Abstract
Background
Iron deficiency anemia is represented in colorectal cancer (CRC) patients. Iron surplus load to increase non-transferrin bound iron (NTBI), and NTBI promotes cancer progression and influences microbiota. This study investigated whether preoperative serum iron status was associated with prognosis after CRC resection.
Methods
We evaluated preoperative iron and transferrin saturation (TSAT), which was calculated as iron divided by total iron-binding capacity, in 327 patients who underwent surgery for Stage II–III CRC. Fe < 60 μg/dl and TSAT > 40% were defined as low and high iron, respectively. The associations between iron status and overall survival (OS) were evaluated in univariate and multivariate Cox proportional hazards analysis.
Results
Of the 327 patients, 179 (54.7%), 124 (37.9%) and 24 (7.3%) had low, normal and high iron, respectively. In univariate analysis, low iron was associated with shorter OS (hazard ratio [HR] 2.821, 95% confidence interval [CI] 1.451–5.485, P = 0.002). High iron was also associated with shorter OS (HR 3.396, 95% CI 1.359–8.489, P = 0.009). In multivariate analysis, high age (P = 0.002), depth of invasion pT4 (P = 0.012), lymph-node metastasis presence (P = 0.035), low albumin (P = 0.011), low iron (HR 2.282, 95% CI 1.163–4.478, P = 0.016) and high iron (HR 3.757, 95% CI 1.486–9.494 P = 0.005) were independently associated with shorter OS. High iron was associated with the amount of intratumoral Fusobacterium nucleatum compared with normal iron.
Conclusion
Both low and high preoperative iron in Stage II–III CRC patients were associated with unfavorable OS in univariate and multivariate analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed malignant disease in men and the second in women worldwide [1]. CRC patients are likely to suffer from iron deficiency anemia. The most effective treatment for resectable CRC is surgical resection with lymph-node (LN) dissection [2], but CRC patients with anemia often receive perioperative blood transfusion, which has been associated with unfavorable short- and long-term prognosis in many studies [3, 4]. Therefore, iron supplementation has been performed to improve anemia and thus to avoid perioperative blood transfusion. In a few studies, preoperative iron supplementation has been reported to decrease perioperative blood transfusion and improve short-term outcome [5]. However, the association between preoperative iron status and long-term outcome has remained unclear.
Iron deficiency anemia has been observed in CRC patients; however, iron surplus can contribute to both tumor initiation and tumor growth. Iron also plays a role in the tumor microenvironment and in metastasis. Reprogramming of iron metabolism is a crucial aspect of tumor cell survival. Hypoxia-inducible factor and WNT pathways may contribute to altered iron metabolism in cancer [6]. Iron surplus leads to increased non-transferrin bound iron (NTBI), and NTBI promotes cancer progression [7] and influences microbiota. High transferrin saturation (TSAT) results in elevated NTBI, and elevating NTBI leads to unfavorable side effects at the highly interactive host–microbe interface of the human gastrointestinal tract. Microbial populations change in the gut in response to increased luminal iron concentration [8]. Microbial change has been associated with prognosis in many gastrointestinal cancers [9,10,11].
Fusobacterium nucleatum (F. nucleatum) is associated with unfavorable prognosis after curative resection in colorectal cancer. To our knowledge, no association between F. nucleatum and iron status has been reported. However, the Western dietary pattern has been strongly associated with F. nucleatum-positive colorectal cancer [12], and Western dietary patterns are characterized by red and processed meats, which contain large amounts of iron [13, 14].
The aim of this study is to evaluate the relationship between preoperative iron status and long-term outcome in Stage II and III CRC patients who underwent colorectal resection. This study may lead to novel insights into the association between preoperative iron status and prognosis of the CRC, and iron and transferrin saturation should be measured to ‘to inform decisions about whether to perform iron supplementation.
Materials and methods
Patients and evaluation for iron status
Four hundred thirty-six Stage II and III CRC patients underwent surgery at the Department of Gastroenterological Surgery, Kumamoto University between January 2005 and March 2018. Blood iron and unsaturated iron-binding capacity were measured in 327 of these 436 patients. TSAT was calculated as iron divided by total iron-binding capacity. The lower limit of the normal range of iron was 60 μg/dl, as specified by the recommendations of the measuring kit our institute adopted. TSAT > 40% was defined as high iron in accordance with a previous report [7]. Clinical data, including age, gender, body mass index, depth of invasion (pT), presence of metastatic lymph node (LN), pathological type, lymphatic invasion, and vascular invasion, were retrospectively available for all 327 patients. The laboratory data were collected less than 2 months before surgery. Laboratory measurements included carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), white blood cells (WBC), serum total protein, albumin, CRP, hemoglobin, platelets, peripheral neutrophils, and lymphocytes. Each cut-off value was defined according to the recommendations of the measuring kits we used. The cut-off values of hemoglobin in males and females were 13.0 and 12.0 g/dl, respectively, as defined by the World Health Organization. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), as inflammatory markers, were evaluated, because the association between these markers and metastatic CRC patient prognosis has been reported. Cut-off values of NLR and PLR were determined as 5 and 150, respectively, in accordance with a previous study [15]. Complication after surgery was evaluated in accordance with the Clavien–Dindo classification [16]. Written informed consent was obtained from all patients for the treatments and the study was approved by the Institute Review Board of the Graduate School of Medical Science, Kumamoto University.
Treatment strategy and follow-up evaluation
The treatment strategy followed the Japanese colorectal cancer guidelines, which recommend surgery with LN dissection for Stage II and Stage III CRC. Patients were followed up at 3-month intervals. Recurrence was confirmed by clinical examinations, including computed tomography (CT). Tumor marker levels were measured every 3 months for 5 years after surgery. CT scanning studies that included the neck to the pelvis were performed at least twice a year for 3 years after surgery.
Quantitative polymerase chain reaction (PCR) for intratumor Fusobacterium nucleatum
Genomic DNA was extracted from CRC tissue using a QIAamp DNA Mini Kit (Qiagen). Quantitative PCR assays were performed to measure the amount of tissue DNA of F. nucleatum. Custom TaqMan primer/probe sets (Applied Biosystems) for the nusG gene of F. nucleatum and the reference human gene SLCO2A1 were used as previously described [17]. Each 10-μl reaction contained 12.5 ng of genomic DNA, 1 × final concentration LightCycler 480 Probes Master (Roche). Amplification and detection of DNA were performed with a LightCycler 480 Instrument II (Roche). The primer and probe sequences for each TaqMan Gene Expression Assay were as follows: F. nucleatum forward primer, 5′-TGGTGTCATTCTTCCAAAAATATCA-3′; F. nucleatum reverse primer, 5′-AGATCAAGAAGGACAAGTTGCTGAA-3′; F. nucleatum FAM probe, 5′-ACTTTAACTCTACCATGTTCA-3′. Each specimen was analyzed in duplicate in a single batch, and we used the average of the two cycle threshold (Ct) values. The amount of F. nucleatum in each specimen was calculated as a relative unitless value normalized with SLCO2A1 using the 2-ΔCt method as previously described [17].
Statistical analysis
The association of transferrin with recorded clinical and pathological characteristics was determined by chi-squared tests. All P values were two-sided; P < 0.05 was considered significant. Analysis of risk factors for survival included age, gender, depth of invasion, LN metastasis, albumin, CRP, hemoglobin, NLR, PLR and iron. Mortality was estimated from cancer-specific survival (CSS) and overall survival (OS). The log-rank test and Wilcoxon test were used in the survival analysis; the Kaplan–Meier method was used to assess cumulative survival. Cox proportional hazards regression models were utilized to calculate hazard ratios (HR) and 95% confidence interval (CI). We performed multivariate Cox proportional hazards regression analysis to compute an HR according to age ≥ 70, male sex, depth of invasion (pT4), the presence of LN metastasis, low albumin (< 4.1 g/dl), CPR (≥ 0.14 mg/dl), low hemoglobin, NLR (≥ 5), PLR (≥ 150) and iron status. Backward stepwise elimination (likelihood method) with a threshold of P = 0.20 was used to select variables for the final model. Prognostic analysis was performed following the REMARK Guidelines [18]. Probability values < 0.05 were considered significant. All data were processed and analyzed using SPSS and JMP 11 software programs.
Results
Association between iron and clinicopathological findings
Of the 327 patients, 179 (54.7%), 124 (37.9%) and 24 (7.3%) had low, normal and high iron, respectively. For these patients, age (P = 0.016), gender (P = 0.016), depth of invasion (P < 0.001), CEA (P = 0.024), CA19-9 (P = 0.028), WBC (P = 0.001), albumin (P = 0.001), CRP (P < 0.001), hemoglobin (P < 0.001), NLR (P = 0.001) and PLR (P = 0.001) were significantly different between the three groups (Table 1). For these patients, low iron was significantly associated with high age (P = 0.0087), right-sided tumor location (P = 0.0241), depth of invasion T4 (P < 0.0001), high CEA (P = 0.0064), high CA19-9 (P = 0.0121), high WBC (P = 0.0021), low albumin (P = 0.0013), high CRP (P < 0.0001), low hemoglobin (P < 0.0001), high NLR (P = 0.0001), and high PLR (P = 0.0003) compared with normal iron using Bonferroni-adjusted p values in univariate analysis (Supplementary Table S1). High age (risk ratio (RR) 1.896, 95%CI 1.087–3.307, P = 0.024), depth of invasion T4 (RR 2.293, 95%CI 1.123–4.682 P = 0.023), high CRP (RR 2.646, 95%CI 1.514–4.625, P < 0.001) and low hemoglobin (RR 4.509, 95%CI 2.600–7.820, P < 0.001) were independently associated with low iron in multivariate binomial logistic analysis. High iron was not significantly associated with any clinicopathological factors compared with normal iron (Supplementary Table S2).
Association of iron with blood transfusion and postoperative complication
The association of the prognosis of CRC patients after surgery with blood transfusion and postoperative complication was previously reported [19, 20]. Of the 327 patients, 19 received blood transfusion 7 days before surgery, and 57 received intraoperative blood transfusion. Preoperative and intraoperative blood transfusion was more frequently performed for patients with low iron than for those with normal iron (P = 0.0303 and P < 0.0001, respectively) (Table 2). Postoperative complication was evaluated with the Clavien–Dindo classification. Low iron was not associated with anastomotic leakage (P = 0.5297) or postoperative complication with Clavien–Dindo classification ≥ 3 (P = 0.3167) compared with normal iron. Preoperative high iron was associated with preoperative blood transfusion (P = 0.0066), but not with intraoperative blood transfusion or with any postoperative complication.
Association between iron status and survival
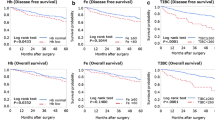
The 327 patients, of whom 61 (18.7%) were dead and 35 (10.7%) were cancer-specific deaths, were monitored over a median follow-up of 54.7 months. Five-year CSS and OS rates were 89.1% and 81.3%. We evaluated the association between survival and iron status with the Kaplan–Meier method. Low iron was significantly associated with shorter OS (log-rank test P = 0.002, Wilcoxon test P < 0.001), and tended to result in shorter CSS (log-rank test P = 0.095, Wilcoxon test P = 0.001) compared with normal iron. Five-year OS rates of CRC patients with low and normal iron were 75.1% and 93.7%, respectively. Five-year CSS rates of CRC patients with low and normal iron were 84.9% and 95.9%, respectively (Fig. 1).
High iron was significantly associated with shorter OS (log-rank test P < 0.001, Wilcoxon test P < 0.001) but not with CSS (log-rank test P = 0.541, Wilcoxon test P = 0.103) compared with normal iron. Five-year OS rates of CRC patients with low and normal iron were 66.2% and 93.7%, respectively. Five-year CSS rates of CRC patients with high and normal iron were 85.0% and 95.9%, respectively (Fig. 1C, D).
Association between clinicopathological factors and survival
Many clinicopathological factors are associated with OS after colorectal resection. Both low and high iron were also significantly associated with shorter OS (HR 2.821, 95%CI 1.451–5.485, P = 0.002 and HR 3.396, 95%CI 1.359–8.489, P = 0.009, respectively) compared with normal iron, in univariate Cox proportional hazards regression analysis. Preoperative or intraoperative blood transfusion was not associated with prognosis after colorectal resection (Supplementary Figure S1). High age (P = 0.002), depth of invasion pT4 (P = 0.012), LN metastasis presence (P = 0.035), low albumin (P = 0.011), low iron (HR 2.282, 95%CI 1.163–4.478, P = 0.016) and high iron (HR 3.757, 95%CI 1.486–9.494 P = 0.005) were independently associated with shorter OS in multivariate Cox proportional hazards regression analysis (Table 3).
Association between iron status and Fusobacterium nucleatum
Recent work has demonstrated that TSAT is associated with microbiota in the gut [8]. Of the 327 CRC patients, frozen samples were available for 142. The quantity of F. nucleatum within the primary tumor of 142 patients was measured. These patients consisted of 80 with low iron, 48 with normal iron and 14 with high iron. F. nucleatum was more frequently detected in high-iron patients compared with normal-iron patients (P = 0.0138) (Fig. 2A). An association between F. nucleatum and prognosis of CRC patients has been reported. In accordance with that previous study, we divided the patients into three groups, F. nucleatum-negative, -low and -high [9]. The proportions of F. nucleatum-negative, -low and -high were 43.8%, 31.3% and 25.0%, respectively, in the primary tumor of normal-iron patients, whereas they were 21.4%, 28.6% and 50.0%, respectively, in the tumor of high-iron patients (Fig. 2B).
Association of intratumor Fusobacterium nucleatum with iron status. The quantity of F. nucleatum within the primary tumor of the 142 patients was measured by real-time PCR (A). The 142 patients were divided into three groups, Fusobacterium nucleatum-negative, -low and -high. Association between F. nucleatum and iron status is shown (B)
Discussion
Preoperative low iron levels tended to correlate with unfavorable CSS and were significantly associated with shorter OS of Stage II and III CRC patients. Iron supplementation may improve prognosis of CRC patients with iron deficiency anemia. However, preoperative high iron was also associated with shorter OS. These data indicated that Stage II and III CRC patients who displayed both a lack and a surplus of iron had unfavorable prognoses, and further research on this finding will be required.
Preoperative anemia was significantly associated with shorter OS and disease-free survival in rectal cancer in a meta-analysis [21]. In our study, anemia was associated with shorter CSS and OS in univariate analysis. The treatment of anemia encompasses three strategies: erythropoietin-stimulating agents, blood transfusion and iron supplementation. Recombinant human erythropoiesis-stimulating agents improve anemia in many studies [22,23,24]; however, a total of 13,933 patients with cancer in 53 trials were surveyed in a meta-analysis of randomized trials, and erythropoiesis-stimulating agents increased mortality (HR 1.17, 95%CI 1.06–1.30) [25]. Erythropoietin-stimulating agents and blood transfusion are effective in increasing hemoglobin levels, but both modalities should be given with caution to cancer patients [26]. However, perioperative blood transfusion was also associated with unfavorable long-term prognosis and increased short-term complications after CRC surgery in another meta-analysis. A total of 174,036 patients in 36 trials were analyzed and perioperative blood transfusion was associated with shorter OS (HR 0.33, 95%CI 0.24–0.41, P < 0.0001) and shorter CSS (HR 0.34, 95%CI 0.21–0.47, P < 0.0001). Perioperative blood transfusion was associated with postoperative infectious complications, pulmonary complications, cardiac complications, anastomotic complications, reoperation and general complications [4].
Iron supplementation is another strategy for treatment of anemia. Clinical trials have revealed that iron supplementation improved short-term outcome in CRC patients who underwent surgery. Oral ferrous sulfate (200 mg twice daily for 2 weeks) or no iron therapy before surgery was compared in a randomized-controlled trail (RCT). The iron-supplemented group had higher hemoglobin than the non-iron-treated group (13.1 g/dl, range 9.6–17 g/dl versus 11.8 g/dl) at the time of admission to hospital [27]. Intravenous iron infusion, as a single 1000-mg dose of ferric carboxymaltose, was preoperatively administered in the outpatient clinic to CRC patients with anemia. Iron infusion was associated with increased hemoglobin levels and reduced allogeneic red blood cell transfusions use [28]. In other studies, intravenous iron could also increase hemoglobin level and reduce red blood cell transfusion [27, 29, 30]. Postoperative intravenous iron administration also improved the recovery of hemoglobin level at postoperative day 30 without increasing postoperative complication [31]. Iron supplementation has been found to reduce blood transfusion and improve short-term outcome in many studies, but the association between iron supplementation and long-term outcome remains unclear; 1000–2000 mg preoperative intravenous iron therapy had little effect on long-term OS or disease-free survival in CRC patients with anemia [32]. In our study, low iron was not associated with postoperative complication, although preoperative low iron was significantly associated with anemia and perioperative blood transfusion. Iron supplementation may therefore reduce perioperative blood transfusion.
Low iron levels were associated with shorter OS than were normal iron levels in the present study, as well as with both nutritional and inflammatory status. In turn, nutritional and inflammatory status were correlated with NLR and PLR, which were associated with prognosis in many types of cancers. Therefore, we performed survival analysis with these factors to test for confounding. However, low iron levels were independently associated with an unfavorable prognosis.
Iron status has been associated with cancer initiation and progression. Iron homeostasis is regulated via several mechanisms and may promote tumor growth or cell death. Mutations in APC are present in more than 80% of sporadic colon cancers, while mutations in β-catenin are present in approximately 10% of colon cancers [33]. Wnt signaling is activated by iron in APC-mutant malignant cell lines [34]. Cancer cells exhibit an increased dependence on iron compared with normal cells [35]. The mean concentration of iron in CRC (46.1 μg/g) was higher than in polyps (43.2 μg/g), as determined by the total-reflection X-ray fluorescence method [36]. In a case–control study within a prostate, lung, colorectal and ovarian cancer screening trial, patients with lower iron had a reduced risk of developing colorectal adenoma [37]. Higher TSAT or serum iron concentrations were associated with increased nonskin cancer risk and increased risk of cancer death in 1597 men and 1795 women [38]. The relative risk of CRC was 1.18 (95%CI 1.06–1.32) for patients in the highest category of heme iron intake compared with those in the lowest category [39]. Qualitative requirements for iron of normal and neoplastic cells are similar. Anemia can develop in states of iron deficiency, whereas iron excess increases oxidative stress in body tissues [33] High TSAT results in elevated NTBI, which has unfavorable side effects at the highly interactive host–microbe interface of the human gastrointestinal tract. Microbial composition changes in the gut in response to increased luminal iron concentration [8]. Boyer E. et al. reported that significant correlations were found between TSAT and the proportions of microbiota when TSAT exceeded 45% [40]. NTBI was disappeared when TSAT was less than 35% [41]. We determined cut-off value as 40% in this study in accordance with these studies. In this study, intratumor F. nucleatum in CRC patients was associated with high iron. This result may indicate that microbial change is induced by NTBI. Lactoferrin is a secreted, iron-binding glycoprotein originally discovered as a component of milk, and daily intake of 3 g of lactoferrin has been suggested as a clinically beneficial adjunct to colorectal polyp extraction [42]. Morita et al. reported that F. nucleatum on the tongue was significantly less frequent in patients receiving lactoferrin and lactoperoxidase-containing tablets for 8 weeks than in those receiving placebo tablets [43].
The aim of iron supplementation in perioperative CRC patients is to improve anemia and decrease blood transfusion. However, the association between perioperative iron supplementation and long-term prognosis remained unclear. This study revealed that preoperative low iron was associated with shorter OS compared with normal iron in univariate and multivariate analysis. However, high iron was also associated with an unfavorable prognosis and surplus iron supplementation may lead to an unfavorable prognosis. Therefore, iron supplementation should be performed in conjunction with monitoring TSAT.
This study had limitations. It was a retrospective study and 109 of the 436 CRC patients (24.9%) were not evaluated for their iron level before surgery. This potentially leads to selection bias. The supplied iron agents depended on the attending doctor, because the doctors had not participated in any trials involving iron support. The administration of iron supplementation could not be accurately monitored before and after surgery.
In conclusion, both low and high preoperative iron in patients who underwent CRC resection was identified as being associated with an unfavorable prognosis by univariate and multivariate analyses. Suppling iron to alleviate iron deficiency anemia, together with monitoring TSAT to avoid surplus iron, may improve the prognosis of patients who receive CRC resection.
References
Torre LA, Bray F, Siegel RL et al (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Hashiguchi Y, Muro K, Saito Y et al (2020) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25:1–42
Acheson AG, Brookes MJ, Spahn DR (2012) Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg 256:235–244
Pang QY, An R, Liu HL (2019) Perioperative transfusion and the prognosis of colorectal cancer surgery: a systematic review and meta-analysis. World J Surg Oncol 17:7
Hallet J, Hanif A, Callum J et al (2014) The impact of perioperative iron on the use of red blood cell transfusions in gastrointestinal surgery: a systematic review and meta-analysis. Transfus Med Rev 28:205–211
Torti SV, Torti FM (2013) Iron and cancer: more ore to be mined. Nat Rev Cancer 13:342–355
Brissot P, Ropert M, Le Lan C et al (2012) Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta 1820:403–410
Yilmaz B, Li H (2018) Gut microbiota and iron: the crucial actors in health and disease. Pharmaceuticals 11:98
Mima K, Nishihara R, Qian ZR et al (2016) Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65:1973–1980
Yamamura K, Baba Y, Nakagawa S et al (2016) Human microbiome fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin Cancer Res 22:5574–5581
Mima K, Ogino S, Nakagawa S et al (2017) The role of intestinal bacteria in the development and progression of gastrointestinal tract neoplasms. Surg Oncol 26:368–376
Mehta RS, Nishihara R, Cao Y et al (2017) Association of dietary patterns with risk of colorectal cancer subtypes classified by fusobacterium nucleatum in tumor tissue. JAMA Oncol 3:921–927
Cross AJ, Ferrucci LM, Risch A et al (2010) A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res 70:2406–2414
Bastide NM, Chenni F, Audebert M et al (2015) A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res 75:870–879
Rossi S, Basso M, Strippoli A et al (2017) Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer 16:264–274
Katayama H, Kurokawa Y, Nakamura K et al (2016) Extended Clavien–Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today 46:668–685
Mima K, Sakamoto Y, Kosumi K et al (2020) Mucosal cancer-associated microbes and anastomotic leakage after resection of colorectal carcinoma. Surg Oncol 32:63–68
McShane LM, Altman DG, Sauerbrei W et al (2005) REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93:387–391
Wu HL, Tai YH, Lin SP et al (2018) The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: a propensity score analysis of 4,030 patients. Sci Rep 8:13345
Miyamoto Y, Hiyoshi Y, Tokunaga R et al (2020) Postoperative complications are associated with poor survival outcome after curative resection for colorectal cancer: a propensity-score analysis. J Surg Oncol. https://doi.org/10.1002/jso.25961
Wilson MJ, van Haaren M, Harlaar JJ et al (2017) Long-term prognostic value of preoperative anemia in patients with colorectal cancer: a systematic review and meta-analysis. Surg Oncol 26:96–104
Braga M, Gianotti L, Vignali A et al (1995) Evaluation of recombinant human erythropoietin to facilitate autologous blood donation before surgery in anaemic patients with cancer of the gastrointestinal tract. Br J Surg 82:1637–1640
Levine EA, Laborde C, Hambrick E et al (1999) Influence of erythropoietin on transfusion requirements in patients receiving preoperative chemoradiotherapy for rectal cancer. Dis Colon Rectum 42:1065–1069 (discussion 1069–1071)
Christodoulakis M, Tsiftsis DD (2005) Preoperative epoetin alfa in colorectal surgery: a randomized, controlled study. Ann Surg Oncol 12:718–725
Bohlius J, Schmidlin K, Brillant C et al (2009) Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet 373:1532–1542
Pascual M, Bohle B, Alonso S et al (2013) Preoperative administration of erythropoietin stimulates tumor recurrence after surgical excision of colon cancer in mice by a vascular endothelial growth factor-independent mechanism. J Surg Res 183:270–277
Lidder PG, Sanders G, Whitehead E et al (2007) Pre-operative oral iron supplementation reduces blood transfusion in colorectal surgery—a prospective, randomised, controlled trial. Ann R Coll Surg Engl 89:418–421
Keeler BD, Simpson JA, Ng S et al (2014) The feasibility and clinical efficacy of intravenous iron administration for preoperative anaemia in patients with colorectal cancer. Colorectal Dis 16:794–800
Quinn M, Drummond RJ, Ross F et al (2010) Short course pre-operative ferrous sulphate supplementation–is it worthwhile in patients with colorectal cancer? Ann R Coll Surg Engl 92:569–572
Kam PM, Chu CW, Chan EM et al (2020) Use of intravenous iron therapy in colorectal cancer patient with iron deficiency anemia: a propensity-score matched study. Int J Colorectal Dis 35:521–527
Laso-Morales MJ, Vives R, Gomez-Ramirez S et al (2018) Intravenous iron administration for post-operative anaemia management after colorectal cancer surgery in clinical practice: a single-centre, retrospective study. Blood Transfus 16:338–342
Wilson MJ, Dekker JWT, Buettner S et al (2018) The effect of intravenous iron therapy on long-term survival in anaemic colorectal cancer patients: results from a matched cohort study. Surg Oncol 27:192–199
Padmanabhan H, Brookes MJ, Iqbal T (2015) Iron and colorectal cancer: evidence from in vitro and animal studies. Nutr Rev 73:308–317
Brookes MJ, Boult J, Roberts K et al (2008) A role for iron in Wnt signalling. Oncogene 27:966–975
Chen Y, Fan Z, Yang Y et al (2019) Iron metabolism and its contribution to cancer (Review). Int J Oncol 54:1143–1154
Kucharzewski M, Braziewicz J, Majewska U et al (2003) Iron concentrations in intestinal cancer tissue and in colon and rectum polyps. Biol Trace Elem Res 95:19–28
Cross AJ, Sinha R, Wood RJ et al (2011) Iron homeostasis and distal colorectal adenoma risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Prev Res 4:1465–1475
Chua AC, Knuiman MW, Trinder D et al (2016) Higher concentrations of serum iron and transferrin saturation but not serum ferritin are associated with cancer outcomes. Am J Clin Nutr 104:736–742
Bastide NM, Pierre FH, Corpet DE (2011) Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res 4:177–184
Boyer E, Le Gall-David S, Martin B et al (2018) Increased transferrin saturation is associated with subgingival microbiota dysbiosis and severe periodontitis in genetic haemochromatosis. Sci Rep 8:15532
Loreal O, Gosriwatana I, Guyader D et al (2000) Determination of non-transferrin-bound iron in genetic hemochromatosis using a new HPLC-based method. J Hepatol 32:727–733
Kozu T, Iinuma G, Ohashi Y et al (2009) Effect of orally administered bovine lactoferrin on the growth of adenomatous colorectal polyps in a randomized, placebo-controlled clinical trial. Cancer Prev Res 2:975–983
Morita Y, Ishikawa K, Nakano M et al (2017) Effects of lactoferrin and lactoperoxidase-containing food on the oral hygiene status of older individuals: a randomized, double blinded, placebo-controlled clinical trial. Geriatr Gerontol Int 17:714–721
Acknowledgements
The authors thank Drs. Kenichi Iyama and Yoshiki Mikami for the pathological diagnoses. The authors also thank Mototsugu Shimokawa for the statistical analysis of the data.
Funding
This work was supported by JSPS KAKENHI (Grant Numbers 19K09199).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any conflict of interest.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. All patients gave informed consent for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10147_2021_1995_MOESM2_ESM.pptx
Supplementary Figure S1. Kaplan–Meier curve of overall survival in patients with colorectal cancer who have undergone colorectal resection according to preoperative (A) and intraoperative (B) blood transfusion (PPTX 125 KB)
About this article
Cite this article
Sawayama, H., Miyamoto, Y., Mima, K. et al. Preoperative iron status is a prognosis factor for stage II and III colorectal cancer. Int J Clin Oncol 26, 2037–2045 (2021). https://doi.org/10.1007/s10147-021-01995-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01995-9