Abstract
The Schwann-like cells can be considered as promising in stem cell therapies, at least in experimental models. Human adipose-derived stem cells (ADSCs) are induced into Schwann-like cells (SC-like cells) and are cultured on either a plastic surface or laminin-coated plates. The findings here reveal that laminin is a critical component in extracellular matrix (ECM) of SC-like cells at in vitro. The survival rate of SC-like cells on a laminin matrix are measured through MTT assay and it is found that this rate is significantly higher than that of the cells grown on a plastic surface (P < 0.05). Schwann cell markers and the myelinogenic ability of SC-like cells at the presence versus absence of laminin are assessed through immunocytochemistry. The analysis of GFAP/S100β and S100β/MBP markers indicate that laminin can increase the differentiated rate and myelinogenic potential of SC-like cells. The expression levels of SCs markers, myelin basic proteins (MBP), and neurotrophic factors in two conditions are analyzed by real-time reverse transcription polymerase chain reaction (RT-PCR). The findings here demonstrated that gene expression of SCs markers, MBP, and brain-derived neurotrophic factors (BDNF) increase significantly on laminin compared to plastic surface (P < 0.01). In contrast, the nerve growth factor (NGF) expression is downregulated significantly on laminin-coated plates (P < 0.05). The obtained data suggest that production of neurotrophic factors in SC-like cell in presence of laminin can induce appropriate microenvironment for nerve repair in neurodegenerative diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative diseases and nerve injuries are among the range of life-threatening disorders mostly concerned with demyelination of nerve fibers. Demyelination is defined as the poor neural recovery and may result in loss of motor or sensory functions in both the peripheral and central nervous systems (Nave 2010).
Nerve regeneration is a major concern in regenerative medicine. One of the approaches in regenerative medicine is stem cell therapy which introduces new strategies to replace impaired tissues with autologous stem cells. The cell type is an important factor in therapeutic strategies (Zhu et al. 2014).
One of the appropriate candidates for autologous stem cell therapy is the mesenchymal stem cells (MSCs). MSCs are multipotent, fast proliferating, and involved in treatment of bone, cartilage, and myocardium defects in addition to skeletal treatment and neurological disorders (Zuk et al. 2002).
Among human MSCs, adipose-derived stem cells (ADSCs) can constitute a proper source of autologous adult stem cells due to their abundant, accessible, and non-invasive procedures without ethical problem (Dhar et al. 2007). ADSCs are able to become differentiated into Schwann-like cells (SC-like cells) and they can be effective in treatment of neurological diseases (di Summa et al. 2012; Razavi et al. 2013a; Razavi et al. 2015).
Schwann cells are myelinating, exhibiting axon guidance, and producing neurotrophic factors and can migrate in the peripheral nerves for regeneration (Armati and Mathey 2014; Chen et al. 2015). SCs play an essential role in nerve injury repair and neurodegenerative disease treatment (Woodhoo et al. 2007).
Schwann cells synthesize extracellular matrix component like laminin. This protein is a heterotrimeric protein localized in the basement membrane and contributes to regulating axonal sorting and is necessary for increasing proliferation and regulating the morphology of Schwann cells (Chen and Strickland 2003; Yu et al. 2009). It is revealed that signals omitted by laminin are essential for radial sorting and myelination of the peripheral nerves (Chernousov et al. 2008; Nodari et al. 2007). By consuming laminin, a specific component, the potential enhancement of peripheral nervous regeneration is determined (Chen and Strickland 2003).
Schwann cells are able to secrete neurotrophic factors (NTFs), a main group of polypeptides contributing to the maintenance of neuronal cells, promoting development, and providing cellular guidance for axons regeneration. Schwann cells can release NTFs to the impaired nerves (Madduri and Gander 2010).
ADSCs induce SC-like cells to secrete many types of neurotrophins, like nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), and glial cell-derived neurotrophic factor (GDNF) (Faroni et al. 2013). These neurotrophic factors promote the neural survival, differentiation, and neuritogenesis (Crigler et al. 2006; Razavi et al. 2013b; Heuckeroth et al. 1998).
Lack of neurotrophic factors and extracellular matrix in the injury point or in the region of neural degeneration would disturb nerve regeneration (McCall et al. 2012). Laminin-coated surface(s), can advance cell viability and increase neural cell adhesion (Tate et al. 2009).
The objective of this study is to induce ADSCs into SC-like cells and assess the effect of laminin-coated culture on the efficiency of SC-like cells differentiation, the myelination potential, and effects of laminin on the expression of neurotrophic factors in SC-like cells derived from adipose stem cells in vitro.
Materials and Methods
Isolation and Culture of Human ADSCs
The human adipose tissue is provided from lipoaspirate samples of abdominal fat of the female donors (age range 20–50). This procedure is run in accordance with Razavi et al.’s 2012, 2013a) description. The tissue samples are washed by phosphate-buffered saline (PBS) containing 2 % penicillin/streptomycin (P/S) in order to remove contaminated debris and blood cells. Tissues are placed in a sterile 50-ml tube on which 0.075 % collagenase type І prepared in PBS is poured. This tube is incubated for 30 min at 37 °C, 5 % CO2. Next, collagenase type І activity in the samples are naturalized by the same volume ratio of DMEM:F12/10 % fetal bovine serum (FBS) and centrifuged for 10 min at 1700 rpm. The yield cellular pellet is re-suspended by DMEM:F12/10 % FBS, and 5000 cells/cm2 of which are plated in T25 flasks which contains DMEM:F12 medium supplemented with 10 % FBS and 1 % P/S.
Twenty-four hours after plating, this medium is aspirated from the flask to remove the non-attached cells, while the attached ADSCs are expanded after several passages. In this study, the cells obtained after the 3–4 passages are used for experiments.
All procedures of this study are applied in accordance with the Ethics Committee of the Medical Faculty in Isfahan University of Medical Sciences. Most of the chemicals are purchased from Sigma-Aldrich, St. Louis, MO, USA.
Induction of Human ADSCs into Schwann-Like Cells
Characterization of isolated hADSCs is determined through flow cytometry. The induction of hADSCs into Schwann-like cells is carried out in accordance with Razavi et al.’s (2013a) procedure. The hADSCs are maintained in a humidified tissue culture incubator at 37 °C with 5 % CO2 with a change of medium at every 2–3 days until the cells reached 80–90 % confluence. The harvested cells are induced into neurospheres. Human ADSCs are treated with 0.25%Trypsin/EDTA (Gibco, BRL, Paisley, UK) and plated in a medium of DMEM:F12 supplemented with 20 ng/ml human epidermal growth factor (hEGF), 20 ng/ml human basic fibroblast growth factor (bFGF), and 2 % B27 (1:50, Gibco) at 37 °C with 5 % CO2. After 7 days, the neurospheres derived from hADSCs are re-plated in laminin-coated flasks in the presence of differentiation medium consisting of DMEM:F12/10% FBS supplemented with 5 μm forskolin, 5 ng/ml platelet-derived growth factor-AA (PDGF), 10 ng/ml bFGF, and 200 ng/ml recombinant human heregulin-beta. The cells are retreated with the abovementioned factors every 3 days until day 7. It should be noted that here laminin is absent in the control group.
3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide Assay
The 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) assay is run to examine the viability of SC-like cells in the presence or absence of laminin at 7 days post induction. A 5-mg/ml MTT in PBS solution is added to the medium of DMEM:F12 at 1:10 ratio. The MTT solution in each one of the 24-well plate contains 4× 103 cells and are incubated at 37 °C under 5 % CO2 for 4 h; next, the MTT assay media is discarded and 200 μl of dimethyl sulfoxide (DMSO) is added to extract the MTT formazan. The MTT is metabolized by mitochondrial enzyme in the living cells in order to produce formazan. The absorbance of each well is detected by micro plate reader (Hiperion MRP 4+, Germany) at 540 nm wavelength.
Immunocytochemistry Staining
The human ADSCs are differentiated for 7 days and then are fixed by 4 % paraformaldehyde in 30 min time. These cells are permeabilized by using 0.2 % Triton x-100 at room temperature (RT) in 30 min time then incubated with primary and secondary antibodies diluted in blocking solution containing 10 % goat serum and 1 mg/ml bovine serum albumin (BSA) in PBS. The following primary antibodies of: anti- S-100 β (1:500; Abcam, UK), anti-glial fibrillary acidic protein (anti-GFAP, 1:300; Abcam, UK) and anti-major basic protein (anti-MBP, 1:500; Abcam, UK) are consumed for overnight incubated at RT. After washing the slides three times with PBS, they are exposed to conjugated secondary antibody, rabbit anti-mouse FITC (1:500; Abcam, UK) and rabbit anti-mouse PE (1:1000; Abcam, UK) at RT for 1 h. The 4,6-diamidino-2-phenylindole (DAPI) is consumed for nuclear counter staining. The cells are examined through fluorescence microscope (Olympus BX51, Japan). To perform quantitative analysis, a minimum of 200 cells per slide are selected by fluorescence microscope in order to analyze the number of immune-positive cells by ImageJ 1.42 (NH). Experiments are run in triplicate and the percentage of positive cells is obtained by counting several non-overlap fields.
Real-Time Reverse Transcription Polymerase Chain Reaction
For gene expression studies, cells are collected after 7 days of transdifferentiation. Total RNA is isolated from cells by applying High Pure RNA isolation kit (Roche, Germany). Isolated RNA is dissolved in RNase-free water, the RNA quantity and quality is determined by measuring absorbance at 260–280 nm with a nanodrop. The RNA samples are treated of DNase І (Roche) in order to avoid potential contamination with genomic DNA. Total RNA is consumed to synthesis double-stranded cDNA through Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific) and oligo dT primer. The primers for all assessed genes are designed by applying Allele ID 7.6 (Primer Biosoft). The list of primer sequences are tabulated in Table 1. The qRT-PCR is run with gene specific primers and Maxima SYBR Green/ROX qPCR Master Mix 2X (Thermo Scientific) and the steponeplus™ Real time PCR detection system (Applied Biosystems). The PCR amplification takes 10 min at 95 °C followed by 40 cycles of denaturation steps at 95 °C for 15 s per cycle. The annealing and extending processes takes 1 min at 60 °C. Melting curve analysis is used to determine the melting temperature of specific amplification products and the primer. These experiments are run in triplicates and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is consumed as the endogenous control. The expression level of each target gene is calculated as 2−∆∆CT method.
Statistical Analysis
The data are presented as the mean value ± standard error of the mean (SEM) from the independent cell cultures. Independent student t test is run to compare the mean values of the samples. Levels of the statistical significance are set at *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Isolation and Differentiation of hADSCs into SC-like Phenotype
The human ADSCs are isolated from the adipose tissue, and cells are adhered to tissue culture plastic and are grown to 80–90 % confluence. The hADSCs revealed the typical fibroblast-like flat phenotype after 1–2 passage (Fig. 1a). These cells are re-plated in pre induction medium, where a clump of floating cells is shown 7 days after induction to neurospheres (Fig. 1b). The neurospheres are dissociated, and the single cells are re-plated in the terminal differentiation medium. Seven days after induction, the phenotype of the differentiated ADSCs are converted from large and flat into a bi-polar or tri-polar, spindle shape in the absence laminin (Fig. 1c) and in the presence laminin (Fig. 1d).
Phase contrast image of human ADSCs induced to SC-like cells. a Human ADSCs before induction. b Neurospheres exploited from human ADSCs 7 days after induction. Human ADSCs differentiated to Schwann cells phenotype show bi- or tri-polar and spindle-like morphology after induction c without laminin and d with laminin. Scale bars in a = 200 μm, in c = 100 μm, and in b and d = 150 μm
Influence of Laminin on Cell Viability
The viability of SC-like cells is assessed in the presence or absence of laminin coated surface through MTT assay. The mean of optical density for laminin and non-coated cell cultures are 0.236 ± 0.02 and 0.182 ± 0.01, respectively. There exists a statistically significant difference in the mean of SC-like cell viability in the presence of the laminin compared to the control surfaces (P < 0.05) (Fig. 2).
Immunocytochemical Staining
Immunofluorescence is performed to assess the expression of various markers, including Schwann cell markers (GFAP, S100β) and myelinating potential marker, myelin basic protein (MBP). Seven days after differentiation, fixation of the differentiated cells is done and the induced cells are detected through Schwann cells and myelinating potential markers of, GFAP/S100β and S100β/MBP, respectively (Fig. 3).
Immunocytochemistry of SC-like cells; GFAP (red) (a and a’), S100β (green), and MBP (red) proteins; S100β/GFAP (b and b’) and S100β/MBP (c and c’) co-markers (yellow to brown). The yellow to brown color is an overlap of green and red showing positively stained cells for both markers and cell nuclei are exposed with DAPI (blue) in non-coated (a, b, c) and coated laminin surface (a’, b’, c’). Scale bars in a’ = 200 μm but in a, b, b’, c, and c’ = 100 μm
Immunocytochemistry analysis revealed that the mean percentage of GFAP in differentiated cells in the presence of laminin-coating surface is 70.24 ± 3.37 % and in absences of laminin-coating surface is 67.41 ± 2.64 %. The mean percentage of co-markers GFAP/S100β-positive cells in the presence of laminin-coating surface is 64.91 ± 3.81 % and in the absence of laminin-coating surface is 63.26 ± 3.28 %. The mean percentage of cells that expressed the co-markers S100β/MBP increased significantly in the presence of laminin-coating surface by (74.92 ± 2.42 %) as compared to cells in the absence of laminin-coating surface which decreased by (64.72 ± 3.77 %) (P < 0.05) (Fig. 4).
Real time RT-PCR Analysis
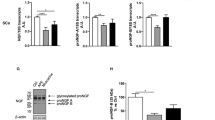
The effect of laminin on differentiation, myelinating ability, and the expression of BDNF, NGF, CNTF, and GDNF in SC-like cells are examined by real time RT-PCR analysis (Fig. 5). The expression of S100β and GFAP markers of Schwann cells indicate a significant upregulation of GFAP expression observed in the presence of laminin (2.70 ± 0.63) in relation to the control group (1.09 ± 0.03) (P < 0.05). A significant upregulation for S100β is observed in the presence of laminin at (3.75 ± 0.62 ) as compared to cell cultures without laminin (1.72 ± 0.24) (P < 0.01). In addition, the result of MBP protein expression to determine the myelination potential of SC-like cells in the presence of laminin is 3.62 ± 0.40 while without laminin it is 2.04 ± 0.18 (P < 0.01).
Comparative analysis of S100β, GFAP, and MBP markers in SC-like cells in the presence or absences of laminin-coating surface examined by RT-PCR. The expression of GFAP and S100β genes, markers of Schwann cells, are significantly upregulated in SC-like cells in the presence of laminin as compared to the absence of laminin (mean ± SEM, *P < 0.05, **P < 0.01). The expression of MBP, marker of myelinization of laminin, is significantly upregulated in SC-like cells in the presence of laminin in relation to the absence of laminin (mean ± SEM, **P < 0.01)
The expression of BDNF is significantly upregulated in SC-like cells in the presence of laminin at (5.14 ± 0.27) as compared to the absence of laminin (1.05 ± 0.26) (P < 0.01), whereas the expression of NGF in SC-like cells in the presence of laminin is downregulated at (0.30 ± 0.03) in relation to the cell culture without laminin (0.52 ± 0.09) (P < 0.05). The level of GDNF expression showed partial upregulation in SC-like cells in the presence of laminin as compared to without laminin with no significant difference. Moreover, the expression of CNTF is no significant difference between two conditions (Fig. 6).
Comparative analysis of BDNF, NGF, CNTF, and GDNF markers in SC-like cells in the presence or absences of laminin-coating surface examined by RT-PCR. The expression level of BDNF genes is significantly upregulated in laminin-coating cell culture as compared to cell culture without laminin (mean ± SEM, **P < 0.01). The expression level of NGF genes is significantly downregulated in both the conditions (mean ± SEM, *P < 0.05)
Discussion
The in vitro investigation in this study revealed that human ADSCs can transdifferentiate into SC-like cells. Morphometric analysis revealed that human ADSCs change morphology into SC-like phenotype. In the presence of laminin, the SC-like cells more survive, and the expression of Schwann cell markers of GFAP, S100, and the myelin basic protein (MBP) increase. In addition, it is found that laminin coating stimulates differentiated human ADSCs to upregulate expression of a number of neurotrophic factors, like BDNF and GDNF, and downregulate NGF and CNTF.
ECM and SCs are necessary for peripheral nerve regeneration. The ECM consists of components like the following: macromolecules and specialized proteins including laminin and fibronectin (Platt et al. 2003). When a peripheral nerve is damaged, laminin expression increases and this phenomenon can have a role during regeneration (Chen et al. 2007). Laminin, as a natural substance, has many applications in tissue engineering. This glycoprotein, present in basal lamina of Schwann cell, could act as a scaffold to absorb and bind factors. According to Yu et al. (2009), laminin is essential for SCs proliferation in addition to regulating SC morphology.
The transdifferentiation of MSCs can have a critical in improving nerve regeneration, be differentiated into SC-like cells in in vitro and show ability to differentiate into SC-like cells at the site of injury (Chen et al. 2006).
In addition, MSCs promote neural repair process by producing cytokines, growth factors, and neuroregulatory molecules (Guo et al. 2015).
Neurotrophic factors and extracellular matrix proteins are the two basic growth-inducing factors with major contribution in Schwann cell survival, proliferation, differentiation, even stimulate the outgrowth (Guo et al. 2015).
The MSCs sources include the following: the bone marrow, adipose-derived stem cells, and the human umbilical cord (huc). The bone marrow stromal cells were the first cells induced towards Schwann cells and, consequently, utilized for nerve regeneration trials (Dezawa et al. 2001). The adipose-derived stem cells successfully differentiate into SC-like phenotype (Kingham et al. 2007). However, other mesenchymal stem cell populations such as huc MSCs are used for peripheral nerve repair (Guo et al. 2015). Both the SCs and human differentiated adipose-derived stem cells (dADSCs) contain similar molecular markers of GFAP, S100, and P75 (Faroni et al. 2011; Kingham et al. 2007; Tomita et al. 2012). Mature SCs have the capacity for myelin formation which is a definitive criterion for native SCs (Jessen and Mirsky 2005). SC-like cells induced from ADSCs could produce myelin basic protein (MBP) to form myelin structures in vitro (Mantovani et al. 2010; Razavi et al. 2012; Tomita et al. 2012; Tomita et al. 2013; Xu et al. 2008; Yu et al. 2009). Likewise, MSCs from the bone marrow can form myelin in vitro (Keilhoff et al. 2006; Mantovani et al. 2010) and in vivo (Dezawa et al. 2001). A suitable source of MSCs is obtained for transplantable cells for the use of neural repair.
In this study, whether the laminin may regulate the expression of neurotrophin factors in SC-like cells induced from human adipose-derived stem cells is being assessed.
A mixture of glial growth factors containing the following: forskolin, bFGF, PDGF, and also heregulin for induction is introduced by Kingham et al. (2007) and Xu et al. (2008). Forskolin can elevate the expression of mitogenic genes (Fortino et al. 2002) and also upregulate NGF gene expression levels in Schwann cells (Yamamoto et al. 1993). The bFGF upregulates BDNF gene expression in retinal ganglion cells (Soto et al. 2006). Kingham et al. (2007) showed that the mixture bFGF and forskolin could lead to the expression of glia cell markers GFAP, S100, and P75. The BDNF secretion from the transplanted dADSCs could promote myelin formation by native SCs (Tomita et al. 2012). Neurogulin-1 is a transmembrane molecule of neurons that is inserted into axonal membranes, as a secreted growth factor; it is also named heregulin or neu differentiation factors (NDF). This axonally derived signal is essential for maintenance, proliferation, and differentiation of Schwann cells (Garratt et al. 2000).
Human ADSCs can be induced into SC-like cells with spindle-shaped morphology similar to SCs. The survival capacity rate of SC-like cells found in this study is significantly stronger in laminin-coated surfaces versus uncoated surfaces, which is in agreement with the findings of other studies (di Summa et al. 2012; Worth and Parsons 2008). The cell adhesion to ECM is important for cell survival. Cell attachment to biomaterials is critical for regenerative therapy (di Summa et al. 2012).
The abovementioned findings could induce SC-like cells to produce major basic proteins; therefore, SC-like cells derived from adipose tissue are detectable by double immunofluorescence staining of both S100β/GFAP and S100β/MBP. It is observed that the presence of laminin significantly promotes not only the SC differentiation but the myelinating potential of differentiated cells. The expression of SC markers S100, GFAP, and MBP proteins are confirmed in SC-like cells.
MSCs release variety of secretory factors. A group of proteins are expressed at significantly high levels and identified as neurotrophic factors like BDNF, NGF, GDNF, LIF, NT-3, and CNTF as expressed by native SCs (Firouzi et al. 2006). The expression levels of neurotrophic factors and the biological timing are critical in promoting axon regeneration (Gordon 2009), thus neurotrophic factors have profound effect on a wide variety of phenomena, like regeneration and myelination (Blesch and Tuszynski 2003; Chao 2003).
Some of neurotrophic factors produced by Schwann cells, including BDNF, NGF, GDNF, and CNTF play important roles in neurogenesis during the development, neuronal survival, and regeneration process of peripheral nerve injury (Fu and Gordon 1997; Gordon 2009; Terenghi 1999).
According to Faroni et al. (2013), using baclofen, which is the specific agonist for GABA receptors, could regulate the expression and the secretion of neurotrophin factors where the SC-like cells similar to SCs reveal that baclofen influences the expression and the secretion of BDNF and NGF. Moreover, GABA and its receptors modulate development and myelination in PNS.
di Summa et al. (2012) revealed the effect of ECM molecules on SC-like cells differentiated from ASCs. In their study, fibronectin and laminin as components of ECM have affected the viability and cell attachment in differentiated ADSCs. Furthermore, when SC-like cells are seeded on fibronectin and or laminin cultures of dorsal root ganglion (DRG) neurons, higher expression levels of both neurotrophin BDNF and NGF are detected.
The expression of BDNF, NGF, CNTF, and GDNF in SC-like cells after 7 days of exposure to the laminin is assessed here. It is found that mRNA transcripts of BDNF are upregulated in SC-like cells exposed to the laminin. In contrast to BDNF, observations here revealed that the mRNA levels for NGF and CNTF are downregulated in SC-like cells in the presence of laminin compared to controls. It is found that reduced gene expression of NGF, is in agreement with findings of Faroni et al. (2015). They assessed the stability of SC-like cells derived from ASCs by changing their condition, withdrawing differentiation medium for 3 days after 18 days of differentiation. They reported a significant change in gene and protein expression of growth factors. In presence of differentiation medium, BDNF increased but during withdrawal of induction medium the BDNF decreased. In their study, NGF in differentiation medium is decreased and the same happened in this study. These existed different reports on the level of NGF expression in SCs and SC-like cells in vitro and in vivo (Matsuoka et al. 1991; Meyer et al. 1992; Zafra et al. 1991). These contrary results may be due to differences in media collection intervals. The results may be influenced by assessing the neurotrophic levels; the evaluation of gene expression is made by real time RT-PCR (Faroni et al. 2015). In this respect, some researchers adopt the qualitative reverse transcriptase polymerase chain reaction method introduced by (Kingham et al. 2013). Furthermore, consumption of neuregulin in differentiation process is critical. In this study, the HRG-β1 (recombinant human neuregulin-1β (rh. NRG-1β)) is consumed. Here, it is observed that SC-like cells express GDNF, with a partially upregulated reveal in the SC-like cells in laminin-coated conditions with no statistical significant difference.
Conclusion
SC-like cells at the presence of laminin could produce myelin in vitro condition and may have the capacity to act as the native SCs in terms of myelination and neurotrophic secretion. This function is of great importance in therapeutic strategy with respect to injured tissues in PNS and CNS. This phenomenon suggests that the stem cells harvested from adipose tissue could be applied in peripheral nerve repair and the potential of being applied in neurodegenerative diseases. The produced neurotrophic factors during in vitro differentiation could lead to paracrine signaling between SC-like cells and neurons.
References
Armati PJ, Mathey EK (2014) Clinical implications of Schwann cell biology. J Peripher Nerv Syst 19:14–23
Blesch A, Tuszynski MH (2003) Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol 467:403–417
Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4:299–309
Chen Q et al. (2015) A fibrin matrix promotes the differentiation of EMSCs isolated from nasal respiratory mucosa to myelinating phenotypical Schwann-like cells. Molecules and cells 38:221
Chen X, Wang XD, Chen G, Lin WW, Yao J, Gu XS (2006) Study of in vivo differentiation of rat bone marrow stromal cells into Schwann cell-like cells. Microsurgery 26:111–115
Chen Z-L, Strickland S (2003) Laminin γ1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol 163:889–899
Chen Z-L, Yu W-M, Strickland S (2007) Peripheral regeneration. Annu Rev Neurosci 30:209–233
Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S (2008) Regulation of Schwann cell function by the extracellular matrix. Glia 56:1498–1507
Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG (2006) Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol 198:54–64
Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H (2001) Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci 14:1771–1776
Dhar S, Yoon ES, Kachgal S, Evans GR (2007) Long-term maintenance of neuronally differentiated human adipose tissue-derived stem cells. Tissue Eng 13:2625–2632
di Summa PG, Kalbermatten DF, Raffoul W, Terenghi G, Kingham PJ (2012) Extracellular matrix molecules enhance the neurotrophic effect of Schwann cell-like differentiated adipose-derived stem cells and increase cell survival under stress conditions. Tissue Eng A 19:368–379
Faroni A, Calabrese F, Riva MA, Terenghi G, Magnaghi V (2013) Baclofen modulates the expression and release of neurotrophins in schwann-like adipose stem cells. J Mol Neurosci 49:233–243. doi:10.1007/s12031-012-9813-6
Faroni A, Mantovani C, Shawcross SG, Motta M, Terenghi G, Magnaghi V (2011) Schwann-like adult stem cells derived from bone marrow and adipose tissue express γ-aminobutyric acid type B receptors. J Neurosci Res 89:1351–1362
Faroni A, Smith RJ, Lu L, Reid AJ (2015) Human Schwann-like cells derived from adipose-derived mesenchymal stem cells rapidly de-differentiate in the absence of stimulating medium European Journal of Neuroscience
Firouzi M, Moshayedi P, Saberi H, Mobasheri H, Abolhassani F, Jahanzad I, Raza M (2006) Transplantation of Schwann cells to subarachnoid space induces repair in contused rat spinal cord. Neurosci Lett 402:66–70
Fortino V, Torricelli C, Gardi C, Valacchi G, Paccani SR, Maioli E (2002) ERKs are the point of divergence of PKA and PKC activation by PTHrP in human skin fibroblasts. Cellular and Molecular Life Sciences CMLS 59:2165–2171
Fu SY, Gordon T (1997) The cellular and molecular basis of peripheral nerve regeneration. Molecular neurobiology 14:67–116
Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C (2000) A dual role of erbB2 in myelination and in expansion of the schwann cell precursor pool. J Cell Biol 148:1035–1046
Gordon T (2009) The role of neurotrophic factors in nerve regeneration. Neurosurg Focus 26:E3
Guo Z-y, Sun X, Xu X-l, Zhao Q, Peng J, Wang Y (2015) Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural regeneration research 10:651
Heuckeroth RO, Lampe PA, Johnson EM, Milbrandt J (1998) Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitorsin vitro. Dev Biol 200:116–129
Jessen K, Mirsky R (2005) Symposium S01: molecules and mechanisms in Schwann cell development. J Neurochem 94:2–3
Keilhoff G, Stang F, Goihl A, Wolf G, Fansa H (2006) Transdifferentiated mesenchymal stem cells as alternative therapy in supporting nerve regeneration and myelination. Cell Mol Neurobiol 26:1233–1250
Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G (2007) Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol 207:267–274
Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M (2013) Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev 23:741–754
Madduri S, Gander B (2010) Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J Peripher Nerv Syst 15:93–103
Mantovani C, Mahay D, Kingham PJ, Terenghi G, Shawcross SG, Wiberg M (2010) Bone marrow-and adipose-derived stem cells show expression of myelin mRNAs and proteins. Regen Med 5:403–410
Matsuoka I, Meyer M, Thoenen H (1991) Cell-type-specific regulation of nerve growth factor (NGF) synthesis in non-neuronal cells: comparison of Schwann cells with other cell types. J Neurosci 11:3165–3177
McCall J, Weidner N, Blesch A (2012) Neurotrophic factors in combinatorial approaches for spinal cord regeneration. Cell Tissue Res 349:27–37
Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H (1992) Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol 119:45–54
Nave K-A (2010) Myelination and support of axonal integrity by glia. Nature 468:244–252
Nodari A et al. (2007) β1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol 177:1063–1075
Platt C, Krekoski C, Ward R, Edwards D, Gavrilovic J (2003) Extracellular matrix and matrix metalloproteinases in sciatic nerve. J Neurosci Res 74:417–429
Razavi S, Ahmadi N, Kazemi M, Mardani M, Esfandiari E (2012) Efficient transdifferentiation of human adipose-derived stem cells into Schwann-like cells: a promise for treatment of demyelinating diseases. Advanced biomedical research 1:12
Razavi S, Mardani M, Kazemi M, Esfandiari E, Narimani M, Esmaeili A, Ahmadi N (2013a) Effect of leukemia inhibitory factor on the myelinogenic ability of Schwann-like cells induced from human adipose-derived stem cells. Cell Mol Neurobiol 33:283–289
Razavi S, Razavi MR, Kheirollahi-Kouhestani M, Mardani M, Mostafavi FS (2013b) Co-culture with neurotrophic factor secreting cells induced from adipose-derived stem cells: promotes neurogenic differentiation. Biochem Biophys Res Commun 440: 381–7
Razavi S, Zarkesh-Esfahani H, Morshed M, Vaezifar S, Karbasi S, Golozar MA (2015) Nanobiocomposite of poly (lactide-co-glycolide)/chitosan electrospun scaffold can promote proliferation and transdifferentiation of Schwann-like cells from human adipose-derived stem cells. Journal of Biomedical Materials Research Part A 103:2628–2634
Soto I, Rosenthal JJ, Blagburn JM, Blanco RE (2006) Fibroblast growth factor 2 applied to the optic nerve after axotomy up-regulates BDNF and TrkB in ganglion cells by activating the ERK and PKA signaling pathways. J Neurochem 96:82–96
Tate CC, Shear DA, Tate MC, Archer DR, Stein DG, LaPlaca MC (2009) Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. Journal of tissue engineering and regenerative medicine 3:208–217
Terenghi G (1999) Peripheral nerve regeneration and neurotrophic factors. J Anat 194:1–14
Tomita K, Madura T, Mantovani C, Terenghi G (2012) Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J Neurosci Res 90:1392–1402
Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K (2013) Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience 236:55–65
Woodhoo A et al. (2007) Schwann cell precursors: a favourable cell for myelin repair in the central nervous system. Brain 130:2175–2185
Worth DC, Parsons M (2008) Adhesion dynamics: mechanisms and measurements. Int J Biochem Cell Biol 40:2397–2409
Xu Y et al. (2008) Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Res 1239:49–55
Yamamoto M, Sobue G, Li M, Arakawa Y, Mitsuma T, Kimata K (1993) Nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and low-affinity nerve growth factor receptor (LNGFR) mRNA levels in cultured rat Schwann cells; differential time-and dose-dependent regulation by cAMP. Neurosci Lett 152:37–40
Yu W-M, Chen Z-L, North AJ, Strickland S (2009) Laminin is required for Schwann cell morphogenesis. J Cell Sci 122:929–936
Zafra F, Castren E, Thoenen H, Lindholm D (1991) Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci 88:10037–10041
Zhu T, Tang Q, Gao H, Shen Y, Chen L, Zhu J (2014) Current status of cell-mediated regenerative therapies for human spinal cord injury. Neurosci Bull 30:671–682
Zuk PA et al. (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Acknowledgments
The authors are grateful to the Iranian Council of Stem Cell Technology, Isfahan University of Medical Sciences for financial support (Grant No. 193070).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures of this study are applied in accordance with the Ethics Committee of the Medical Faculty in Isfahan University of Medical Sciences.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zarinfard, G., Tadjalli, M., Razavi, S. et al. Effect of Laminin on Neurotrophic Factors Expression in Schwann-Like Cells Induced from Human Adipose-Derived Stem Cells In Vitro. J Mol Neurosci 60, 465–473 (2016). https://doi.org/10.1007/s12031-016-0808-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-016-0808-6