. The χ2-test manifests a significant difference in the vimentin positive expression between the gastric cancer group and the normal gastric mucosa group (P < 0.01) (Table 1). Vimentin immunohistochemical staining is shown in Fig. 1g, h.
Correlations between EphA2, E-cadherin, β-catenin and vimentin expression and clinicopathologic characteristics
The correlations between the expression of EphA2, E-cadherin, β-catenin and vimentin and the clinicopathological characteristics of gastric cancer patients are shown in Table 2. The overexpression of EphA2 was closely related to the depth of invasion (χ2 = 6.459, P = 0.011), TNM stage (χ2 = 4.590, P = 0.032) and lymph node metastasis (χ2 = 4.444, P = 0.035), but no significant correlation was present with patients’ age, gender, tumor size, location and differentiation (P > 0.05). The down-regulated expression of E-cadherin was associated with tumor differentiation (χ2 = 4.435, P = 0.035), depth of invasion (χ2 = 7.162, P = 0.007), TNM stage (χ2 = 9.251, P = 0.002) and lymph node metastasis (χ2 = 5.664, P = 0.017), but no correlation was present with patients’ age, gender, tumor size and location (P > 0.05). The positive expression of β-catenin correlated only with lymph node metastasis (χ2 = 5.754, P = 0.016), but no association with patients’ age, gender tumor size, location, differentiation, depth of invasion and TNM stage was present (P > 0.05). The ectopic expression of β-catenin correlated with tumor differentiation (χ2 = 6.607, P = 0.010), depth of invasion (χ2 = 4.084, P = 0.043), TNM stage (χ = 7.041, P = 0.008) and lymph node metastasis (χ2 = 4.029, P = 0.045), but no association with patients’ age, gender tumor size and location was present (P > 0.05). A significant correlation between the positive expression of vimentin and tumor differentiation (χ2 = 8.375, P = 0.004), depth of invasion (χ2 = 5.431, P = 0.020), TNM stage (χ2 = 4.457, P = 0.035) and lymph node metastasis (χ2 = 6.652, P = 0.010) was present, but no correlation existed with patients’ age, gender, tumor size and location (P > 0.05).
Correlation between EphA2, E-cadherin, β-catenin and vimentin expression in gastric cancer
The Spearman rank test indicated that the positive expression of EphA2 was negatively correlated with E-cadherin (r = −0.625, P < 0.001) and that the expression of EphA2 was positively associated with β-catenin ectopic expression (r = 0.375, P < 0.001) and was positively correlated with vimentin expression (r = 0.330, P < 0.001) (Table 3).
Survival analysis
In the present study, by using the Kaplan–Meier method with the log-rank test, the overall 5-year survival rate of patients with positive EphA2 expression was significantly lower than those with negative expression (P = 0.000, Fig. 2a). The patients with a loss of E-cadherin expression had a shorter survival duration (P = 0.000, Fig. 2b). Similarly, the patients with ectopic β-catenin expression had a significantly lower overall 5-year survival rate than those with normal expression (P = 0.020, Fig. 2c). However, positive β-catenin expression had no significant effect on patients’ 5-year survival rate (P = 0.085, Fig. 2d). Compared with the group with negative vimentin expression, those patients with positive vimentin expression had a lower 5-year survival rate (P = 0.029, Fig. 2e).
The 5-year survival curves of patients with gastric cancer according to the immunostaining results of EphA2, E-cadherin, β-catenin and vimentin determined by the Kaplan–Meier analysis. a The EphA2 positive expression group (median 19 months) had a shorter survival duration than the EphA2 negative expression group (median 31 months) (P = 0.000). b The E-cadherin positive expression group (median 35 months) had a longer survival duration than the EphA2 negative expression group (median 19 months) (P = 0.000). c The β-catenin ectopic expression group (median 18 months) had a shorter survival duration than the β-catenin normal expression group (median 23 months) (P = 0.020). d No significant difference in survival duration between the β-catenin positive expression group (median 22 months) and the β-catenin negative expression group (median 24 months) (P = 0.085). e The vimentin positive expression group (median 18 months) had a shorter survival duration than the vimentin negative expression group (median 23 months) (P = 0.029)
By way of the COX multivariate analysis, TNM stages, lymph node metastasis, EphA2 expression, E-cadherin expression and β-catenin ectopic expression were found to be independent prognostic factors for human gastric cancer after resection (P < 0.05, Table 4).
Discussion
In the present study, we detected the expression of EphA2 and the EMT-related proteins E-cadherin, β-catenin and vimentin in gastric cancer, and we exposed the relationship between these markers and the prognosis of the patients.
We found that the expression of EphA2 was significantly higher in gastric cancer than in normal gastric mucosa. Furthermore, the expression patterns of EphA2 correlated with tumor progression and lymphogenous metastasis in gastric cancer.
Moreover, the overexpression of EphA2 was also found to be significantly associated with the prognosis of the patients who had undergone operations. These findings are consistent with our previous study [6] and some other studies of hepatocellular carcinoma [19, 20], nonsmall cell lung cancer [21], glioblastoma multiforme [22], endometrial cancer [23], ovarian cancer [24] and colorectal cancer [25].
Although there have been many studies of EphA2 expression in carcinomas, the research has seldom investigated the relationship between EphA2 and EMT. EMT plays an important role in tumor progression of epithelial cancers [26–28]. The loss of a polarized epithelial phenotype and the acquisition of mesenchymal properties endow tumor cells with the capacity to invade surrounding tissues and metastasize [27]. Therefore, we focused our efforts on assessing the expression of three EMT-related proteins, E-cadherin, β-catenin and vimentin, in gastric cancer.
The loss of E-cadherin is defined as a significant characteristic of EMT [29]. This study found that E-cadherin was down-regulated in gastric cancer compared with normal gastric mucosa and its down-regulation of expression was closely associated with tumor differentiation, depth of invasion, TNM stage and lymph node metastasis. According to Almeida [30], the expression patterns of E-cadherin significantly correlate with the histotypes of gastric carcinoma. Reduced expression of E-cadherin has been reported to be positively related to poor tumor differentiation and a deeper tumor invasion [31]. The detection of missing E-cadherin may be helpful in predicting the capability of tumor metastasis in gastric cancer. In approximately 50% of diffuse-type carcinomas, such as diffuse gastric carcinoma, an inactivating mutation in E-cadherin exists. However, in the most common nondiffuse-type carcinomas, a common mechanism is probably the silencing of E-cadherin as a result of hypermethylation of the E-cadherin promoter [32].
Immunohistochemistry was performed to detect β-catenin expression in gastric cancer tissues and to determine whether expression correlates with clinicopathologic characteristics. The ectopic expression of β-catenin was closely related to tumor differentiation, depth of invasion, TNM stage and lymph node metastasis. In addition, ectopic β-catenin expression predicted a poor outcome. Under normal conditions, β-catenin was associated with E-cadherin to maintain cell-to-cell adhesion at the cell membrane. β-Catenin has an established role as an essential mediator of the canonical WNT signal pathway [33]. In the absence of WNT signals, cytosolic β-catenin is targeted for proteasomal breakdown by the APC–Axin–GSK complex. When the WNT pathway is activated, binding of WNT molecules to the frizzled receptors inhibits the activity of the destruction complex and allows β-catenin to accumulate and to translocate to the nucleus [34–36]. After translocation to the nucleus and binding to TCF, β-catenin promotes transcriptional activation of specific target oncogenes, such as cyclin D1 or c-myc, which are associated with proliferation [37, 38]. In gastric cancer, strong nuclear accumulation of β-catenin was detected at the invasive front, demonstrating that nuclear translocation of β-catenin plays an essential role in the process of tumor invasion [39] and that abnormal β-catenin would be a useful prognostic marker for gastric carcinoma patients [40]. Similar results have also been found in other carcinomas, such as colorectal cancer [41] and ampullary cancer [42].
Our study demonstrates that vimentin positive expression in gastric cancer is associated with tumor differentiation, depth of invasion, TNM stage and lymph node metastasis. These results suggest that vimentin acts as a mesenchymal marker and may play an important role in tumor invasion and metastasis in gastric cancer. Our results were identical to a previous study that discovered that the acquisition of vimentin was associated with poorly differentiated histology, advanced stage, lymph node metastasis and lymphatic invasion in gastric cancer [43, 44]. Additionally, in epithelial cancer, vimentin expression and a perturbation of E-cadherin-mediated cell adhesion both appear to be markers of EMT-associated events in cell invasion and migration [45]. Moreover, vimentin is also a target of the β-catenin/TCF pathway, and its regulation plays a role during epithelial cell migration [46].
On the basis of the above results, we assessed the connection between EphA2 and EMT-related hallmarks, E-cadherin, β-catenin and vimentin. We found that the expression of EphA2 was inversely correlated with that of E-cadherin. However, the mechanism of interaction between EphA2 and E-cadherin is not yet clear. In human mammary epithelial cells, the overexpression of EphA2 weakens E-cadherin-mediated cell–cell adhesion [47]. Conversely, VE-cadherin can regulate the expression of EphA2 at the cell membrane by mediating its ability to become phosphorylated through interactions with its membrane-bound ligand, ephrin-A1 [48]. Furthermore, EphA2 expression was positively associated with β-catenin ectopic expression and vimentin expression. To the best of our knowledge, this is the first report that investigates the relationship between EphA2 and EMT-related proteins in gastric cancer.
The survival analysis demonstrated that the gastric cancer patients with negative EphA2 expression had higher survival rates than those with positive EphA2 expression, and EphA2 expression was an independent prognostic factor. This result is consistent with our previous studies in gastric carcinoma [49] and hepatocellular carcinoma [19]. In addition, negative E-cadherin expression, ectopic β-catenin expression and positive vimentin expression also correlated with a poor outcome for the gastric cancer patients. Similar results have been reported in a study by Kim [43].
In conclusion, a high expression level of EphA2 is associated with EMT-related markers in gastric cancer tissues. The overexpression of EphA2, the loss of epithelial markers and the appearance of mesenchymal markers indicate poor prognosis. EphA2, as an epithelial cell kinase, may play a role in the process of EMT.
References
Hohenberger P, Gretschel S. Gastric cancer. Lancet. 2003;362:305–15.
Dicken BJ, Bigam DL, Cass C, et al. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39.
Hess AR, Seftor EA, Gardner LM, et al. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2). Cancer Res. 2001;61:3250–5.
Ireton RC. Chen J: EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5:149–57.
Lu C, Shahzad MM, Wang H, et al. EphA2 overexpression promotes ovarian cancer growth. Cancer Biol Ther. 2008;7:1098–103.
Yuan W, Chen Z, Wu S, et al. Expression of EphA2 and E-cadherin in gastric cancer: correlated with tumor progression and lymphogenous metastasis. Pathol Oncol Res. 2009;15:473–8.
Thiery JP, Acloque H, Huang RY, et al. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139:871–90.
Micalizzi DS, Farabaugh SM, Ford HL. Epithelial–mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–34.
Tomita K, van Bokhoven A, van Leenders GJ, et al. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60:3650–4.
Schmalhofer O, Brabletz S, Brabletz T. E-cadherin beta-catenin and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–66.
Benjamin JM, Kwiatkowski AV, Yang C, et al. AlphaE-catenin regulates actin dynamics independently of cadherin-mediated cell–cell adhesion. J Cell Biol. 2010;189:339–52.
Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-Mesenchymal Transition in Cancer: Parallels Between Normal Development and Tumor Progression. J Mammary Gland Biol Neoplasia. 2010;15:117–34.
Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58.
Thaker PH, Deavers M, Celestino J, et al. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145–50.
Lin YG, Han LY, Kamat AA, et al. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer. 2007;109:332–40.
Jawhari A, Jordan S, Poole S, et al. Abnormal immunoreactivity of the E-cadherin-catenin complex in gastric carcinoma: relationship with patient survival. Gastroenterology. 1997;112:46–54.
Zhou Y, Ran J, Tang C. Effect of celecoxib on E-cadherin, VEGF. Microvessel density and apoptosis in gastric cancer. Cancer Biol Ther. 2007;6:269–75.
Prudkin L, Liu DD, Ozburn NC, et al. Epithelial–mesenchymal transition in the development and progression of adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol. 2009;22:668–78.
Yang P, Yuan W, He J, et al. Overexpression of EphA2, MMP-9 and MVD-CD34 in hepatocellular carcinoma: implications for tumor progression and prognosis. Heptol Res. 2009;39:1169–77.
Cui XD, Lee MJ, Yu GR, et al. EFNA1 ligand and its receptor EphA2: potential biomarkers for hepatocellular carcinoma. Int J Cancer. 2010;126:940–9.
Brannan JM, Dong W, Prudkin L, et al. Expression of the receptor tyrosine kinase EphA2 is increased in smokers and predicts poor survival in non-small cell lung cancer. Clin Cancer Res. 2009;15:4423–30.
Wang LF, Fokas E, Bieker M, et al. Increased expression of EphA2 correlates with adverse outcome in primary and recurrent glioblastoma multiforme patients. Oncol Rep. 2008;19:151–6.
Kamat AA, Coffey D, Merritt WM, et al. EphA2 overexpression is associated with lack of hormone receptor expression and poor outcome in endometrial cancer. Cancer. 2009;115:2684–92.
Merrit WM, Thaker PH, Landen CN Jr, et al. Analysis of EphA2 expression and mutant p53 in ovarian carcinoma. Cancer Biol Ther. 2006;5:1357–60.
Kataoka H, Igarashi H, Kanamori M, et al. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci. 2004;95:136–41.
Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–18.
Thiery JP. Epithelial-mesenchymal transition in tumor progression. Nat Rev Cancer. 2002;2:442–54.
Nieto MA. Epithelial–mesenchymal transitions in development and disease: old views and new perspectives. Int J Dev Biol. 2009;53:1541–7.
Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33.
Almeida PR, Ferreira VA, Santos CC, et al. E-cadherin immunoexpression patterns in the characterisation of gastric carcinoma histotypes. J Clin Pathol. 2010;63:635–9.
Zhou Y, Li G, Wu J, et al. Clinicopathological significance of E-cadherin, VEGF, and MMPs in gastric cancer. Tumour Biol. 2010;31:549–58.
Guarino M, Rubino B, Ballabio G. The role of epithelial–mesenchymal transition in cancer pathology. Pathology. 2007;39:305–18.
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26.
Liu C, Li Y, Semenov M, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47.
Song S, Mazurek N, Liu C, et al. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009;69:1343–9.
Fukuyama R, Niculaita R, Ng KP, et al. Mutated in colorectal cancer, a putative tumor suppressor for serrated colorectal cancer, selectively represses beta-catenin-dependent transcription. Oncogene. 2008;27:6044–55.
Lovatt M, Bijlmakers MJ. Stabilisation of β-catenin downstream of T cell receptor signaling. PLoS One. 2010;5:e12794.
Wang Q, Sun ZX, Allgayer H, et al. Downregulation of E-cadherin is an essential event in activating beta-catenin/Tcf-dependent transcription and expression of its target genes in Pdcd4 knockdown cells. Oncogene. 2010;29:128–38.
Miyazawa K, Iwaya K, Kroda M, et al. Nuclear accumulation of beta-catenin in intestinal-type gastric carcinoma: correlation with early tumor invasion. Virchows Arch. 2000;437:508–13.
Zhou YN, Xu CP, Han B, et al. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8:987–93.
Wong SC, Lo ES, Lee KC, et al. Prognostic and diagnostic significance of beta-catenin nuclear immunostaining in colorectal cancer. Clin Cancer Res. 2004;10:1401–8.
Hsu HP, Shan YS, Jin YT, et al. Loss of E-cadherin and beta-catenin is correlated with poor prognosis of ampullary neoplasms. J Surg Oncol. 2010;101:356–62.
Kim MA, Lee HS, Lee HE, et al. Prognostic importance of epithelial-mesenchymal transition-related protein expression in gastric carcinoma. Histopathology. 2009;54:442–51.
Fuyuhiro Y, Yashiro M, Noda S, et al. Clinical significance of vimentin-positive gastric cancer cells. Anticancer Res. 2010;30:5239–43.
Polette M, Gilles C, de Bentzmann S, et al. Association of fibroblastoid features with the invasive phenotype in human bronchial cancer cell lines. Clin Exp Metastasis. 1998;16:105–12.
Gilles C, Polette M, Mestdagt M, et al. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res. 2003;63:2658–64.
Fang WB, Ireton RC, Zhuang G, et al. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci. 2008;121:358–68.
Hess AR, Seftor EA, Gruman LM, et al. VE-cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biol Ther. 2006;5:228–33.
Yuan WJ, Ge J, Chen ZK, et al. Over-expression of EphA2 and EphrinA-1 in human gastric adenocarcinoma and its prognostic value for postoperative patients. Dig Dis Sci. 2009;54:2410–7.
Acknowledgments
This study was supported by the National Nature Foundation of China (No. 81172297).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, F., Yuan, W., Huang, J. et al. Overexpression of EphA2 correlates with epithelial–mesenchymal transition-related proteins in gastric cancer and their prognostic importance for postoperative patients. Med Oncol 29, 2691–2700 (2012). https://doi.org/10.1007/s12032-011-0127-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-011-0127-2