Abstract
The involvement of iron and inflammation parameters on overall survival in non-small-cell lung cancer (NSCLC) patients was studied. Furthermore, transferrin receptors 1 (TfR1) and ferritin expression in tumor tissue, tumor stroma, and normal lung tissue were analyzed. Iron metabolism and inflammation parameters were determined by automated laboratory measurements at the time of diagnosis. TfR1 and ferritin expression were determined by immuno-histochemical methods. About 50% of patients survived 12 months only. At the time of diagnosis more than half of the patients had anemia and significantly elevated serum ferritin. Iron content of serum ferritin (ICF) was below the reference values in 90% of patients. Furthermore, ICF showed positive correlation with iron metabolic parameters and survival but negative correlation with serum ferritin and ESR. The expression of TfR1 and ferritin in tumor cells was observed in 88% or 62% of patients, respectively. Tumor stroma was TfR1 negative and sporadically ferritin positive. Tumor tissue ferritin expression showed negative correlation with serum iron and hematokrit (Ht), and positive correlation with ferritin, erythrocyte sedimentation rate (ESR), α-1 globulin, and α-2 globulin. Positive correlation was found between TfR1 expression in tumor tissue and α-globulin. The correlation between TfR1/ferritin expression in tumor tissue and ICF or survival was not observed. Therefore, we conclude that elevated serum ferritin in sera of NSCLC patients is the result of inflammation and oxidative stress rather than body iron overload. Higher expression of ferritin in tumor tissue may be the consequence of iron deficiency or local toxicity induced by environmental factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alterations in whole-body iron metabolism are known to occur in patients with cancer [1–4]. Overabundance of iron is associated with increased risk of neoplasia at the site of metal deposition. Iron could participate in carcinogenesis by (i) catalyzing the formation of mutagenic oxygen radicals, (ii) suppressing anti-tumor action of host defense cells, and (iii) serving as essential element for tumor cell multiplication [5].
Iron can catalyze generation of highly reactive hydroxyl radicals via the Haber–Weiss reaction. The lungs are afforded some protection from iron-induced injury by the association of iron with iron-binding proteins ferritin and transferrin. But there is recent evidence that reductants present in cigarette smoke can readily mobilize iron from ferritin, leading to lipid peroxidation and cell damage [6].
The association of body iron stores with the risk of cancer has been inconsistently explored. Regulation of intracellular iron homeostasis is mainly based on regulation of iron uptake, its utilization, and storage. Iron excess and iron deprivation have deleterious effects; therefore iron homeostasis within cells is highly regulated by proteins responsible for iron uptake (transferrin, TfR1) and storage (ferritin) [7]. TfR1 and ferritin synthesis, in turn, are regulated by the interaction of their respective mRNAs with cytoplasm iron regulatory proteins [8].
The expression of TfR1 is related to iron requirements associated with cell proliferation. Immuno-histochemical staining for TfR1 has been used extensively to estimate the proliferation rate of cells and is thought to be of prognostic value in several types of malignant tumors [9–11]. The density of receptor at the cell surface can be greatly increased when cells become malignant. This association of receptor density with active growth was reported in fibroblasts [12], lymphocytes [13], breast tumor cells [14], and leukemia cells [15].
Excess intracellular iron is stored in ferritin complexes. The sequestration of iron within the shell of the ferritin molecule prevents formation of toxic free radical species that otherwise cause cellular damage. The function of serum ferritin has yet to be clarified, because certain disease processes (such as inflammation, acute and chronic liver disease, and some tumors) are associated with increased serum ferritin independent of the size of body iron stores [16].
To date, the relationships between the expression of TfR1 and ferritin in lung cancer, and hematological findings have not been investigated. In this study we have examined whether the expression of ferritin and TfR1 in lung cancer tissue shows a relationship with the iron status, tumor type, and the clinical status of NSCLC patients. Furthermore, the relationship between the iron and inflammation parameters with respect to ICF, serum iron and ferritin, ESR and survival were investigated.
Materials and methods
Subjects
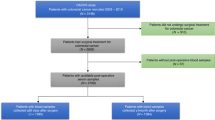
The study includes all the consecutive patients who underwent radical surgery for NSCLC at the Clinical Hospital for Lung Diseases “Jordanovac” from 2000 to 2008. A total of 125 Croatian male patients with histopathology proven lung carcinoma were studied: 58 patients with adeno carcinoma (AC) and 67 patients with squamous cell carcinoma (SCC). At the time of diagnoses, patients were from 37 to 88 years of age (median 60). Before therapy, all patients gave a complete history and had a physical examination, complete blood count, a full chemistry profile, electrocardiogram, chest radiograph, and computed tomography scan of the chest and upper abdomen.
Samples, data collection, and protection of human subjects
Clinical and histopathologic data were obtained from the Clinical Hospital for Lung Diseases “Jordanovac”, Zagreb, Croatia. Ten blood parameters determined by automated laboratory measurements at the time of diagnosis were included in this study, 5 of them were iron parameters (Hb = hemoglobin, Ht = hematokrit, Fe = serum iron, TIBC = total iron binding capacity, and transferin saturation) and 5 were inflammation parameters (serum ferritin, ESR, albumin, α-1 globulin, and α-2 globulin).
Furthermore, 42 lung tissue samples from operable patients were collected prospectively during surgery and used for TfR1 and ferritin determination. Additional 24 tumor samples were collected retrospectively from existing database and analyzed for the ferritin presence.
All samples and data used in this study were maintained in accordance with institutional patient care, quality assurance policies. The use of these data for this investigational purpose was reviewed and approved by the University and medical center institutional review board. Written informed consent was obtained from every subject after a full explanation of the study that was approved by the Ethics Committee of the Clinical Hospital for Lung Diseases “Jordanovac”. All procedures were in accordance with the recommendations found in the Helsinki Declaration of 1975.
ICF (iron content of serum ferritin) calculation
We calculated ICF values as suggested by Yamanishy et al. [17]. Briefly, ICF values were calculated by subtracting the background values (physiologic saline) from the serum sample values according the following equations:
The factor of 300 corrects for serum dilutions and unit conversions.
Histopathology examination
Tumor and the surrounding normal lung tissue obtained by surgery were immediately stored at −20°C. Histological sections (5 μm) of frozen tissue were fixed immediately in acetone for 10 min, and then rehydrated in phosphate buffered saline. The sections were examined by a pathologist well experienced in the histopathology of lung cancer but without prior knowledge of the history of patients.
The pathologic stage of each tumor was established according to the World Health Organization [18]. The staging was performed according to TNM classification as described in Sobin and Wittekind [19].
Immunohistochemical detection of ferritin and TfR1
Prospectively collected frozen tissue samples were cut in 5 μm cryostat sections, transferred on glass slides, air-dried overnight, and analyzed for ferritin and TfR1 expression. Retrospectively, 5 μm paraffin sections were deparaffinized, rehydrated, and analyzed for ferritin expression only.
Subsequently, all sections were quenched for endogenous peroxidase activity with a 3% hydrogen peroxide solution in methanol at room temperature for 30 min. Non-specific binding sites were blocked by incubation with wash buffer containing 10% of normal serum at 37°C for 30 min. The sections were then incubated overnight at 4°C with a mouse monoclonal anti-human transferrin antibody (CD 71, Dako, and Glostrup, Denmark) or with peroxidase-conjugated rabbit anti-human ferritin antibody (Dako, Glostrup, Denmark) at a dilution of 1:20 or 1:150, respectively. The slides for TfR1 staining were then rinsed and incubated with a peroxidase-conjugated secondary antibody (rabbit-anti-mouse, Dako, Denmark) diluted in phosphate buffered saline (1:10) and incubated for 1 h. The 3,3′-diaminobenzidine tetrahydrocloride (DAB, Sigma, USA) in phosphate buffer saline solution (0.0025%) was used as chromogen. The sections were counterstained with hematoxylin [20, 21]. Negative control slides were included in all experiments in which test antibody was omitted and replaced by control irrelevant diluents.
Assessment of transferrin receptors
Staining of the TfR1 was graded (blinded to the patient outcome) on a scale from 0 to 4 as follows: Grade 0 if no staining was observed, Grade 1 if only the cellular membrane was positive, Grade 2 if there was weak and focal positive reaction to TfR1, Grade 3 represent diffuse reaction in the cytoplasm, and Grade 4 if the tissue stained intensely in a diffuse pattern [20]. For purpose of analysis, TfR1 staining was categorized as absent (Grades 0 and 1) and strong (Grades 2, 3, and 4).
Assessment of ferritin content
The expression of ferritin was graded (blinded to the patient outcome) according to the percentage of positive cells as follows: Grade 0 if no staining was observed, Grade 1 if there was less then 5% positive cells, Grade 2 if there was between 5% and 25% positive cells present, Grade 3 represent the number of positive cells between 25% and 50%, Grade 4 if there was more than 50% positive cells [21]. For practical purposes, the expression of ferritin was roughly categorized as absent or weak (grades 0 and 1) and strong (grades 2, 3, and 4).
Statistical analysis
Histopathologic results were compared using the χ2-test. When over 20% of the expected values were less than 5, Fisher exact test was used. Biochemical parameters are presented as the mean value (in the bracket minimal and maximal value). Some of data were statistically analyzed by the Student’s t-test for two tailed equal values. A P < 0.05 was considered to be statistically significant. Correlation between parameters analyzed was done by Pearson rank correlation.
Results
Patients’ characteristics and survival
From 2000 through 2007, 125 male patients underwent radical surgery for NSCLC at the University Hospital for Lung Diseases “Jordanovac”. Median age at surgery was 60 years (range 37–88). Adeno carcinoma (AC) was diagnosed in 58 patients (46%) and squamous cell carcinoma (SCC) in 67 (54%) patients. Table 1 summarizes patients’ characteristics. Most of the patients (69%) were active smokers, 22% of them were former smokers, and only 9% patients were nonsmokers. At the moment of diagnoses more than 60% of examined patients were in stage III.
The survivals of the patients are shown in Fig. 1. Fifty percentage of 125 patients survived 12 months only. First 3 years after diagnosis there was no significant difference in survival between SCC and AC patients. However, overall 5 years survival was gently better in SCC patients than in AC patients. Almost 20% of all patients survived over 70 months (Fig. 1).
Parameters of iron metabolism and inflammation
Distribution of NCSLC patients with low, normal, or high baseline values of iron metabolism and inflammation are shown in Table 2. There were no significant differences in value of examined parameters with regard to the carcinoma type and furthermore, the cumulative results are shown. According to the National Cancer Institute (NCI) hemoglobin values below 140 g/l for man were used as the criteria of anemia. More than 50% of examined patients had anemia at diagnosis (Table 2). Decreased Hb and Ht values were found in about 56% of them. Serum iron and TIBC were below the reference values in about 50% or 52% of patients, while in 64% of the patients serum ferritin was above the reference value (180 ng/ml) [22]. On the other hand, baseline inflammation parameters ESR and α-1 globulin were above the reference values in almost 70% or 68% of the patients. Alpha-2 globulin was above the reference values in more than 87% of the patients (Table 2).
ICF and ferritin relevance in NSCLC patients
ICF values for NSCLC patients are shown in Fig. 2. ICF was below the reference values in 113 of 125 patients (more than 90%), in 10 patients it was in the range of the reference values and only 2 patients had high ICF values (Fig. 2). ICF is in positive correlation with iron metabolic parameters (P < 0.05) and in negative correlation with serum ferritin and ESR (P < 0.001) (Table 3). Contrary serum ferritin is in negative correlation with iron metabolic parameters (P < 0.05), and in positive correlation with ESR (P < 0.001). ESR is in strong negative correlation with iron metabolic parameters and serum ferritin (P < 0.001), and in positive correlation with α-2 globulin (P < 0.05) (Table 3).
Pearson correlation between 9 laboratory test and survival, ICF, ferritin, Fe, and ESR for patients who died during the study trial are shown in Table 4. ICF and all iron metabolic parameters are in positive correlation (P < 0.01) with months of survival. (Table 4). However, inflammation parameters and ferritin were in negative correlation with months of survival but only ESR reached significant negative correlation (P < 0.001) (Table 4). ICF is in positive correlation with Fe, TIBC, and transferin saturation (P < 0.05), and in negative correlation with serum ferritin and ESR (P < 0.001) (Table 4). Serum iron for patients who died is in strong positive correlation with all iron metabolic parameters, and in negative correlation with ESR (P < 0.001). Contrary serum ferritin is in negative correlation with ICF, Ht, and TIBC (P < 0.001), and in positive correlation with only with ESR (P < 0.001) (Table 4).
TfR1 and ferritin expression
Ferritin and TfR1 distribution in tumor cells, tumor stroma, and normal lung tissue are shown in Tables 4 and 5, respectively. There were no significant differences in expression of ferritin or TfR1 between different types (SCC or AC) of lung carcinoma. Strong ferritin expression was observed in 42 of 68 (62%) tumor samples, while it was relatively rare in tumor stroma or normal lung tissue samples (12% or 9%, respectively, (P < 0.001) (Table 5). Thirty-seven of 42 tumor samples (88%) showed strong TfR1 reaction, whereas tumor stroma was consistently negative (P < 0.001). In normal lung tissue TfR1 were expressed in 5 of 42 samples (12%) only (P < 0.001) (Table 6). Distribution of patients with weak/absent or strong ferritin or TfR1 score in tumor, tumor stroma, or lung tissue of NSCLC according to tumor type, T status, N status, and M status at the diagnosis (before therapy) are shown in Tables 5 and 6. The expression of ferritin and TfR1 were more frequently seen in T2, N2 and significantly in M0 status (P < 0.001) (Tables 5, 6).
Association between ferritin or TfR1 expression in tumor tissue and some sera biochemical parameters of patients with lung cancer are shown in Table 7. Patients with significantly lower serum iron and Ht compared to reference values had high amount of ferritin in tumor tissue (P < 0.01) (Table 7). Higher ferritin expression in tumor tissue was found in patients with lower transferrin saturation, while the patients with higher transferrin saturation did not express the ferritin in tumor cells, albeit the values of transferrin saturation in that both cases was in range of reference values. The strong expression of ferritin in tumor tissue was significantly associated with higher values of systemic parameters of inflammation (like serum ferritin, ESR, α-1 globulin, and α-2 globulin).
The higher expression of TFR1 on tumor cells was associated with higher values of α-1 globulin and α-2 globulin and with lower ESR values. Furthermore, high serum ferritin, ESR, and α-1 globulin were found in patients who did not express TfR1 on tumor cells (Table 7).
Discussion
In period from 2000 through 2007, we have collected and analyzed data from 125 male patients (58 from AC and 67 from SCC diagnosed patients). Female patients are not included in the study, because the iron metabolism in female is different, especially before menopause and incidence of lung tumor is significantly lower.
In the present study, we observed that 1-year survival for whole group of 125 (NSCLC) patients was about 50% and was similar compared with results described earlier [23, 24]. In our study overall 5-years survival was less than 25%, which is slightly lower compared to results described by Fujimoto et al. [24]. Generally there was no significant difference between survival of SCC and AC. The survival was significantly decreased by advanced pathologic T classification, higher tumor grade, unsuccessful resection of tumor, and worse results of therapy accompanied with severe anemia and inflammation.
At the time of diagnosis half of examined patients with NSCLC had mild symptoms of anemia of chronic disease. Most of the patients had Hb, Ht, and serum iron values below the reference, contrary serum ferritin values were above the reference. We suppose that elevated serum ferritin in lung cancer patients is the result of inflammation rather than of the body iron overload. Namely, inflammation might reflect condition in which high oxidative stress products and serum ferritin may be responsively increased in them [16]. Low ICF in 113 NSCLC patients additionally supports our hypothesis. Namely, serum ferritin with low content of iron has anti-oxidative function rather then body iron stores function. Most of examined NSCLC patients had serum ferritin with low content of iron which means that the body store of iron is not preserved. Pearson correlation between serum ferritin and months of survival did not reach statistical significance for patients who died in period of 1 year after diagnosis. This opinion suggests that ICF has a better prognostic importance than serum ferritin value. It means that the ferritin with lower iron content plays a significant role in patient survival and vice versa the NSCLC patients with higher ICF have a better prognosis.
Majority of the patients with NSCLC who died during 1 year after the diagnosis had severe anemia and higher values of inflammation parameters than the patients who survived for longer period. This corresponds well with data described by Yovino et al. [25]. They have shown better survival of NSCLC patients with Hb ≥ 12 mg/dl than of those with lower value of Hb. Recently, Berardi et al. reported that lung cancer anemia represents a prognostic factor not only for chemotherapy and/or radiotherapy but also for surgery in radically resected patients [26].
Patients with cancer often develop “anemia of chronic disease”, in which the body store of iron are preserved but the utilization is impaired [27]. In our previous study we have shown that more than 70% of the colorectal patients had anemia at the moment of diagnosis [28]. Anemia in patients with colorectal cancer is a complex condition. Patients could have anemia due to chronic bleeding from gastrointestinal tract or they could have anemia as a type of “chronic disease”, where body stores of iron are preserved but the utilization of iron is depleted. Both conditions have low serum iron, and distinctive parameter is ferritin, which is normal or higher in anemia of chronic disease, and low in anemia due to blood loss [29]. The difference between anemic condition of lung carcinoma and colon carcinoma patients could be due to chronic bleeding from gastrointestinal tract of patients with colon carcinoma.
Recently, novel functions of ferritin were described. Ferritin was described as a signaling molecule [30], immune regulator in humans [31] and that H-ferritin chain (PLIF: placenta immunomodulator ferritin) suppresses myelopoiesis and T cell by modulating the cytokine–chemokine network [32]. These findings reveal a new insight in increased serum ferritin values in lung cancer patients. Serum ferritin, especially H-ferritin chain, could explain anemia and immunosupression in NSCLC patients.
Serum ferritin, α-1 globulin, and α-2 globulin values were above the reference values for both tumor types and throughout the period of observation. These parameters could be useful prognostic factors for NSCLC patients, because all patients who died during period of 1 year of observation had higher values of those parameters. Inflammation is a known side effect of lung cancer, but it is not clear whether it reflects host response to the tumor or the accompanying infections. A number of researchers have reported cytotoxicity of neutrophils toward tumor targets under in vitro conditions [33–36], and in vivo [37]. On the other hand, there is evidence suggesting that inflammatory cells and cytokines in tumor stroma contribute to tumor development and progression rather than to an effective anti-tumor response [38, 39].
Serum iron parameters of the NSCLC patients were in significant negative correlation with the higher expression of ferritin in tumor tissue. Serum iron, Ht, and transferrin saturation were significantly lower in the patients with high expression of ferritin in tumor cells. Perhaps, tumor cells in need a higher amount of iron for their metabolism in anemic condition store more ferritin which could be used as a source of intracellular iron.
In the present study, we describe correlation between high TfR1 expression on tumor cells with high α-1 and α-2 globulin but with lower ESR. Furthermore, the expression of TfR1 on tumor cells was not associated with most of parameters of iron metabolism and association with parameters of inflammation is not consistent. It means that higher expression of TFR1 on tumor cells was associated with higher values of inflammation parameters (α-1 globulin and α-2 globulin), and lower ESR values. On contrary, higher serum ferritin, ESR, and α-1 globulin were found in patients who did not express TfR1 on tumor cells. At the moment we cannot give an adequate explanation for these findings.
In our investigation correlation between expression of TfR1 and ferritin in cancer cells and survival of NSCLC patients was not detected (data are not shown). Possible reason for that could be the different metabolism of cancer and normal cells in lung. Also, the difference in expression of TfR1 and ferritin between cancer cells and stroma or normal lung tissue could be explained with metabolic differences between malignant and normal cells. The iron metabolism in normal and cancer cells is also different. We have shown earlier higher sensitivity of colon carcinoma SW620 cells to ferric-sorbitol-citrate (FSC iron), whereas Wi38 fibroblasts were not sensitive [40]. Increased iron uptake by colon carcinoma cells was noticed in the first 3 h of the incubation with FSC iron, whereas higher FSC iron concentrations and longer incubation also impaired ferritin expression in SW260 colon carcinoma cells. The anticancer ability of FSC could result from its higher initial utilization of iron and consecutive negative signal for the expression of TfR1 in tumour cells. Tumor cells containing lower amounts of ferritin are probably more sensitive to oxidative stress caused by iron overload. Those data support our hypothesis that the systemic iron metabolic alteration are not in relationship with iron metabolism in cancer tissue.
Generally, tumor stroma and normal lung tissue showed weak expression of TfR1 but the density of receptors in NSCLC was increased. These findings are similar to those we described earlier for colorectal carcinoma [28]. The difference was observed in ferritin amount. In present studies most of lung carcinoma (42 of 68, 61%) expressed high amount of ferritin while less than 50% colon carcinoma expressed ferritin [28]. Normal lung tissue of lung carcinoma patients expressed ferritin sporadically (6 of 68, 9%), while most of samples of colon mucosa of patients with colon cancer showed strong expression of ferritin (47 of 63) [28]. The high ferritin content in colon mucosa where tumor cells have penetrated serosa and perivascular fat may be related to lower amount of serum iron or differential inflammatory response to metastasis and primary tumors.
Ferritin expression in lung cancer tissue is higher in NSCLC patients having lymph node involvement. Strong ferritin expression was detected in 13 patients of N1 status and in 23 patients of N2 status but only in 7 patients without lymph node metastasis. That finding may be related to anemia or to differences in the inflammatory response to primary tumors and to metastasis. Namely, in systemic inflammatory responses iron accumulates at the place of inflammation (liver, spleen, and bone marrow) [41]. Thus, iron accumulation at the place of inflammation could be also one of explanation of chronic anemia of patients with cancer. There was no difference in TfR1 expression in patients with or without lymph node metastasis.
The majority of carcinoma tissue samples exhibiting high TfR1 expression (23 of 26 patients) at T2 status, although this could be due to the high incidence of T2 status amongst patients in the study.
Finally, we conclude that prognosis of patients with NSCLC is evidently related to the degree of anemia and inflammation. Elevated serum ferritin in sera and higher expression of ferritin in tumor tissue of NSCLC patients is the result of inflammation and oxidative stress rather than higher body iron accumulation. It may be consequence of local toxicity induced by environmental factors. In that case ferritin may have an anti-oxidative role in tumor cells. Most of examined NSCLC patients had serum ferritin with low content of iron it means that the body store of iron are not preserved. Thus, ICF may be a better prognostic factor for NSCLC patients than ferritin. Assessment of TfR1 in lung tumors may therefore identify patients who could benefit from adjuvant therapy. Namely, blocking of the TfR1 in that stage of lung cancer might diminish tumor cell proliferation because of iron restriction.
References
Stevens RG, Jones DY, Micozzi MS, Taylor PR. Body iron stores and the risk of cancer. N Engl J Med. 1988;319(16):1047–52.
Weinberg ED. Association of iron with respiratory tract neoplasia. J Trace Elem Exp Med. 1993;6:117–23.
Stevens RG, Graubard BI, Micozzi MS, Neriishi K, Blumberg BS. Moderate elevation of body iron level and increased risk of cancer occurrence and death. Int J Cancer. 1994;56(3):364–9. doi:10.1002/ijc.2910560312.
Knekt P, et al. Body iron stores and risk of cancer. Int J Cancer. 1994;56(3):379–82. doi:10.1002/ijc.2910560315.
Weinberg ED. The role of iron in cancer. Eur J Cancer Prev. 1996;5(1):19–36. doi:10.1097/000199608469-199609001-00004.
Lapenna D, et al. Cigarette smoke, ferritin, and lipid peroxidation. Am J Respir Crit Care Med. 1995;151(2Pt 1):431–5.
Richardson DR, Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim Biophys Acta. 1997;1331(1):1–40.
Haile DJ. Regulation of genes of iron metabolism by the iron-response proteins. Am J Med Sci. 1993;318(4):230–40. doi:10.1097/00000441-199910000-00003.
Faulk WP, Hsi BL, Stevens PJ. Transferrin and transferrin receptors in carcinoma of the breast. Lancet. 1980;2(8191):390–2.
Habeshaw JA, Lister TA, Stansfeld AG, Greaves MF. Correlation of transferrin receptor expression with histological class and outcome in non-Hodgkin lymphoma. Lancet. 1983;1(8323):498–501. doi:10.1016/S0140-6736(83)92191-8.
Wrba F, Ritzinger E, Reiner A, Holzner JH. Transferrin receptor (TrfR) expression in breast carcinoma and its possible relationship to prognosis. An immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1986;410(1):69–73. doi:10.1007/BF00710908.
Octave JN, Schneider YJ, Hoffmann P, Trouet A, Crichton RR. Transferrin protein and iron uptake by cultured rat fibroblasts. FEBS Lett. 1979;108(1):127–30. doi:10.1016/0014-5793(79)81193-X.
Larrick JW, Cresswell P. Transferrin receptors on human B and T lymphoblastoid cell lines. Biochim Biophys Acta. 1979;583(4):483–90.
Larson SM, et al. Common pathway for tumor cell uptake of gallium-67 and iron-59 via a transferrin receptor. J Natl Cancer Inst. 1980;64(1):41–53.
Delia D, et al. Modulation of T leukaemic cell phenotype with phorbol ester. Int J Cancer. 1982;29(1):23–31. doi:10.1002/ijc.2910290106.
Ponka P, Beaumont C, Richardson DR. Function and regulation of transferrin and ferritin. Semin Hematol. 1998;35(1):35–54.
Yamanishi H, Iyama S, Yamaguchi Y, Kanakura Y, Iwatani Y. Relation between iron content of serum ferritin and clinical status factors extracted by factor analysis in patients with hyperferritinemia. Clin Biochem. 2002;35(7):523–9. doi:10.1016/S0009-9120(02)00380-6.
Travis WD, Colby TV, Corrin B, et al. Histological typing of lung and pleural tumours. World Health Organization International Histological Classification of Tumors, XIII. 3rd ed. Berlin/Heidelberg: Springer-Verlag; 1999.
Sobin LH, Wittekind CL. TNM classification of malignant tumors. 6th ed. New York: John Wiley & Sons, Inc.; 2002.
Whitney JF, Clark JM, Griffin TW, Gautam S, Leslie KO. Transferrin receptor expression in nonsmall cell lung cancer. Histopathologic and clinical correlates. Cancer. 1995;76(1):20–5. doi:10.1002/1097-0142(19950701)76:1<20::AID-CNCR2820760104>3.0.CO;2-3.
Yang HB, et al. Adenoma-carcinoma sequence: a reappraisal with immunohistochemical expression of ferritin. J Surg Oncol. 1995;60(1):35–40. doi:10.1002/jso.2930600108.
Nakano M, et al. Oxidative DNA damage (8-hydroxydeoxyguanosine) and body iron status: a study on 2507 healthy people. Free Radic Biol Med. 2003;35(7):826–32. doi:10.1016/S0891-5849(03)00432-5.
Zaniboni A, et al. Phase II study oftaxol combined with ifosfamide and carboplatin in the treatment of stage IIIb-IV non-small-cell lung cancer. Am J Clin Oncol. 2003;26(1):84–8. doi:10.1097/00000421-200302000-00016.
Fujimoto T, et al. Completely resected N1 non-small-cell lung cancer: factors affecting recurrence and long-term survival. J Thorac Cardivasc Surg. 2006;132(3):499–506. doi:10.1016/j.jtcvs.2006.04.019.
Yovino S, Kwok Y, Krasna M, Bangalore M, Suntharalingam M. An association between preoperative anemia and decreased survival in early-stage non-small-cell lung cancer patients treated with surgery alone. Int J Radiat Oncol Biol Phys. 2005;62(5):1438–43. doi:10.1016/j.ijrobp.2004.12.038.
Berardi R, et al. Perioperative anemia and blood transfusions as prognostic factors in patients undergoing resection for non-small cell lung cancers. Lung Cancer. 2005;49(3):371–6. doi:10.1016/j.lungcan.2005.04.011.
Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Rev. 2002;16(2):87–96. doi:10.1054/blre.2002.0193.
Prutki M, et al. Altered iron metabolism, transferrin receptor 1 and ferritin in patients with colon cancer. Cancer Lett. 2006;238(2):188–96. doi:10.1016/j.canlet.2005.07.001.
Hyman GA, Harvey JE. The pathogenesis of anaemia in patients with carcinoma. Am J Med. 1955;19(3):350–6. doi:10.1016/0002-9343(55)90123-6.
Li R, Luo C, Mines M, Zhang J, Fan GH. CV hemocine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and translocation of ferritin heavy chain. J Biol Chem. 2006;281(49):37616–27. doi:10.1074/jbc.M607266200.
Harada T, Baba M, Torii I, Morikawa S. Ferritin selectively suppresses delayed-type hypersensitivity responses at induction or effector phase. Cell Immunol. 1987;109(1):75–88. doi:10.1016/0008-8749(87)90293-0.
Moroz C, et al. Treatment of human bone marrow with recombinant placenta immunomodulator ferritin results in myelopoiesis a T-cell suppression through modulation of the cytokine-chemokine networks. Exp Hematol. 2006;34(2):159–66. doi:10.1016/j.exphem.2005.10.006.
Dallegri F, Patrone F, Frumento G, Sacchetti C. Antibody-dependent killing of tumor cells by polymorphonuclear leukocytes. Involvement of oxidative and nonoxidative mechanisms. J Natl Cancer Inst. 1984;73(2):331–9.
Dallegri F, Frumento G, Ballestrero A, Goretti R, Patrone F. Relationship between antibody-dependent tumour cell lysis and primary granule exocytosis by human neutrophils. Clin Exp Immunol. 1987;70(2):479–83.
Valerius T, et al. Involvement of the high-affinity receptor for IgG (Fc gamma RI; CD64) in enhanced tumor cell cytotoxicity of neutrophils during granulocyte colony-stimulating factor therapy. Blood. 1993;82(3):931–9.
Reali E, et al. Interferon-γ enhances monoclonal antibody 17-1A-dependent neutrophil cytotoxicity toward colorectal carcinoma cell line SW11-16. Clin Immunol Immunopathol. 1994;71(1):105–12. doi:10.1006/clin.1994.1058.
Katano M, Torisu M. Neutrophil-mediated tumor cell destruction in cancer ascites. Cancer. 1982;50(1):62–8. doi:10.1002/1097-0142(19820701)50:1<62::AID-CNCR2820500113>3.0.CO;2-0.
Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi:10.1016/S0140-6736(00)04046-0.
O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer. 2001;85(4):473–83. doi:10.1054/bjoc.2001.1943.
Prutki M, Poljak-Blazi M, Mihaljevic B, Orescanin V, Zarkovic N. Uptake of anti-anemic substance ferric-sorbitol-citrate by normal and malignant cells and its effects on expression of transferrin receptor 1 and ferritin. Cancer Biother Radiopharm. 2006;21(6):636–44. doi:10.1089/cbr.2006.21.636.
Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 2nd ed. Oxford: Clarendon Press; 1989. 466.
Acknowledgments
The study was supported by the Croatian Ministry of Science, Education, and Sport.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kukulj, S., Jaganjac, M., Boranic, M. et al. Altered iron metabolism, inflammation, transferrin receptors, and ferritin expression in non-small-cell lung cancer. Med Oncol 27, 268–277 (2010). https://doi.org/10.1007/s12032-009-9203-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-009-9203-2