Abstract
Background
Epithelial-mesenchymal transition (EMT), when epithelial cells convert to mesenchymal cells, influences cancer invasion and metastasis. Smad interacting protein 1 (SIP1) is an EMT trigger, which is inversely correlated with E-cadherin in some carcinomas. To elucidate the role of SIP1 in esophageal squamous cell carcinoma (ESCC), the status of EMT and the clinicopathological features were evaluated.
Methods
Immunohistochemical (IHC) analyses of 111 human ESCC tissue specimens for SIP1 and E-cadherin were performed, and the relationships between the expression and clinicopathological features were evaluated.
Results
IHC analyses of esophageal tumors showed the expression of SIP1 and E-cadherin to be significantly inversely correlated. Significant correlations between the SIP1 expression and clinicopathological variables such as differentiation, depth of invasion, vascular invasion, and pathological stage were also seen. Conversely, tumors with a weak expression of E-cadherin tended to exhibit greater histological differentiation. Logistic regression analyses revealed a positive SIP1 expression, lymphatic invasion, and vascular invasion to be factors predicting lymph node (LN) metastasis. Univariate survival analyses revealed a positive SIP1 expression predicted a poorer overall survival than a negative expression.
Conclusion
These results suggest that SIP1 is correlated with LN metastasis and may therefore be an independent marker for metastasis in patients with ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Esophageal squamous cell carcinoma (ESCC) is still associated with a poor prognosis because of the high frequency of lymph node (LN) metastasis and invasion to neighboring organs.1 One of the most representative features of a malignant tumor is its invasiveness and tendency to metastasize.
The process by which an epithelial cell loses its epithelial properties and acquires the characteristics of mesenchymal cells (such as fibroblasts)2–4 is a phenomenon referred to as epithelial-mesenchymal transition (EMT). The loss of E-cadherin is one of the most important features of EMT, which is considered to contribute to cancer metastasis.5
E-cadherin is expressed mainly on the epithelial cell membrane and is required for cell–cell adhesion;6 therefore, E-cadherin plays a crucial role in the suppression of tumor invasion.7 Because the functional loss of E-cadherin leads to the disruption of adherens junctions, cell migration from the original location, marked phenotypic changes, and a highly motile fibroblastoid, mesenchymal phenotype, allowing the cells to move through the extracellular matrix, a loss of E-cadherin is believed to be one of the hallmarks of EMT.8 The transcriptional repression is one of the most important mechanisms in the downregulation of E-cadherin expression.
The expression of E-cadherin is directly or indirectly regulated by various factors, such as snail, slug, and twist, and is repressed via their cell- or tissue-type-dependent binding to the promoter of E-cadherin.9–12 In other words, these factors are considered to trigger EMT. Among these proteins, Smad interacting protein 1 (SIP1), identical to ZEB2, which has been implicated in transforming growth factor-β signaling,13,14 binds to the E-cadherin promoter, thus resulting in downregulation of its promoter activities.14–16 As such, SIP1 is also one of the key proteins that trigger EMT.17
The downregulation of E-cadherin expression by snail promotes the invasiveness of human hepatocellular carcinoma.18 The expression of snail, slug, and twist have been reported to be inversely correlated with that of E-cadherin in ESCC, and all of these contribute to the aggressive clinicopathological features and poor prognosis associated with the disease.12,19,20 The expression of SIP1 also contributes to tumor progression and poor prognosis in oral squamous cell carcinoma (SCC).21 Nevertheless, the role of SIP1 in ESCC is unknown. We hypothesized that SIP1 expression in ESCC is involved in EMT, and consequently leads to cancer invasion and metastasis.
Materials and Methods
Patients
Overall, 111 patients with ESCC were examined in this study. All ESCC tissues were obtained from patients who underwent esophagectomy without any preoperative therapy in the Department of Surgery and Science, Kyushu University Hospital from 1992 to 2002. Adjuvant chemotherapy was not applied routinely. Ethical approval for this study was obtained from the Institutional Review Board (#21-95) of Kyushu University. Histopathological diagnosis was determined according to the TNM classification.22
Immunohistochemistry
Specimens with the deepest site of the cancerous lesion were selected for immunohistochemical (IHC) staining. IHC staining of SIP1 and E-cadherin was analyzed as previously described.23 Briefly, sections measuring 3-μm thickness were deparaffinized and rehydrated.
For SIP1, microwave heat-induced epitope retrieval was performed for 15 min at 95 °C in citrate buffer (pH 6), followed by immersion in 3 % hydrogen peroxide in 100 % ethanol for 30 min to inhibit the endogenous peroxidase activity. After being incubated with normal rabbit serum for 10 min, the sections were then incubated with a goat polyclonal antibody against SIP1 (1:50; sc-18392: Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4 °C. The streptavidin–biotin method and Histofine SAB-PO (goat) kits (Nichirei Corporation, Tokyo, Japan) were used. The sections were then incubated with biotinylated rabbit anti-goat immunoglobulins G, A, and M (Nichirei Corporation) for 20 min. The slides were treated with peroxidase-conjugated streptavidin for 20 min. The slides were developed by immersion into 0.01 % H2O2 and 0.05 % diaminobenzidine tetrahydrochloride for 4 min, and counterstained with Meyer’s hematoxylin.
For E-cadherin, the epitope retrieval was performed with an autoclave at 121 °C for 15 min in citrate buffer (pH 6). The sections were incubated with a mouse anti-human monoclonal E-cadherin antibody (2 μg/ml; Takara Bio Inc., Shiga, Japan) overnight at 4 °C and labeled with the Envision detection system (Dako Ltd., Glostrup, Denmark). The slides were developed by immersion into 0.01 % H2O2 and 0.05 % diaminobenzidine tetrahydrochloride for 5 min, and counterstained with Meyer’s hematoxylin.
Evaluation of Immunohistochemical Staining
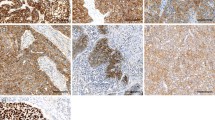
To evaluate the SIP1 and E-cadherin expression, the staining intensity was scored as 0, 1 (weak), 2 (medium), or 3 (strong) (Fig. 1). The extent of staining was scored as 0 (0 %), 1 (1–25 %), 2 (26–50 %), 3 (51–75 %), or 4 (76–100 %) according to the percentage of the positively stained area relative to the total tumor area.24 The value obtained by multiplication of the intensity and extent scores was used as the final staining score (0–12) for SIP1 and E-cadherin. For SIP1 staining, tumors having a final staining score of 0–2 or 3–12 were considered to be negative or positive for expression, respectively. On the other hand, a final staining score of 0–2 or 3–12 was considered to be weak or retained expression for E-cadherin, respectively. The scoring procedure was carried out twice each by three independent observers (RY, MM, YN; each blinded to each others’ scores) without any knowledge of the clinical parameters or other prognostic factors.
The immunohistochemical detection of SIP1 and E-cadherin in esophageal squamous cell carcinoma (a–d). a Positive expression of SIP1 was detected in the cytoplasma. b Negative expression of SIP1. c Retained expression of E-cadherin was detected on the cell membrane. d Weak expression of E-cadherin. SIP1 Smad interacting protein 1
Statistical Analysis
Student’s t test, the Chi square test, and Fisher’s exact test, when appropriate, were used to compare the clinicopathological data. A p value <0.05 was considered to indicate a statistically significant difference. The independent factors associated with LN metastasis were evaluated with a logistic regression analysis. Survival rates were calculated using the Kaplan–Meier method, and differences between survival curves were examined with the log-rank test. All statistical analyses were performing using the statistical package StatView version 5.0 (SAS Institute Inc. Cary, NC, USA).
Results
Expression of Smad Interacting Protein 1 (SIP1) and E-cadherin
SIP1 expression was detected in the cytoplasm of the cancer cells, whereas E-cadherin expression was detected at the cell membrane (Fig. 1). Thirty-five tumors (31.5 %) had positive IHC expression of SIP1. On the other hand, membranous expression of E-cadherin was retained in 53 tumors (47.7 %) (Table 1). The E-cadherin expression was weak in 24 (68.6 %) of the 35 cases positive for SIP1 expression, and weak in 34 (44.7 %) of the 76 cases negative for SIP1. Therefore, the expression of SIP1 and E-cadherin was significantly inversely correlated (p = 0.0195).
Clinicopathological Analysis
The association between the clinicopathological variables and IHC expression of SIP1 and E-cadherin are shown in Table 1. Significant correlations were observed between SIP1 expression and clinicopathological variables such as histological differentiation, histological depth of invasion, LN metastasis, lymphatic invasion, vascular invasion, and pathological stage (p = 0.0014, 0.0003, 0.0007, 0.0322, 0.0046, and 0.0014, respectively), although there were no relationships between SIP1 expression and age, sex, and distant metastasis. On the other hand, only histological differentiation was related to tumors with weak expression of E-cadherin (p = 0.0395).
A logistic regression analysis revealed that positive SIP1 expression, lymphatic invasion, and vascular invasion were factors predicting LN metastasis (Table 2).
Univariate Analysis for Survival
Survival analysis according to the Kaplan–Meier method revealed that both the overall survival and disease-free survival rate of patients in the SIP1-positive group were significantly poorer than those of patients in the SIP1-negative group (p = 0.019 and 0.001, respectively; Fig. 2). The 5-year survival rate was 31.7 % in the SIP1-positive group compared with 60.3 % in the SIP1-negative group. The disease-free survival rate was 27.4 % in the SIP1-positive group compared with 58.6 % in the SIP1-negative group.
Comparison of the Kaplan–Meier survival curves in the SIP1-positive group versus the SIP1-negative group. a Overall survival rates. b Disease-free survival rates. Both survival rates of patients with SIP1-positive tumors (bold line) were significantly worse than those of patients with SIP1-negative tumors (thin line) (p = 0.019 and 0.001, respectively). SIP1 Smad interacting protein 1
Discussion
The aim of this study was to elucidate the relationship between SIP1 and E-cadherin, and to determine the clinical significance of SIP1 in ESCC. We have immunohistochemically shown that SIP1 expression is inversely correlated with E-cadherin expression, and that its expression is associated with advanced tumor properties and a poor prognosis in ESCC. The IHC inverse correlation between SIP1 and E-cadherin expression has previously been found in gastric cancer, renal cell carcinoma, and oral SCC.11,21,25,26 We previously demonstrated that SIP1 is inversely associated with E-cadherin and that SIP1-positive patients have a poorer prognosis in lung cancer. The relationship was more remarkable in SCC than in adenocarcinoma.23 As a result, our data showing a reciprocal correlation between SIP1 and E-cadherin in ESCC are consistent with the findings of the previous immunohistochemical study. In addition, SIP1 messenger RNA (mRNA) expression has previously been reported to be inversely correlated with E-cadherin mRNA in glioma, pancreatic cancer,and bladder cancer cell lines.27–29
Considering the role of SIP1 as a repressor of E-cadherin, SIP1 expression and concurrent repression of E-cadherin probably result in an advanced tumor profile. Snail, another repressor of E-cadherin, was also reported to promote cancer invasion and metastasis.15 We herein demonstrated that SIP1 expression shows relationships with clinicopathological factors, including histological differentiation, histological depth of invasion, LN metastasis, pathological stage, lymphatic invasion, and vascular invasion. Histologically, poorly differentiated SCC was significantly predominant in terms of the SIP1-positive cases. Miyoshi et al..30 reported that SIP1 caused the repression of E-cadherin expression and the morphological change from differentiation to dedifferentiation. Vandewalle et al..17 reported that the exogenous expression of SIP1 in a colon cancer cell line resulted in E-cadherin repression and a morphological change from an epithelial to a mesenchymal phenotype. Our results might therefore also be consistent with the conversion of epithelial cells to mesenchymal-like cells during the EMT process. EMT plays an important role during the progression of tumor cells to dedifferentiation.2
The process of cancer metastasis requires cell migration and invasion, cell-substrate adhesion, intravasation and extravasation, as well as the cell survival and re-growth of malignant cells at a distant location.31,32 EMT that occurs concomitantly with the downregulation of E-cadherin expression33,34 is facilitated in terms of the process of the acquisition of migratory properties, the intravasation of tumor cells into the blood or lymph vessels, and the subsequent formation of distant metastasis.35 ESCC cells with SIP1 expression might have a facilitated EMT-like process, and promote cells to have a more infiltrative phenotype, leading to deep invasion and metastasis. Therefore, we analyzed the relationship between SIP1 expression and lymphatic or vascular invasion. In this study, positive SIP1 cases were found to have invaded deeper than negative cases. Furthermore, we demonstrated that tumors positive for SIP1 expression had more lymphatic or vascular invasion than those without SIP1 expression. Our results support that the loss of E-cadherin by SIP1 induces cancers that are more invasive to lymphvascular vessels.
SIP1 expression significantly correlated with the presence of LN metastasis in the univariate analysis. Moreover, the multivariate analysis revealed that the expression of SIP1 is an independent predictive factor for the presence of LN metastasis. The association of LN metastasis with SIP1 is in agreement with the hypothesis that SIP1 could play an important role in tumor progression and metastasis. The snail expression in breast cancer tissue was previously reported to correlate with LN metastasis via the repression of E-cadherin.10 Taken together, these findings suggest that SIP1 might be responsible for advanced clinicopathological properties, and may also be a predictive marker for the presence of LN metastasis.
The survival rate of patients with positive SIP1 expression was significantly poorer than that of patients with negative expression in the univariate analysis. SIP1 was demonstrated to correlate with aggressive features, as described above. Therefore, the survival rate would be expected to be poorer in the patients with these features. These data are in agreement with another previous report showing that SIP1 is related to a poor prognosis in patients with oral SCC, gastric cancer, breast cancer, and ovarian cancer.21,26,36–38 Furthermore, SIP1 is a poor prognostic factor in bladder cancer patients treated with postoperative radiotherapy. SIP1 also has the antiapoptotic activity independent of its effects on cell adhesion, thus suggesting that SIP1 may promote tumor progression.26
Conclusions
Our data showed that SIP1 expression is associated with aggressive tumor properties and a poor prognosis, and suggest that SIP1 is an independent marker of LN metastasis and is also useful for predicting the malignant properties of ESCC. Therefore, SIP1 might be a candidate molecule that can be targeted to decrease tumor progression. The present study may serve as a stepping stone for improving prognosis via the suppression and prevention of distant metastasis in the future. Further in vitro studies are necessary to clarify the contribution of SIP1 to the EMT process.
References
Ando N, Ozawa S, Kitagawa Y, Shinozawa Y, Kitajima M. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg. 2000; 232(2):225-32.
Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442-54.
Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548-58.
Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66(17):8319-26.
Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429-37.
Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153(2):333-9.
Inada S, Koto T, Futami K, Arima S, Iwashita A. Evaluation of malignancy and the prognosis of esophageal cancer based on an immunohistochemical study (p53, E-cadherin, epidermal growth factor receptor). Surg Today. 1999;29(6):493-503.
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871-90.
Peinado H, Portillo F, Cano A. Transcriptional regulation of cadherins during development and carcinogenesis. Int J Dev Biol. 2004;48(5-6):365-75.
Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21(20):3241-6.
Rosivatz E, Becker I, Specht K, et al. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002; 61(5):1881-91.
Lee KW, Kim JH, Han S, et al. Twist1 is an independent prognostic factor of esophageal squamous cell carcinoma and associated with its epithelial-mesenchymal transition. Ann Surg Oncol. 2012;19(1):326-35.
Bindels S, Mestdagt M, Vandewalle C, et al. Regulation of vimentin by SIP1 in human epithelial breast tumor cells. Oncogene. 2006;25(36):4975-85.
Verschueren K, Remacle JE, Collart C, et al. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5′-CACCT sequences in candidate target genes. J Biol Chem. 1999;274(29):20489-98.
Comijn J, Berx G, Vermassen P, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7(6):1267-78.
Remacle JE, Kraft H, Lerchner W, et al. New mode of DNA binding of multi-zinc finger transcription factors: deltaEF1 family members bind with two hands to two target sites. Embo J. 1999;18(18):5073-84.
Vandewalle C, Comijn J, De Craene B, et al. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 2005;33(20):6566-78.
Sugimachi K, Tanaka S, Kameyama T, et al. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin Cancer Res. 2003;9(7):2657-64.
Natsugoe S, Uchikado Y, Okumura H, et al. Snail plays a key role in E-cadherin-preserved esophageal squamous cell carcinoma. Oncol Rep. 2007;17(3):517-23.
Uchikado Y, Natsugoe S, Okumura H, Setoyama T, Matsumoto M, Ishigami S, et al. Slug expression in the E-cadherin preserved tumors is related to prognosis in patients with esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11(3):1174-80.
Maeda G, Chiba T, Okazaki M, et al. Expression of SIP1 in oral squamous cell carcinomas: implications for E-cadherin expression and tumor progression. Int J Oncol. 2005;27(6):1535-41.
Sobin L, Gospodarowicz M, Wittekind C; International Union Against Cancer. TNM classification of malignant tumours. 7th ed. New York: Wiley; 2009.
Miura N, Yano T, Shoji F, et al. Clinicopathological significance of Sip1-associated epithelial mesenchymal transition in non-small cell lung cancer progression. Anticancer Res. 2009;29(10):4099-106.
Kyo S, Sakaguchi J, Ohno S, et al. High Twist expression is involved in infiltrative endometrial cancer and affects patient survival. Hum Pathol. 2006;37(4):431-8.
Sakamoto K, Imanishi Y, Tomita T, et al. Overexpression of SIP1 and downregulation of E-cadherin predict delayed neck metastasis in stage I/II oral tongue squamous cell carcinoma after partial glossectomy. Ann Surg Oncol. 2012;19(2):612-9.
Fang Y, Wei J, Cao J, et al. Protein expression of ZEB2 in renal cell carcinoma and its prognostic significance in patient survival. PLoS One. 2013;8(5):e62558.
Imamichi Y, Konig A, Gress T, Menke A. Collagen type I-induced Smad-interacting protein 1 expression downregulates E-cadherin in pancreatic cancer. Oncogene. 2007;26(16):2381-5.
Xia M, Hu M, Wang J, Xu Y, Chen X, Ma Y, et al. Identification of the role of Smad interacting protein 1 (SIP1) in glioma. J Neurooncol. 2010;97(2):225-32.
Sayan AE, Griffiths TR, Pal R, et al. SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proc Natl Acad Sci USA. 2009;106(35):14884-9.
Miyoshi A, Kitajima Y, Sumi K, Sato K, Hagiwara A, Koga Y, et al. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004;90(6):1265-73.
Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):314-23.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420-8.
Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98(10):1512-20.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-74.
Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118(Pt 19):4325-6.
Elloul S, Elstrand MB, Nesland JM, et al. Snail, slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103(8):1631-43.
Karihtala P, Auvinen P, Kauppila S, Haapasaari KM, Jukkola-Vuorinen A, Soini Y. Vimentin, zeb1 and Sip1 are up-regulated in triple-negative and basal-like breast cancers: association with an aggressive tumour phenotype. Breast Cancer Res Treat. 2013;138(1):81-90.
Okugawa Y, Inoue Y, Tanaka K, et al. Smad interacting protein 1 (SIP1) is associated with peritoneal carcinomatosis in intestinal type gastric cancer. Clin Exp Metastasis. 2013;30(4):417-29.
Acknowledgment
The authors would like to thank Brian Quinn for assisting with the preparation of the manuscript.
Disclosure
Rintaro Yoshida, Masaru Morita, Fumihiro Shoji, Yuichiro Nakashima, Naoko Miura, Keiji Yoshinaga, Tadashi Koga, Eriko Tokunaga, Hiroshi Saeki, Eiji Oki, Yoshinao Oda, and Yoshihiko Maehara have no competing financial interests related to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, R., Morita, M., Shoji, F. et al. Clinical Significance of SIP1 and E-cadherin in Patients with Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 22, 2608–2614 (2015). https://doi.org/10.1245/s10434-014-4314-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-4314-1