Abstract
Purpose The goal of this study was to compare the sensitivity of MRI and scintigraphy for detecting metastatic bone disease involving the axial skeleton. Patients and Methods A total of 59 patients (58 women and 1 man, age range 28–83 years, mean age 53.0 years) with histopathologically proven breast cancer during a 15-month period (between April 2003 and January 2004) were included in the study. All the patients underwent scintigraphy and MRI examinations for staging, follow-up, or evaluation of bone pain. Results MR imaging revealed 59 metastases in 59 patients (sensitivity, 95%; specificity, 100%; positive predictive value, 100%). Four lesions detected by MRI were classified as of uncertain origin (grade 2) and 36 lesions were regarded as definitely benign (grade 1). Scintigraphy revealed 44 metastases in 59 patients (sensitivity, 70%; specificity, 94%; positive predictive value, 95%). A total of 29 lesions were considered as of uncertain origin (grade 2), and 26 lesions were regarded as definitely benign (grade 1). About five lesions were graded as grade 2 in scintigraphy, while MRI graded them as degeneration or benign compression (Grade 1). For 11 lesions the same grade was regarded in both MRI and scintigraphy. Two lesions graded as grade 3, and eleven lesions graded as grade 2 in scintigraphy demonstrated no pathological signal intensity in MRI. In total, 18 lesions with no activity in scintigraphy were graded as grade 3 lesions in MRI. Conclusion MRI is more sensitive than scintigraphy in the detection of bone metastases. MRI appears to be able to screen patients more effectively than scintigraphy if the spine and pelvis are included because metastases merely outside the axial skeleton are rare.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer represents the most common tumor in women [1] and bone is a common site of metastatic disease in breast cancer [2, 3]. Metastases are predominantly found in the axial skeleton [4]. Patients with skeletal metastases have a favorable prognosis compared to patients with extraskeletal metastases [5, 6]. Rapid therapy by early diagnosis [7] may decrease morbidity arising from bone pain [8] or spinal cord compression.
Scintigraphy using technetium 99-m methylene diphosphonate enables imaging of the entire skeleton with high sensitivity, but limited specificity [9]. New imaging techniques such as single-photon-emission computed tomography (SPECT), positron emission tomography (PET) and magnetic resonance imaging (MRI) can identify bone metastases at an earlier stage [10, 11]. MRI is sensitive to bone marrow abnormalities [12–14] and yields information on tumor extent, vertebral morphology, spinal cord compression, or medullary metastases. Such information cannot be obtained by scintigraphy. MRI may differentiate metastases from osteoporotic fractures [15] and other causes of increased bone metabolism such as degenerative disease. Several studies have compared scintigraphy with MR imaging in tumor detection and characterization [16–18]. Although most of these studies of localized areas conclude that MR imaging is both more sensitive and more specific than scintigraphy, its use as an alternative to scintigraphy for whole-body imaging has been limited by cost, acquisition time, and convenience [16–18].
The goal of this study was to compare the sensitivity of MRI and scintigraphy for detecting metastatic bone disease involving the axial skeleton.
Subject and methods
Patient data
A total of 59 patients (58 women and 1 man, age range 28–83 years, mean age 53.0 years) with histopathologically proven breast cancer during a 15-month period (between April 2003 and January 2004) were included in the study. All the patients underwent scintigraphy and MRI examinations for staging, follow-up or evaluation of bone pain. Exclusion criteria were based on contraindications to MR imaging, such as the presence of a cardiac pacemaker. The mean interval between the MRI and scintigraphy examinations was 15 days (range 10–40 days).
Imaging protocol
The MR examinations were performed on a 1.5-T system (Symphony; Siemens Medical Solutions, Issaquah, WA, USA). For MR studies high-field strength scanners were used.
The cervical vertebrae, acromioclavicular and glenohumeral joints, proximal part of humeral diaphyses, dorsolumbar vertebrae, sternum and costae, sacrum and femur were evaluated by MRI.
In all the patients, sagittal-T1 weighted spin-echo (TR 616 ms, TE 20 ms) and STIR (TR 6,660 ms, TE 81 ms) MR sequences were used. A matrix size of 256, rectangular field-of-view, slice thickness between 5 and 7 mm, interslice gap 1.5 mm was used.
Whole-body scintigraphy was performed 2–3 h after injection of 550 MBq of Tc-99 m MDP in anterior and posterior projections by use of a large-field gamma camera equipped with all-purpose collimators.
Image interpretation
MR images and scintigrams were initially reviewed by two experienced observers who were unaware of the results of the corresponding imaging technique, with interpretation based on consensus. Subsequently, images were reread with bone scan and corresponding MR images side to side to ensure that concordant lesions were truly concordant and that discordance actually existed. Each lesion was graded from 1 to 3 (1 = benign, 2 = uncertain origin, and 3 = malignant) based on the probability of being malignant.
On MRI a lesion was considered to be malignant when there was a focal or diffuse hypointensity on the T1-weighted scan and corresponding intermediate to high signal intensity on the STIR image. To differentiate metastases from benign lesions, additional criteria like the bull’s eye and halo sign were considered as described by Schweitzer et al. [19]. For the spine, additional criteria for malignant infiltration included bulging of the posterior margin of the vertebral body, signal intensity changes extending into the pedicles, and paraosseous tumor extension. A lesion was considered as uncertain in origin when the differentiation between a metastatic and a benign process, such as osteoporotic fracture or bone marrow reconversion, was not possible. A lesion was regarded to be benign when it was located directly adjacent to degenerative changes of the vertebral endplates or near joint surfaces or when the lesion displayed high signal intensity on T1-weighted scans.
On bone scintigraphy, criteria for lesion characterization were distribution of tracer accumulation as well as localization, shape, and intensity of focal tracer uptake. A lesion was regarded to be benign and of degenerative origin when focal tracer accumulation occurred adjacent to joint surfaces. Well-circumscribed linear tracer uptake involving the thoracic or lumbar spine or symmetrical tracer uptake of adjacent ribs was considered to be a benign process caused by osteoporotic or traumatic fractures. A focal lesion was regarded to be malignant when the distribution and pattern of focal accumulation could not be explained by degenerative or posttraumatic changes. Findings for which a differentiation between degenerative, posttraumatic, or tumorous origin of tracer uptake was impossible were considered as uncertain.
To determine the diagnostic potential of both modalities in different anatomic regions MR images and bone scans were evaluated separately for the following areas: thoracic spine, lumbar spine, pelvis including sacrum, proximal parts of upper and lower extremities, rib cage including sternum, and shoulder girdle including scapula and clavicles. To confirm or rule out the initial findings and to classify findings into benign and malignant lesions, follow-up studies were performed with MRI, bone scintigraphy, CT, and radiographs over a 3- to 6-month period. A lesion was considered as metastatic when it showed progression on the same imaging modality on follow-up examination or fulfilled typical criteria of a malignant lesion on a follow-up examination with another imaging modality.
Statistical analysis
For the purpose of our study, a gold standard was established by correlation with clinical outcome (minimum follow-up, 1 year), additional imaging (plain graphy, CT, and MRI). Using the established gold standard, sensitivity, specificity, and positive predictive values were calculated for each technique. Using the Mc Nemar test, we compared the sensitivities of the two imaging techniques.
Results
Both MR and scintigraphic imagings were well tolerated by all patients. With the imaging protocol used in our study, the axial skeleton could be imaged within 25–30 min of acquisition time. The complete examination, including patient positioning and changing of MR coils, required 45 min of room time.
MR imaging revealed 59 metastases in 59 patients (sensitivity, 95%; specificity, 100%; positive predictive value, 100%). About 59 lesions were graded as definitely being metastatic on MRI. All of these lesions demonstrated low signal intensity on T1-weighted sequences and intermediate to high signal intensity on STIR images. Of 59 lesions, 54 (91.5%) were located in the axial skeleton (spine, pelvis), whereas 5 metastatic lesions (8.5%) were detected in other parts of the skeleton (extremities, ribs, and sternum). Four lesions detected by MRI were classified as of uncertain origin (grade 2) and 36 lesions were regarded as definitely benign (grade 1).
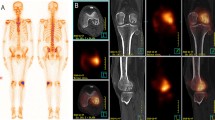
Scintigraphy revealed 44 metastases in 59 patients (sensitivity, 70%; specificity, 94%; positive predictive value, 95%). Bone scintigraphy detected 44 lesions that fulfilled the criteria of a metastatic deposit. Of the 44 lesions, 36 (82%) were located in the axial skeleton and 8 (18%) in the extremities, ribs, sternum. A total of 29 lesions were considered as of uncertain origin (grade 2), and 26 lesions were regarded as definitely benign (grade 1). About 5 lesions were graded as grade 2 in scintigraphy, while MRI graded them as degeneration or benign compression (Grade 1) (Fig. 1). For 11 lesions the same grade was regarded in both MRI and scintigraphy (3 lesion grade 3; 2 lesion grade 2; 6 lesion grade 1) (Fig. 2). Two lesions graded as grade 3, and 11 lesions graded as grade 2 in scintigraphy demonstrated no pathological signal intensity in MRI (Fig. 3). A total of 18 lesions with no activity in scintigraphy were graded as grade 3 lesions in MRI. In pelvic region the number of MRI grade 3 lesions was 25, while in scintigraphy it was 3. The 17 lesions depicted in sacrum, pubis, and ischion in MRI demonstrated no activity in scintigraphy.
A 62 years old female with back pain operated 5 years ago for right breast cancer. Whole-body scintigram (anterior view) (a) shows metastatic activity increase in D8 vertebrae (arrow) MR images shows height loss in the D8 vertebrae body, T1 hypointense (b), STIR hyperintense, and (c) pathological signal changes (arrows). Note almost complete regression of the pathological signal changes in the follow-up MR images after 45 days (d) due to benign osteoporotic compression fracture (arrow)
A 63 years old female operated 4 years ago with left breast cancer. Whole-body scintigram shows suspect activity increase in the right sternoclavicular region. In the sagittal T1 (b) and STIR (c) weighted MR sequences for the dorsolumbar region depicts T1 hypo-STIR hyperintense metastatic lesions in the dorsal and lumbar vertebrae (arrows). In the coronal T1 (d) and STIR (e) weighted sequences for the pelvis, multiple T1 hypo-STIR hyperintense metastatic lesions in the right side of the sacrum, ilium, and ischium, intertrochanteric region of the right femur and the left collum femoris (arrows)
Exact correlation between both techniques was found in 46 (78%) of the 59 patients. Discrepancy between the two techniques was observed in 13 patients (22%) (Mc Nemar test, 0.001; P = .016).
Additional findings demonstrated by MRI were lung metastases and pleural effusion (two patients), ovarian cyst (one patient), renal cyst (one patient), enchondromas in proximal diaphysis of femora (one patient), spondylolisthesis and degenerative changes (two patients), and degenerative cyst in right caput humeri (one patient).
Discussion
Bone is a common site of metastatic disease in breast cancer. While the frequency of bone metastases is 1–2% at the time of primary diagnosis [2, 3], bone metastases are found in approximately one-third of the patients with recurrent disease. Bone metastases may occur with almost all malignancies, but they are most common in carcinomas of the breast (47–85%), lung (32%), prostate (54–85%), kidney (33–40%), or thyroid (28–60%) [20, 21]. At autopsy, bone metastases are found in more than half of the patients who die of breast cancer [22, 23].
The spine is the most common site of skeletal metastases (39%) because of the abundant vascularization and red bone marrow [15, 18]. Most bone metastases are hematogenous in origin [15, 24]. The initial seeding of metastatic deposits via hematogenous spread is typically localized in the hematopoetic (red) marrow. This location explains the predominance of metastatic bone lesions in the axial skeleton (>90% of metastatic bone lesions) [25].
The imaging of spinal metastatic disease may include conventional radiography, myelography, radionuclide bone scintigraphy, CT, and MR imaging. Radiography, CT, and bone scans assess mainly the bony abnormality, particularly the cortex, whereas MR imaging examines bone marrow, in which the early metastatic deposits frequently occur [24]. Findings of abnormal bone on radiography, bone scintigraphy, and CT mainly result from tumor invasion of the cortical bone rather than of the medullary bony matrix. CT is sensitive in detecting subtle cortical invasion, but is less sensitive for medullary bone or bone marrow involvement [18]. Bone destruction can be difficult to detect on CT in the presence of osteoporosis or degenerative changes, which are common in elderly adults with cancer [26].
The mechanism of abnormal Tc-99 m MDP uptake shown in bone scanning is complex. Abnormal radionuclide uptake is generally believed to increase with regional bone-blood flow, bone remodeling, formation of new bone, and enhanced bone matrix turnover [27, 28]. For many years, bone scintigraphy using technetium-labeled diphosphonate analogs has been used to provide whole-body imaging of the skeleton to detect skeletal metastases, either manifested as focal accumulation (a hot spot) or as the absence of radiotracer (a cold spot) [29]. Although planar scintigraphy is easily performed and is the current method of choice for whole-body skeletal evaluation, it creates a limited representation of the body in a single composite plane or multiple oblique planes with both poor spatial and contrast resolution [29]. Similarly, as technetium-labeled diphosphonate analogs are taken up by chemisorptions onto the phosphorus groups of calcium hidroxyapatite produced by osteoblasts, concentration of isotope at scintigraphy is essentially an indirect marker of tumor, in effect indicating the host osteoblastic response to tumor deposits.
Alternative screening modalities, such as conventional radiography and CT, for the detection of bone marrow metastases have been shown to be less sensitive than skeletal scintigraphy [30]. Bone marrow scintigraphy showed a higher sensitivity for the detection of bone marrow metastases than skeletal scintigraphy [31].
Magnetic resonance imaging is sensitive to bone marrow abnormalities [12–14] and yields information on tumor extent, vertebral morphology, spinal cord compression, or medullary metastases. Such information cannot be obtained by scintigraphy. MRI may differentiate metastases from osteoporotic fractures [15] and other causes of increased bone metabolism such as degenerative disease. In our study five cases were younger than 40 ages, and 38 cases were older than 50 ages. Due to the high prevalence of osteoporosis and degenerative changes, MRI provides better information in the middle and old age group for differentiation of metastases compared to scintigraphy.
For extremities, pelvis, and spine, MR imaging is clearly superior to bone scanning and provides important information about tumor morphology, tumor extension, and neurological complications. Its inferiority in detecting metastases of skull and ribs is likely related to the small and curved marrow spaces of these bones and, in the thoracic region, to additional motion artifacts of respiration and pulsation. In recent studies whole-body MR imaging showed a similar number of lesions in the head–neck region, ribs, scapulae and skull as in scintigraphy and depicted more lesions in the chest wall [32].
There are a number of studies demonstrating the high sensitivity of MRI in the detection of bone marrow metastases and its advantages over bone scintigraphy and conventional radiography [33–36].
In a retrospective study comparing scintigraphy and MR imaging for the detection of spinal metastases in 35 patients, Gosfield et al. [37] described 69 lesions using MRI and 63 lesions using scintigraphy. No patient with positive bone scan results had negative MR scan results, but one patient with positive MR scan results was noted to have a bone scan with completely normal findings. Fujii et al. [38] compared scintigraphy and MR imaging examinations of 36 patients with suspected metastatic prostatic cancer to the spine and found that MR imaging had revealed in six patients metastatic lesions that were not shown on scintigraphy. Likewise, Flickenger et al. [39], in study of 32 patients with metastatic breast cancer, reported a sensitivity of 100% and a specificity of 73% for MR imaging, versus a sensitivity of 62% and a specificity of 100% for scintigraphy. In our study 59 and 44 lesions were diagnosed as definitely malignant in MRI and scintigraphy, respectively. In MRI 54, and in scintigraphy 36 vertebral metastases were depicted in the axial skeleton. 18 MR grade 3 lesions had no activity in scintigraphy, while 2 scintigraphy grade 3 lesions demonstrated no pathological signal intensity in MR.
An important aspect of our study is that with scintigraphy, significantly more lesions were graded as uncertain in origin, especially in the spine. This finding proves that the morphologic information provided by MRI is of great value for the differentiation between benign and malignant lesions. In addition, MRI offers important information for treatment planning and detects imminent tumor associated complications that can lead to early and adequate treatment [40].
While comparing the costs of axial skeletal MRI and bone scintigraphy, one has to consider that because of the aforementioned reasons, the results of nuclear medicine studies often require additional examinations like plain films, CT, or even MRI. The cost is three times higher with MRI plus scintigraphy. Thus MRI alone is not a more expensive imaging method.
The disadvantage remains to be the inability to image the entire skeleton within reasonable time. However, our data depict that metastases merely in remote areas of the axial skeleton are rare. This observation could justify the screening of patients with breast carcinoma by MRI of the axial skeleton [4]. Scintigraphy might be added for staging in patients with positive MRI results to screen remote skeletal regions. New developments in sequence design have led to considerable reduction of acquisition times. For imaging of bone marrow lesions, a combination of T1-weighted and STIR sequences provides all the information required, as these sequences combine anatomical information and high image quality with a high sensitivity and specificity in lesion detection [39, 41]. In comparison with a recent study by Eustace et al. [16], who used only STIR sequences for whole-body imaging, our protocol includes in addition T1-weighted sequences, which we found to be very helpful for the differentiation between benign and malignant lesions. The use of fast MR sequences and a large field-of-view does not have a limiting effect on the lesion detection rate for the bone marrow spaces of extremities, pelvis, and spine. For these areas, MRI was clearly superior to bone scanning and provided important information about tumor morphology, tumor extension, and neurological complications [40].
Accurate comparison of two imaging techniques requires a gold standard, which traditionally has been biopsy or histopathology. The ideal histological correlation, made by biopsy of every presumed lesion, was not performed in this study. Correlation with other imaging techniques; additional imaging after 1 year were used to validate our results (gold standard), similar to many early studies that established the validity of scintigraphy and other comparative studies between scintigraphy and MR imaging [16].
This study reveals the unique potential of axial skeletal MR imaging including to provide skeletal screening in patient with known or suspected bone metastases of breast cancer. Specifically, our study depicts the ability of MR imaging to provide screening for skeletal metastases as an alternative to planar scintigraphy in our institution at a similar cost (total charge), based on unit-time costing. In effect, 25–30 min of MR scan time (the time for an average axial skeletal MR study) is equivalent to the time currently charged for routine MR study of knee and equivalent to the total cost of 99 m-Tc-methylene diphosphonate scintigraphy.
In conclusion, bone marrow imaging MRI is more sensitive than scintigraphy in the detection of bone metastases. MRI appears to be able to screen patients more effectively than scintigraphy if the spine and pelvis are included because metastases merely outside the axial skeleton are rare. Both the methods demonstrate a high specificity in differentiating benign from malignant causes of symptoms [4]. Further studies will have to show if MRI of the entire skeleton will change current concepts in screening tumor patients for bone metastases.
References
Landis SH, Murray T, Bolden S, et al. Cancer statistics. CA Cancer J Clin 1999;49:8–31.
Perez DJ, Powles TJ, Milan J, et al. Detection of breast carcinoma metastases in bone: relative merits of x-rays and skeletal scintigraphy. Lancet 1983;2:613–6.
Rossing N, Munck O, Nielsen SP, Andersen KW. What do early bone scans tell about breast cancer patients? Eur J Cancer Clin Oncol 1982;18:629–36.
Altehoefer C, Ghanem N, Hogerle S, Moser E, Langer M. Comparative detectability of bone metastases and impact on therapy of magnetic resonance imaging and bone scintigraphy in patients with breast cancer. Eur J Radiol 2000;40:16–23.
Sherry MM, Greco FA, Johnson DH, Hainsworth JD. Metastatic breast cancer confined to the skeletal system. Am J Med 1986;81:381–6.
Sherry MM, Greco FA, Johnson DH, Hainsworth JD. Breast cancer with skeletal metastases at initial diagnosis. Distinctive clinical characteristics and favorable prognosis. Cancer 1986;58:178–82.
Schocker JD, Brady LW. Radiation therapy for bone metastasis. Clin Orthoped Rel Res 1982;169:38–43.
Gilbert HA, Kagan R. Evaluation of radiation therapy for bone metastases: pain relief and quality of life. Am J Roentgenol 1977;129:1095.
Kamby C, Vejborg I, Daugaard S, Guldhammer B, Dirkson H, Rossing N, Mouridson HAT. Clinical and radiological characteristics of bone metastases in breast cancer. Cancer 1987;60:2524–31.
Ryan PJ, Fogelmen I. The bone scan: where are we now? Semin Nucl Med 1995;25:76–91.
Rosenthal DI. Radiologic diagnosis of bone metastases. Cancer 1997;80(Suppl 8):1595–607.
Daffner RH, Lupetin AR, Dash N, Deeb ZL, Sefczek RJ, Schapiro RL. MRI in the detection of malignant infiltration of bone marrow. Am. J. Roentgenol 1986;146:353–8.
Hoane BR, Shields AF, Porter BA, et al. Detection of lymphomatous bone marrow involvement with magnetic resonance imaging. Blood 1991;78:728–38.
Sanal SM, Flickinger FW, Caudell MJ, Sherry RM. Detection of bone marrow involvement in breast cancer with magnetic resonance imaging. J. Clin Oncol 1994;12:1415–21.
Yuh WTC, Zachar CK, Barloon TJ, Sato Y, Sickels WJ, Hawes DR. Vertebral compression fractures: distinction between benign and malignant causes with MR imaging. Radiology 1989;172:215–8.
Eustace S, Tello R, DeCarvalho V, Carey J, Wroblicka JT, Melhem ER, Yucel EK. A comparison of whole-body turboSTIR MR imaging and planar 99 mTc-methylene diphosphonate scintigraphy in the examination of patients with suspected skeletal metastases. AJR Am J Roentgenol 1997;169:1655–61.
Kattapuram SV, Khurana JS, Scott JA, El Khoury G. Negative scintigraphy with positive magnetic resonance imaging in bone metastasis. Skeletal Radiol 1990;19:204–7.
Aitchison FA, Poon FW, Hadley MD, Gray HW, Forrester AW. Vertebral metastases and equivocal bone scan: value of magnetic resonance imaging. Nucl Med Commun 1992;13:429–31.
Schweitzer ME, Levine C, Mitchell DG, Gannon FH, Gomella LG. Bull’s-eyes and halos: useful MR discriminators of osseous metastases. Radiology 1993;188:249–52.
Marcove RC, Arlen M. Atlas of bone pathology: with clinical and radiographic correlations. 1st ed. Philadelphia: Lippincott; 1992. p. 14–21.
Galasko CSB. Skeletal metastases. Clin Orthop 1986;210:18–30.
Viadana E, Bross IDJ, Pickren JW. An autopsy study of some routes of dissemination of cancer of the breast. Br J Cancer 1973;27:336–40.
Bunting JS, Hemsted EH, Kremer JK. The pattern of spread and survival in 596 cases of breast cancer related to clinical staging and histological grade. Clin Radiol 1976;27:9–15.
Yuh WT, Quets JP, Lee HJ, et al. Anatomic distribution of metastases in the vertebral body and modes of hematogenous spread. Spine 1996;21:2243–50.
Thrall TH, Ellis BI. Skeletal metastases. Radiol Clin North Am 1987;25:1155–70.
Kamholtz R, Sze G. Current imaging in spinal metastatic disease. Semin Oncol 1991;18:158–69.
Delbeke D, Powers TA, Sandler MP. Correlative radionuclide and magnetic resonance imaging in evaluation of the spine. Clin Nucl Med 1989;14:742–9.
Gold RH, Bassett LW. Radionuclide evaluation of skeletal metastases: practical considerations. Skeletal Radiol 1986;15:1–19.
Gold RH, Seeger LL, Bassett LW, Steckel RJ. An integrated approach to the evaluation of metastatic bone disease. Radiol Clin North Am 1990;28:471–83.
Kosuda J, Kaji T, Yokoyama H, et al. Does bone SPECT actually have lower sensitivity for detecting vertebral metastasis than MRI? J Nucl Med 1996;37:975–8.
Haubold-Reuter BG, Duewell S, Schilcher BR, Marincek B, von Schulthess GK. The value of bone scintigraphy, bone marrow scintigraphy and fast spin-echo magnetic resonance imaging in staging of patients with malignant solid tumours: a prospective study. Eur J Nucl Med 1993;20:1063–9.
Mentzel HJ, Kentouche K, Sauner D, Fleischmann C, Vogt S, Gottschild D, Zintl F, Kaiser WA. Comparison of whole-body STIR-MRI and 99 mTc-methylene-diphosphonate scintigraphy in children with suspected multifocal bone lesions. Eur Radiol 2004;14:2297–302.
Avrahami E, Tadmor R, Dally O, Hadar H. Early MR demonstration of spinal metastases in patients with normal radiographs and CT and radionuclide bone scans. J Comput Assist Tomogr 1989;13:598–602.
Frank JA, Ling A, Patronas NJ, Carrasquillo JA, Horvath K, Hickey AM, Dwyer AJ. Detection of malignant bone tumors: MR imaging vs scintigraphy. AJR Am J Roentgenol 1990;155:1043–8.
Colletti PM, Dang HT, Deseran MW, Kerr RM, Boswell WD, Ralls PW. Spinal MR imaging in suspected metastases: correlation with skeletal scintigraphy. Magn Reson Imaging 1991;9:349–55.
Mehta RC, Wilson MA, Perlman SB. False-negative bone scan in extensive metastatic disease: CT and MR findings. J Comput Assist Tomogr 1989;13:717–9.
Gosfield E 3rd, Alavi A, Kneeland B. Comparison of radionuclide bone scans and magnetic resonance imaging in detecting spinal metastases. J Nucl Med 1993;34:2191–8.
Fujii Y, Higashi Y, Owada F, Okuno T, Mizuno H, Mizuno H. Magnetic resonance imaging for the diagnosis of prostate cancer metastatic to bone. Br J Urol 1995;75:54–8.
Flickinger FW, Sanal SM. Bone marrow MRI: techniques and accuracy for detecting breast cancer metastases. Magn Reson Imaging 1994;12:829–35.
Steinborn MM, Heuck AF, Tiling R, Bruegel M, Gauger L, Reiser MF. Whole-body bone marrow MRI in patients with metastatic disease to the skeletal system. J Comput Assist Tomogr 1999;23:123–9.
Jones KM, Unger EC, Granstrom P, Seeger JF, Carmody RF, Yoshino M. Bone marrow imaging using STIR at 0.5 and 1.5 T. Magn Reson Imaging 1992;10:169–76. .
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yilmaz, M.H., Ozguroglu, M., Mert, D. et al. Diagnostic value of magnetic resonance imaging and scintigraphy in patients with metastatic breast cancer of the axial skeleton: a comparative study. Med Oncol 25, 257–263 (2008). https://doi.org/10.1007/s12032-007-9027-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-007-9027-x