Abstract
In this study, we aimed to establish the effects of chronic corticosterone (CORT) and ethanol administration on mood-related behaviour and the levels of mature brain-derived neurotrophic factor (mBDNF) and its precursor protein proBDNF in mice. C57BL6 male and female mice received drinking water (n = 22), 1% ethanol in drinking water (n = 16) or 100 μg/ml corticosterone in drinking water (containing 1% ethanol, n = 18) for 4.5 weeks. At the end of experimental protocol, the open field test (OFT) and elevated plus maze test were performed. Brain and adrenal tissues were collected and mBDNF and proBDNF were measured by ELISA assays. We found that the mice fed with corticosterone and ethanol developed anxiety-like behaviours as evidenced by reduced time in the central zone in the OFT compared with the control group. Both proBDNF and mBDNF were significantly decreased in the corticosterone and ethanol groups compared with the control group in the prefrontal cortex, hippocampus, hypothalamus and adrenal. The ratio of proBDNF/mBDNF in prefrontal cortex in the corticosterone group was increased compared with the ethanol group. Our data suggest that the ratio of proBDNF/mBDNF is differentially regulated in different tissues. Ethanol and corticosterone downregulate both mBDNF and proBDNF and alter the balance of proBDNF/mBDNF in some tissues. In conclusion, the ethanol and corticosterone may cause abnormal regulation of mBDNF and proBDNF which may lead to mood disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic stress is a major contributor to the aetiology of mood disorders including anxiety and depression. There is evidence that increased blood levels cortisol (corticosterone in rodents) is a consequence of hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis under chronic stress conditions (Pariante and Lightman 2008; Frodl and O’Keane 2013; Cai et al. 2015). Thus, one of the methods to establish a chronic stress rodent model can be achieved by direct administration of corticosterone (CORT) to induce the depression and anxiety-like symptoms (Donkelaar et al. 2014; Mishima et al. 2015; Siopi et al. 2016). One advantage of this method is that it reduces the individual or sex-dependent variability in response to stress, as well as the variability in effectiveness of the various chronic stressors (Zhao et al. 2008). Therefore, administering corticosterone has successfully been used by many researchers in rodents as a means to study the neurobiological mechanism underlying stress-related mood disorders and also used for screening effective anxiolytics and antidepressants (Zhao et al. 2008; Pazini et al. 2016).
Alcohol consumption is another factor that has been linked to mood disorders in humans and animals. Clinical studies suggested that people involved in alcohol misuse tend to have changes in the limbic system especially reduced hippocampal volume which could lead to the affective disorders (Morris et al. 2005; Paul et al. 2008; Morris et al. 2010; Schneier et al. 2010; Sameti et al. 2011; Lee et al. 2016). Animal models also showed that rats chronically exposed to alcohol develop depressive-like behaviour indicated by the reduction in the sucrose preference test (SPT) and decreases the expression of brain-derived neurotrophic factor (BDNF) in the brain (Boden and Fergusson 2011; Briones and Woods 2013; Pedrelli et al. 2016; Awaworyi Churchill and Farrell 2017). However, the mechanisms responsible for such links are not established yet.
Brain-derived neurotrophic factor (BDNF) and its precursor protein proBDNF play opposing roles in neuronal plasticity and mood regulation (Hempstead 2015). ProBDNF is a potent neurodegenerative factor that significantly inhibits proliferation of neural stem cells and reduces the number of differentiated neurons, oligodendrocytes and astrocytes leading to neurite collapse, neuronal apoptosis and suppressed neurogenesis (Sun et al. 2012; Li et al. 2017). There is evidence that mature BDNF is downregulated whereas proBDNF is upregulated in major depression patients and animal models of depression induced by chronic stress (Zhou et al. 2013; Ruan et al. 2014; Yang et al. 2014a; Yang et al. 2014b). We have also shown that the proBDNF/p75NTR/sortilin pathway is activated in peripheral blood of patients with alcohol dependence (Gourley et al. 2008; Ali et al. 2015; Sousa et al. 2015; Zhou et al. 2018). These results suggest that proBDNF may be contributing to chronic stress- and alcohol-induced mood disturbances, and the ratio of proBDNF/mBDNF may be important in the maintenance of brain functions in physiological conditions.
A number of rodent studies have investigated the effects of chronic corticosterone and ethanol administration on mood-related behaviours and BDNF expression in the brain. Many of these studies have shown that the animals develop anxiety and/or depression-like behaviours in parallel with decreased levels of BDNF in the hippocampus (Gourley et al. 2008; Ali et al. 2015; Sousa et al. 2015; Pazini et al. 2016). However, studies investigating the effects of corticosterone or ethanol on the levels of proBDNF or the ratio of proBDNF to BDNF in the brain or periphery are lacking, while this is important because both BDNF and proBDNF have functional roles and may be significant regulators of mood behaviours (Davis 2008; Logrip et al. 2015). Thus, in this study, we hypothesised that chronic corticosterone and ethanol administration to mice would cause changes in proBDNF and mature BDNF in parallel with mood disturbances. Our main aim was to investigate the effects of chronic corticosterone and low level of ethanol consumption (1% administered to mice in drinking water) on the levels of mature BDNF and proBDNF in the prefrontal cortex (PFC), hippocampus and tissues related to HPA axis and examine the anxiety-like behaviours.
Methods
Animals
All animal procedures and behavioural tests were performed in compliance with the protocols approved by the Animal Ethics Committee of the University of South Australia (project number U23-15). Fifty-six 8-week-old C57BL6 mice (male n = 28, female n = 28) were bred in the Reid Building Animal facility (University of South Australia) and maintained under standard conditions (12:12-h light/dark cycle, lights on between 6 a.m. and 6 p.m., temperature of 22 ± 1 °C, humidity of 52 ± 2%). Mice were housed in groups of 4–5 per cage and provided with free access to water and conventional food. Animals were acclimatised to the environment for 1–2 weeks prior to the experiments and then randomly assigned to specific experimental groups such as control group, ethanol group and corticosterone (CORT) group. Mice were weighed before experiments and weekly thereafter. Behavioural tests were carried out during the light phase between 09:00a.m and 5:00 p.m. On completion of this experiment, the mice were deeply anaesthetised and humanely killed for tissue collection. The brain and peripheral tissues including prefrontal cortex, hippocampus, hypothalamus, pituitary and adrenal glands were collected and kept at − 80 °C for further analyses.
Drugs and Treatments
Pure (purity ≥ 98%) corticosterone (CORT) (11β,21-dihydroxy-pregn-4-ene-3,20-dione) was obtained from Cayman Chemical (USA) and was dissolved in ethanol (100%) and added to the drinking water to make a treatment solution containing 1% ethanol and 100 μg/ml of CORT. Mice were divided into three groups for experimental purpose (control, ethanol and CORT); all groups received their treatments via drinking water for a total duration of 4.5 weeks. The control group (n = 22, male = female) received tap water; the ethanol group (n = 16, male = female) received 1% ethanol made in tap water; the CORT group (n = 18, male = female) received CORT solution (in 1% ethanol in tap water). Drinking bottles containing CORT solution were covered by tinfoil and the solution was changed every 3 days to avoid any possible CORT degradation. The amount of consumption was closely monitored and calculated by weighing bottles to ensure the sufficient provision. We housed mice in groups of 4–5/cage and could not measure consumption of liquid by individual mouse in this study. However, we measured total amount of consumed liquid by all mice in each cage on a daily basis during the last 2 weeks of the study and estimated that each mouse consumed about 5 ml/day (on average). Thus, the approximate amount of ethanol and corticosterone consumed by one mouse was 50 μl/day and 0.5 mg/day, respectively.
Behavioural Tests
After 4.5 weeks, all mice were subjected to behavioural tests including open field test (OFT) and elevated plus maze test (EPM) to assess spontaneous locomotor activity and anxiety-like behaviour. Behavioural tests were conducted during light phase between 9:00 a.m. and 5:00 p.m. and only single test was allowed on 1 day. OFT was performed in an open field of white Plexiglas container with the dimensions of 40 cm (long) × 40 cm (wide) × 40 cm (height). The open field was divided into 5 cm × 5 cm equal squares and the middle nine squares (3 × 3) were defined as the central zone. Mouse was placed in the central area of the open field and was able to travel freely in the field for 5 min. The activities of mouse were immediately recorded by an overhead digital camera and results were analysed by ANY-maze software. EPM test used a plus-cross-shaped grey Plexiglas apparatus which was elevated 50 cm above the floor. The apparatus consisted of two closed arms (50 cm long × 10 cm wide × 40 cm height) and two open arms, the same as closed arms but without two walls, as well as a central platform (7 × 7cm). The mouse was placed in the centre of the maze with nose pointing to the closed arm. The travel distance and the number of entries to different arms were recorded for 5 min by the ANY-maze video software elevated to a height of 50 cm, in which two arms were open and two were closed. The number of times the animal entered each of the arms and the time spent in each arm were recorded during the 5-min test period. The procedures were performed in the special behavioural test room in the animal house.
Enzyme-Linked Immunosorbent Assay
ELISA is a highly specific assay developed in our laboratory that allows differential detection of proBDNF and mature BDNF(mBDNF) levels in tissues (Lim et al. 2015). Tissues were homogenised in RIPA buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 0.5% Sodium deoxycholate, pH 7.4) with a protease inhibitor cocktail (Sigma St. Louis, USA), spun for 30 min at 4 °C and supernatants were collected and stored at − 80 °C until ELISA assay (Lim et al. 2015). The protocols of mBDNF and proBDNF sandwich ELISA were described in the previous publication (Lim et al. 2015). The levels of mBDNF and proBDNF and the ratio of proBDNF/mBDNF in different tissues including hippocampus, PFC, hypothalamus, pituitary and adrenal grands were measured and compared.
Western Blot
To validate proBDNF ELISA results, selected samples from different brain regions were used for proBDNF Western blot. Western blot experiments were conducted as described previously (Lim et al. 2015). Results were quantified using Image Quant TL software (GE Healthcare Life Sciences, UK) and the ratios of OD values of proBDNF/β-actin were calculated.
Statistical Analysis
Statistical analyses were performed by using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test as a post hoc analysis using GraphPad Prism 7.03 for Windows, GraphPad Software (San Diego, CA, USA). The correlation between proBDNF levels assayed by ELISA and Western blot methods was tested by Spearman’s correlation analysis. All data were expressed as means + S.E.M (standard errors of the mean) and a value of p < 0.05 was considered statistically significant. Fifty-six samples (male = 28, female = 28) were used in the analysis of behavioural tests and 22–28 (male = female) samples were analysed for the ELISA.
Results
The Effects of Ethanol and CORT on Mice Behavioural Change
Open Field Test
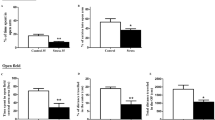
One-way ANOVA was used to analyse the data from OFT. Although the total distance of OFT showed no difference among control, ethanol and CORT groups (Fig. 1a), the travel distance in the central zone was significantly reduced in both ethanol and CORT groups compared with the control group (Fig. 1b). There were significant reductions in the number of entries to the central zone in both ethanol and CORT groups compared with the control group (Fig. 1c). Meanwhile, the mice from the CORT group spent less time in the central zone than the control and ethanol groups (Fig. 1d). The percentage of time in the central zone decreased significantly in the CORT group compared with both ethanol and control groups (Fig. 1e) whereas there was a significant increased percentage of time in the peripheral zone in the CORT group when compared with ethanol and control groups (Fig. 1e). The significant reductions of distance in the central zone and the numbers of entries to the central zone in the ethanol and CORT groups compared with the control group indicated that both ethanol and CORT have effects on these behavioural parameters (Fig. 1b, c).
Effect of ethanol and corticosterone on the behaviours assessed by open field test. a Total travel distance. b Travel distance in the central zone. c Number of entries to the central zone. d Time in the central zone. e Percentage of time spent in the central zone; f Percentage of time spent in the peripheral zone. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with appropriate groups as shown
Elevated Plus Maze
CORT group showed a significant decrease in the total distance and the distance in the closed arm as well as the number of entries to the closed arm compared with the control group (Fig. 2a–e). These decreases were also noticed between CORT and ethanol groups but did not reach the statistical significance (Fig. 2a–e). CORT and ethanol groups showed reduced time and distance travelled in the open arm as well as the number of entries to the open arm compared with the control group but did not reach statistical significance (Fig. 2b–f).
Effect of ethanol and corticosterone on the behaviours assessed by elevated plus maze test. a Total travel distance. b Travel distance in the open arm. c Travel distance in the closed arm. d Number of entries to the open arm. e Number of entries to the closed arm. f Percentage of time spent in the open arm. g Percentage of time spent in the closed arm. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with appropriate groups as shown
The Effects of Ethanol and CORT on BDNF and proBDNF
BDNF and proBDNF Changes in the Brain Tissues of PFC and Hippocampus
In the prefrontal cortex (PFC) and hippocampus, there were significant decreases in the level of mBDNF when comparing CORT and ethanol groups with control group (Fig. 3a). A further significant decrease in the level of mBDNF was found in the PFC when comparing CORT group with the ethanol group (Fig. 3a). The levels of proBDNF in the PFC and hippocampus were also reduced significantly in both CORT and ethanol groups compared with the control group (Fig. 3b, e). There was no significant difference in the levels of proBDNF between CORT and ethanol groups in the PFC and hippocampus (Fig. 3b, e). The ratio of proBDNF/mBDNF increased significantly in the PFC in the CORT group when compared with the ethanol and control groups (Fig. 3c). Both CORT and ethanol groups have shown the tendency of increased ratio of proBDNF/mBDNF in the hippocampus compared with the control group but did not reach the statistical significance (Fig. 3f).
Effect of ethanol and corticosterone on the levels of mature BDNF and proBDNF in the prefrontal cortex (PFC) and hippocampus as determined by ELISA. a mBDNF levels in PFC. b proBDNF levels in PFC. c Ratio of proBDNF/mBDNF in PFC. d mBDNF levels in the hippocampus. e proBDNF levels in the hippocampus. f Ratio of proBDNF/mBDNF in the hippocampus. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with appropriate groups as shown
BDNF and proBDNF Changes in the Tissues of HPA Axis Including Hypothalamus, Pituitary and Adrenals
In the hypothalamus, the level of mBDNF was significantly decreased in the CORT group compared with the control and ethanol groups (Fig. 4a); both ethanol and CORT groups showed significant reductions in the level of proBDNF compared with the control group (Fig. 4b), and the ratio of proBDNF/mBDNF in the ethanol group was significantly decreased vs the control and CORT groups (Fig. 4c). Interestingly, the level of mBDNF in the pituitary was significantly increased in the CORT group compared with the ethanol group and control group (Fig. 4d, Meanwhile, the proBDNF level was significantly decreased in the ethanol group but not in the CORT group compared with the control group (Fig. 4e). There was no difference in the ratio of proBDNF/mBDNF among three groups in the pituitary (Fig. 4f). Both CORT and ethanol groups have shown significant reductions in the levels of mBDNF and proBDNF in the adrenal tissues compared with the control group (Fig. 4g, h). However, the ratio of proBDNF/mBDNF in the adrenals did not change in three groups (Fig. 4i).
Effect of ethanol and corticosterone on the levels of mBDNF and proBDNF in the hypothalamus, pituitary gland and adrenal gland as determined by ELISA. a mBDNF levels in the hypothalamus. b proBDNF levels the hypothalamus. c Ratio of proBDNF/mBDNF in the hypothalamus. d mBDNF levels in the pituitary gland. e proBDNF levels in the pituitary gland. f Ratio of proBDNF/mBDNF in the pituitary gland. g mBDNF levels in the adrenal gland. h proBDNF levels in the adrenal gland. i Ratio of proBDNF/mBDNF in adrenal gland. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, compared with appropriate groups as shown
Discussion
Our main findings are as follows: (a) Mice in both corticosterone and ethanol groups have shown significant anxiety symptoms as indicated in the OFT and EPM; (b) The levels of mBDNF and proBDNF were significantly reduced in the PFC and hippocampus in both CORT and ethanol groups; (c) ethanol has effects on the HPA axis by reducing the levels of proBDNF in hypothalamus, pituitary and adrenal; (d) CORT treatment resulted in the increased ratio of proBDNF/mBDNF in PFC but not in other tissues. Our ELISA results were consistent with the results of Western blots (Fig. 5a) in that both ethanol and CORT reduced the levels of proBDNF in the hippocampus (although only the ethanol group reached statistical significance). Furthermore, the proBDNF levels assayed by the ELISA method were positively correlated with the results produced by Western blot (Fig. 5b). These results suggest that our ELISA assay technique is reliable for the measurement of proBDNF.
Validation of proBDNF ELISA assay by Western blots. a Representative Western blot images on selected samples from three different groups using β-actin as a reference. The levels of proBDNF in the hippocampus were arbitrarily calculated using OD values and expressed as the ratios of proBDNF to β-actin. b proBDNF levels assayed by both ELISA assay and Western blots on selected tissues of hippocampus and prefrontal cortex were analysed by Spearman correlation analysis
In this study, we used ethanol for two purposes: (1) to investigate its own effects on behaviour and biochemistry and (2) to use it as a vehicle control for CORT group (in drinking water) similar to many previous studies investigating mood disorders (Xu et al. 2011; Donkelaar et al. 2014; Mishima et al. 2015). We found that mice treated with 1% ethanol in drinking water exhibited significant anxiety-like behaviours. In the OFT, there were significant decreases in the distance travelled in the central zone and the number of entries into the central zone when comparing the ethanol group with the control group. The total ambulatory distance in open field test showed no difference between groups indicating that locomotor activities were not affected by the ethanol treatment (Seibenhener and Wooten 2015). Therefore, the reductions of activities in the central zone were due to anxiety-like behaviour. Thus, this study demonstrated that chronic low concentration of ethanol (1%) treatment can result in significant anxiety-like behaviours in mice. Our finding is consistent with previous studies that repetitive long-term consumption of alcohol can cause mood changes in both experimental animals and in humans (Kushner et al. 2000; Kliethermes 2005). However, these anxiety-like behaviours induced by ethanol (concentration between 0.2% and 1%) have not been reported in the studies using ethanol as a vehicle for dissolving CORT to develop rodent depression model (Donkelaar et al. 2014; Mishima et al. 2015). Therefore, it should be used with caution as a vehicle for the future studies as ethanol itself even at low concentrations in drinking water (1%) can cause behavioural changes.
In accord with the behavioural changes, we found that animals treated with ethanol also showed reduced mBDNF and proBDNF in the peripheral and central tissues such as PFC, hippocampus, pituitary and adrenals except in the hypothalamus, where mature BDNF was increased. Our results are in agreement with findings from other studies that long-term exposure to ethanol reduces BDNF mRNA in the hippocampus, suggesting this decrease may play a role in neurodegeneration (Davis 2008). Recent studies from Dorit Ron’s laboratory showed that chronic ethanol consumption reduces BDNF in the cortex and hippocampus but increases in the striatum and the changes in BDNF expression levels are associated with heightened ethanol intake (Jeanblanc et al. 2013; Logrip et al. 2015). However, these studies focused on the ethanol addiction by feeding animals with 10% ethanol. Interestingly, the animals consuming very low levels of ethanol (1%) in the present study also showed the reduction of mBDNF and proBDNF expression in the PFC, hippocampus and adrenals suggesting that even low levels of ethanol can cause a decrease in BDNF synthesis in the brain and periphery. The main novelty of our present study is the simultaneous measurement of mBDNF and proBDNF in the regions examined showing their reduction in the most of brain regions and also in the adrenal gland. In addition, by looking at the ratio of proBDNF/mBDNF in different regions, we found that the ratio appears decreased in the hypothalamus, suggesting that low level of ethanol consumption may alter proBDNF processing in this region. Together, these studies indicate that consumption of ethanol even as low as 50 μl/day/mouse can alter the expression levels of mBDNF and proBDNF in different brain regions.
The stress-induced increase of corticosteroids is well known to be associated with mood disorders in humans (Pariante and Lightman 2008; Frodl and O'Keane 2013) and animals (Donkelaar et al. 2014; Pazini et al. 2016; Siopi et al. 2016). In the present study, we found that mice treated with corticosterone dissolved in ethanol (the final concentration of 1% ethanol) showed more obvious anxiety-like behaviours than the control and ethanol groups in some behavioural outcomes. There was a statistical reduction of time in the central zone and increase of time in the peripheral zone in the CORT group versus control and ethanol groups. The percentage of time in the central zone was significantly reduced in the CORT-treated mice compared with the ethanol and control groups (p = 0.001). The CORT-treated mice exhibited significant thigmotaxis, an anxiogenic behaviour, when the mouse tends to avoid open or danger areas and remains close to the walls (Seibenhener and Wooten 2015). Thus, the increase of anxiety-related emotional behaviour in the CORT-treated mice can be concluded (Wang et al. 2017).
Similar to ethanol, it is well known that corticosterone downregulates the levels of mature BDNF and proBDNF in the prefrontal cortex and hippocampus. Our study is consistent with the previous studies which showed corticosteroid is a strong negative regulator of BDNF gene expression in the hippocampus (Schaaf et al. 1997; Schaaf et al. 1998; Vellucci et al. 2001) and other brain regions (Smith et al. 1995). We have made additional findings that the effect of corticosteroid on proBDNF and BDNF expression is not only brain-specific, but also occurs in peripheral tissues such as the adrenal glands where the BDNF gene is expressed. This downregulation response in both central and peripheral systems is understandable, as it is known that corticosteroids are lipophilic molecules which penetrate the brain, and the glucocorticoid/glucocorticoid receptor complex can bind the promotor region of the BDNF gene as a suppressor for its expression in hippocampal neurons (Chen et al. 2017).
Both ethanol and corticosterone have a strong impact on the mood changes such as anxiety and depression. However, their mechanisms underlying the impact on mood are less understood. Here, we have provided the evidence that the downregulation of BDNF gene and abnormal processing of proBDNF may be common mechanisms that explain their effects on the mood. One of the aims of this study was to test the hypothesis that corticosterone and ethanol would cause the imbalance between proBDNF and mature BDNF, seen in the chronically stressed rodents and in patients with depression and alcoholics. Numerous studies in animals and humans have shown that BDNF plays a pivotal role in the mood regulation. proBDNF plays opposite functions to mature BDNF in regulating neuronal cell death, neurite collapse and neuronal migration (Suliman et al. 2013; Zheleznyakova et al. 2016; Castrén and Kojima 2017). Our previous studies showed that proBDNF and its receptors are highly upregulated in the blood of patients with major depression (Zhou et al. 2013) and in alcoholic patients (Zhou et al. 2018) but mature BDNF and trkB are downregulated in these subjects; however, whether similar changes occur in the brain of these patients is not established yet; thus, we cannot make direct comparisons between our study and the previous human studies. Therefore, future studies should focus on investigation of how ethanol and corticosteroids affect mature BDNF and proBDNF levels both in the brain and in the blood to establish if the changes in the blood are representative of the changes in the brain.
In the present study, we showed that the changes in the level of mBDNF and proBDNF were associated with anxiety-like behaviours in response to chronic alcohol consumption. Our findings are in agreement with recent studies showing that alcohol treatment can affect synaptic plasticity and reduce neurogenesis as well as the expression of mature BDNF and proBDNF in the brain of rodents therefore leading to mood changes (Tapia-Arancibia et al. 2001; Miller et al. 2002; Susick et al. 2016; Bazovkina et al. 2017). We found that both ethanol and corticosterone not only led to changes in anxiety behaviours but also triggered differential levels of mature BDNF and proBDNF in the brain and tissues of hypothalamus, pituitary and adrenal gland(HPA axis). However, we found the reduction of only proBDNF but not mature BDNF was observed in the tissues related to HPA axis. There was also a significant increase in the ratio of proBDNF/mature BDNF in the prefrontal cortex but no changes in the other tissues examined, suggesting that corticosteroids may also regulate the conversion of proBDNF to mature BDNF. Our data suggest that corticosteroids may be only responsible for the imbalance of proBDNF/mature BDNF in the brain but not in other tissues. The reason why ethanol and corticosterone cause differential effects on some brain regions is not known but our results are consistent with earlier reports in rodents that CORT treatment suppresses the expression of mature BDNF and proBDNF in the hippocampus and prefrontal cortex (Gourley et al. 2008; Ali et al. 2015; Sousa et al. 2015; Pazini et al. 2016; Sawamoto et al. 2016). Whether corticosteroids affect the expression of proBDNF processing enzymes such as tPA, MMPs and PAI-1 requires further examination.
In conclusion, the treatment with corticosterone and low levels of ethanol caused anxiety-like behaviours and a significant reduction of mature BDNF and proBDNF levels in the brain and HPA axis in mice. CORT increased the proBDNF/mBDNF ratio in PFC, which may contribute to anxiety-like behaviours in response to CORT. Our study suggests that both stress-induced corticosteroids and low levels of ethanol consumption are detrimental and may have a combined effect to cause anxiety by downregulating BDNF gene expression and abnormal conversion of proBDNF to mature BDNF.
References
Ali SH, Madhana RM, K V A et al (2015) Resveratrol ameliorates depressive-like behavior in repeated corticosterone-induced depression in mice. Steroids 101:37–42. https://doi.org/10.1016/j.steroids.2015.05.010
Awaworyi Churchill S, Farrell L (2017) Alcohol and depression: evidence from the 2014 health survey for England. Drug Alcohol Depend 180:86–92. https://doi.org/10.1016/j.drugalcdep.2017.08.006
Boden J, Fergusson D (2011) Alcohol and depression. Addiction 106:906–914. https://doi.org/10.1111/j.1360-0443.2010.03351.x
Briones TL, Woods J (2013) Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience 254:324–334. https://doi.org/10.1016/j.neuroscience.2013.09.031
Cai S, Huang S, Hao W (2015) New hypothesis and treatment targets of depression: an integrated view of key findings. Neurosci Bull 31:61–74. https://doi.org/10.1007/s12264-014-1486-4
Castrén E, Kojima M (2017) Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis 97:119–126. https://doi.org/10.1016/j.nbd.2016.07.010
Chen H, Lombès Ma, Menuet DL , (2017) Glucocorticoid receptor represses brain-derived neurotrophic factor expression in neuron-like cells. Molecular Brain 10 (1)
Davis MI (2008) Ethanol–BDNF interactions: still more questions than answers. Pharmacol Ther 118:36–57. https://doi.org/10.1016/j.pharmthera.2008.01.003
Donkelaar E, Vaessen K, Pawluski J, Sierksma A, Blokland A, Cañete R, Steinbusch H (2014) Long-term corticosterone exposure decreases insulin sensitivity and induces depressive-like behaviour in the C57BL/6NCrl mouse. PLoS One 9:e106960. https://doi.org/10.1371/journal.pone.0106960
Frodl T, O'Keane V (2013) How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol Dis 52:24–37. https://doi.org/10.1016/j.nbd.2012.03.012
Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR (2008) Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biol Psychiatry 64:884–890. https://doi.org/10.1016/j.biopsych.2008.06.016
Hempstead BL (2015) Brain-derived neurotrophic factor: three ligands, many actions. Trans Am Clin Climatol Assoc 126:9–19
Jeanblanc J, Logrip ML, Janak PH, Ron D (2013) BDNF -mediated regulation of ethanol consumption requires the activation of the MAP kinase pathway and protein synthesis. Eur J Neurosci 37:607–612. https://doi.org/10.1111/ejn.12067
Kliethermes CL (2005) Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev 28:837–850. https://doi.org/10.1016/j.neubiorev.2004.11.001
Kushner MG, Abrams K, Borchardt C (2000) The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev 20:149–171. https://doi.org/10.1016/s0272-7358(99)00027-6
Lee J, Im S-J, Lee S-G, Stadlin A, Son JW, Shin CJ, Ju G, Lee SI, Kim S (2016) Volume of hippocampal subfields in patients with alcohol dependence. Psychiatry Res Neuroimaging 258:16–22. https://doi.org/10.1016/j.pscychresns.2016.10.009
Li JY, Liu J, Manaph NPA, Bobrovskaya L, Zhou XF (2017) ProBDNF inhibits proliferation, migration and differentiation of mouse neural stem cells. Brain Res 1668:46–55. https://doi.org/10.1016/j.brainres.2017.05.013
Lim Y, Zhong JH, Zhou XF (2015) Development of mature BDNF-specific sandwich ELISA. J Neurochem 134:75–85. https://doi.org/10.1111/jnc.13108
Logrip ML, Barak S, Warnault V, Ron D (2015) Corticostriatal BDNF and alcohol addiction. Brain Res 1628:60–67. https://doi.org/10.1016/j.brainres.2015.03.025
Miller R, King MA, Heaton MB, Walker DW (2002) The effects of chronic ethanol consumption on neurotrophins and their receptors in the rat hippocampus and basal forebrain. Brain Research 950 (1-2):137-147
Mishima Y, Shinoda Y, Sadakata T, Kojima M, Wakana S, Furuichi T (2015) Lack of stress responses to long-term effects of corticosterone in Caps2 knockout mice. Sci Rep 5:8932. https://doi.org/10.1038/srep08932
Morris EP, Stewart SH, Ham LS (2005) The relationship between social anxiety disorder and alcohol use disorders: a critical review. Clin Psychol Rev 25:734–760. https://doi.org/10.1016/j.cpr.2005.05.004
Morris SA, Eaves DW, Smith AR, Nixon K (2010) Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus 20:596–607. https://doi.org/10.1002/hipo.20665
Pariante CM, Lightman SL (2008) The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31:464–468. https://doi.org/10.1016/j.tins.2008.06.006
Paul CA, Au R, Fredman L, Massaro JM, Seshadri S, Decarli C, Wolf PA (2008) Association of alcohol consumption with brain volume in the Framingham study. Arch Neurol 65:1363–1367. https://doi.org/10.1001/archneur.65.10.1363
Pazini F, Cunha M, Rosa J, Colla A, Lieberknecht V, Oliveira Á, Rodrigues A (2016) Creatine, similar to ketamine, counteracts depressive-like behavior induced by corticosterone via PI3K/Akt/mTOR pathway. Mol Neurobiol 53:6818–6834. https://doi.org/10.1007/s12035-015-9580-9
Pedrelli P, Shapero B, Archibald A, Dale C (2016) Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep 3:91–97. https://doi.org/10.1007/s40429-016-0084-0
Ruan CS, Wang SF, Shen YJ, Guo Y, Yang CR, Zhou FH, Tan LT, Zhou L, Liu JJ, Wang WY, Xiao ZC, Zhou XF (2014) Deletion of TRIM32 protects mice from anxiety- and depression-like behaviors under mild stress. Eur J Neurosci 40:2680–2690. https://doi.org/10.1111/ejn.12618
Sameti M, Smith S, Patenaude B, Fein G (2011) Subcortical volumes in long-term abstinent alcoholics: associations with psychiatric comorbidity. Alcohol Clin Exp Res 35:1067–1080. https://doi.org/10.1111/j.1530-0277.2011.01440.x
Sawamoto A, Okuyama S, Yamamoto K, Amakura Y, Yoshimura M, Nakajima M, Furukawa Y (2016) 3,5,6,7,8,3′,4'-Heptamethoxyflavone, a citrus flavonoid, ameliorates corticosterone-induced depression-like behavior and restores brain-derived neurotrophic factor expression, neurogenesis, and neuroplasticity in the hippocampus. Molecules 21:541. https://doi.org/10.3390/molecules21040541
Schaaf MJ, Hoetelmans RW, de Kloet ER, Vreugdenhil E (1997) Corticosterone regulates expression of BDNF and trkB but not NT-3 and trkC mRNA in the rat hippocampus. Journal of Neuroscience Research 48 (4):334–341
Schaaf MJ, de Jong J, de Kloet ER, Vreugdenhil E (1998) Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Research 813 (1):112–120
Schneier FR, Foose TE, Hasin DS, Heimberg RG, Liu S-M, Grant BF, Blanco C (2010) Social anxiety disorder and alcohol use disorder co-morbidity in the National Epidemiologic Survey on alcohol and related conditions. Psychol Med 40:977–988. https://doi.org/10.1017/s0033291709991231
Seibenhener ML, Wooten MC (2015) Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. https://doi.org/10.3791/52434
Siopi E, Denizet M, Gabellec M-M, de Chaumont F, Olivo-Marin JC, Guilloux JP, Lledo PM, Lazarini F (2016) Anxiety- and depression-like states Lead to pronounced olfactory deficits and impaired adult neurogenesis in mice. J Neurosci 36:518–531. https://doi.org/10.1523/jneurosci.2817-15.2016
Smith MA ,Makino S, Kvetnansky R, Post RM,(1995) Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. The Journal of Neuroscience 15 (3):1768-1777
Sousa CNN, Meneses LN, Vasconcelos GS et al (2015) Reversal of corticosterone-induced BDNF alterations by the natural antioxidant alpha-lipoic acid alone and combined with desvenlafaxine: emphasis on the neurotrophic hypothesis of depression. Psychiatry Res 230:211–219. https://doi.org/10.1016/j.psychres.2015.08.042
Suliman S, Hemmings SM, Seedat S (2013) Brain-derived neurotrophic factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Front Integr Neurosci 7:55. https://doi.org/10.3389/fnint.2013.00055
Sun Y, Lim Y, Li F, Liu S, Lu JJ, Haberberger R, Zhong JH, Zhou XF (2012) ProBDNF collapses neurite outgrowth of primary neurons by activating RhoA. PLoS One 7:e35883. https://doi.org/10.1371/journal.pone.0035883
Susick LL, Chrumka AC, Hool SM, Conti AC,(2016) Dysregulation of TrkB phosphorylation and proBDNF protein in adenylyl cyclase 1 and 8 knockout mice in a model of fetal alcohol spectrum disorder. Alcohol 51:25-35
Tapia-Arancibia L, Rage F, Givalois L, Dingeon P, Arancibia S, Beaug F, (2001) Effects of alcohol on brain-derived neurotrophic factor mRNA expression in discrete regions of the rat hippocampus and hypothalamus. Journal of Neuroscience Research 63 (2):200-208
Vellucci SV, Parrott RF, Mimmack M.L, (2001) Down-regulation of BDNF mRNA, with no effect on trkB or glucocorticoid receptor mRNAS, in the porcine hippocampus after acute dexamethasone treatment. Research in Veterinary Science 70 (2):157-162
Wang Q, Timberlake MA, Prall K, Dwivedi Y (2017) The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry 77:99–109. https://doi.org/10.1016/j.pnpbp.2017.04.008
Xu Z, Zhang Y, Hou B, Gao Y, Wu Y, Zhang C (2011) Chronic corticosterone administration from adolescence through early adulthood attenuates depression-like behaviors in mice. J Affect Disord 131:128–135. https://doi.org/10.1016/j.jad.2010.11.005
Yang CR, Bai YY, Ruan CS et al (2014a) Enhanced aggressive behaviour in a mouse model of depression. Neurotox Res. https://doi.org/10.1007/s12640-014-9498-4
Yang CR, Zhang ZG, Bai YY, Zhou HF, Zhou L, Ruan CS, Li F, Li CQ, Zheng HY, Shen LJ, Zhou XF (2014b) Foraging activity is reduced in a mouse model of depression. Neurotox Res 25:235–247. https://doi.org/10.1007/s12640-013-9411-6
Zhao Y, Ma R, Shen J, Su H, Xing D, Du L (2008) A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol 581:113–120. https://doi.org/10.1016/j.ejphar.2007.12.005
Zheleznyakova GY, Cao H, Schioth HB (2016) BDNF DNA methylation changes as a biomarker of psychiatric disorders: literature review and open access database analysis. Behav Brain Funct 12:17. https://doi.org/10.1186/s12993-016-0101-4
Zhou L, Xiong J, Lim Y, Ruan Y, Huang C, Zhu Y, Zhong JH, Xiao Z, Zhou XF (2013) Upregulation of blood proBDNF and its receptors in major depression. J Affect Disord 150:776–784. https://doi.org/10.1016/j.jad.2013.03.002
Zhou L, Xiong J, Ruan CS, Ruan Y, Liu D, Bao JJ, Zhou XF (2018) ProBDNF/p75NTR/sortilin pathway is activated in peripheral blood of patients with alcohol dependence. Transl Psychiatry 7:2. https://doi.org/10.1038/s41398-017-0015-4
Acknowledgements
Grant sponsor: Lin LY was supported by the University of South Australia Postgraduate Research Award; Luo SY was supported by the Central South University, Xiangya Hospital, Hunan province, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, L.Y., Luo, S.Y., Al-Hawwas, M. et al. The Long-Term Effects of Ethanol and Corticosterone on the Mood-Related Behaviours and the Balance Between Mature BDNF and proBDNF in Mice. J Mol Neurosci 69, 60–68 (2019). https://doi.org/10.1007/s12031-019-01328-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01328-6