Abstract
Neurotrophins are a group of polypeptides that specifically influence neuronal activity during development and adult life in the central and peripheral nervous system (PNS). In particular, Schwann cells (SC) in the PNS exert a neurotrophic role following up-regulation of several growth factors, including nerve growth factor (NGF) and brain derived neurotrophic factor (BDNF). Also SC-like cells derived from adipose tissue (dASC), which have molecular and functional properties similar to SC, can produce and secrete NGF and BDNF. Interestingly, gamma-aminobutyric acid (GABA) and its receptors have been also suggested as modulators of development and myelination in PNS. Therefore, it was interesting to investigate whether the stimulation of the GABA-B receptor may regulate the expression of neurotrophins in SC and dASC. Our findings demonstrated that the specific GABA-B receptors agonist baclofen influences the expression and the secretion NGF and BDNF. In particular, 2 and 24 h of baclofen exposure lead to increased neurotrophins expression in both SC and dASC, as measured by western blot. Moreover, enzyme-linked immunosorbent assay showed that also the levels of released neurotrophins were modified after baclofen treatments. The possibility to modulate the neurotrophic potential of adult stem cell, acting on functional GABAergic receptors, could represent a novel pharmacological approach to improve nerve regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurotrophic factors are a varied group of polypeptides, produced by different cell types, which specifically influence neuronal activity by promoting development and maturation during embryonic life and by sustaining maintenance during adult life and regeneration after injury (Terenghi 1999). Neurotrophins have similar basic structures but also show distinctly different domains that determine the diverse spectra of their neuronal specificity (Meyer et al. 1992). Neurotrophic factors signalling is mediated through two distinct receptor types: the high affinity Trk receptor tyrosine kinases and the low affinity p75 neurotrophin receptor (Chao 2003). In an intact nerve, trophic factors are produced by the target organs and retrogradely transported to the neuronal cell body where they exert a trophic and survival effect (Ide 1996; Terenghi 1999). Following nerve injury, Schwann Cells (SC) loose contact with the axon, switch to a proliferative state and up-regulates several growth factors, including the nerve growth factor (NGF) and the brain derived neurotrophic factor (BDNF), which replace the trophic signal coming from the target organ (Reynolds and Woolf 1993; Snider et al. 2002; Makwana and Raivich 2005). It is evident that the successful regeneration of peripheral nerves depends on the presence of multiple neurotrophic factors acting synergistically or in a well-defined sequence (Terenghi 1999).
In the last decade, an increasing number of evidences suggested the importance of gamma-aminobutyric acid (GABA) in modulating neurotrophins expression levels in adult and developing neurons (Sernagor et al. 2010). The nature and the mechanisms of this modulation are still controversial and seem to involve both the ionotropic GABA-A and the metabotropic GABA-B receptor systems (Zafra et al. 1991; Obrietan et al. 2002; Ghorbel et al. 2005; Khundakar and Zetterstrom 2011). GABA is the main inhibitory neurotransmitter of the adult central nervous system (CNS), but it is also present in the peripheral nervous system (PNS), in particular in SC (Magnaghi et al. 2010). Interestingly, SC express functional GABA receptors which are able to modulate their physiology (Magnaghi et al. 2001; Magnaghi et al. 2004) and to affect the development and myelination of the peripheral nerves (Magnaghi et al. 2008).
Adult stem cells derived from the adipose tissue (ASC) can be easily harvested with non-invasive procedures, rapidly expanded in culture and they have shown multipotential capacity (Zuk et al. 2002; Yoshimura et al. 2007). ASC express general mesenchymal stem cell markers (that is CD29, CD44, CD54, CD90, CD105, and stro-1) and lack expression of the hematopoietic antigens CD14 and CD45 (Kingham et al. 2007; Locke et al. 2009; Kalbermatten et al. 2011; Reid et al. 2011; Faroni et al. 2012). ASC can be differentiated into SC-like cells, which have phenotypical, biochemical, and functional properties similar to SC (Kingham et al. 2007; Jiang et al. 2008; Xu et al. 2008). Differentiated ASC (dASC) produce neurotrophic factors, such as BDNF and NGF, which are responsible for their neurotrophic effects as shown by in vitro co-cultures with NG108-15 neuronal cells (Kingham et al. 2007; Kaewkhaw et al. 2011; Kalbermatten et al. 2011), and with in vivo models of peripheral nerve regeneration (di Summa et al. 2011; Lopatina et al. 2011; Reid et al. 2011). The ease of harvesting and the rapid expansion capability make the ASC cultures ideal candidates as SC replacements in regenerative medicine and peripheral nerve regeneration.
We have recently demonstrated the expression of functional GABA-A and GABA-B receptors in dASC (Faroni et al. 2011; Faroni et al. 2012). Pharmacological stimulation of GABA receptors with specific ligands, such as muscimol or baclofen, affects SC and dASC proliferation (Magnaghi et al. 2004; Faroni et al. 2011), suggesting the GABA ligands as a putative tool to modulate their physiology and differentiation (Faroni et al. 2012).
It is of interest to investigate if the modulation of GABAergic receptors with specific agonist is able to regulate the expression of neurotrophic factors on SC and SC-like stem cells. The possibility to modulate the neurotrophic potential of adult stem cell, acting on functional GABAergic receptors, could represent a novel pharmacological approach to improve nerve regeneration.
Material and Methods
Animals and Cell Cultures
All the experiments requiring animals were performed in accordance with the UK Animals (Scientific Procedures) Act, 1986. Following terminal anaesthesia with CO2 and cervical dislocation, tissues were harvested from the animal and processed as required.
Neonatal Schwann Cells Harvesting and Purification
SC were harvested from the sciatic nerves of neonatal (1–2 day old) Sprague–Dawley rats using a previously established protocol (Brockes et al. 1979; Mosahebi et al. 2001). Briefly, sciatic nerves were collected, enzymatically digested and filtered through a 100-μm BD Falcon™ Cell Strainer (BD bioscience, UK). Cells were plated on poly-d-lysine (PDL; Sigma-Aldrich, UK)-coated 75 cm2 flasks and maintained for 24 h in low glucose Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma-Aldrich, UK) supplemented with 10 % (v/v) of foetal bovine serum (FBS; Biosera, UK), 1 % (v/v) of penicillin-streptomycin solution (P-S; PAA, UK), 10 μM cytosine-β-d-arabinofuranoside (Sigma-Aldrich, UK). This step was carried out to enrich the SC population removing fibroblast contamination. Cultures were maintained in low glucose DMEM supplemented with 10 % FBS (Biosera, UK), 1 % P-S, 14 μM forskolin (fsk; Sigma-Aldrich, UK) and 63 ng ml−1 of glial growth factor-2 (GGF-2; Acorda Therapeutics Inc., USA). When flasks were confluent, a final purification step was performed. Cells were trypsinised and resuspended in media containing a mouse monoclonal antibody directed against the antigen Thy 1.1 (CD90, 1:500; AbD Serotec, UK), which targets fibroblast, but not SC (Brockes et al. 1979). Immunopanning was completed by incubation in freshly thawed rabbit complement (AbD Serotec, UK). Purified neonatal Schwann cells (nSC) were incubated in 5 % CO2 at 37°C and maintained at sub-confluent levels onto PDL-coated 75 cm2 flasks, with medium changes every 72 h.
Adipose Derived Stem Cells Harvesting and Differentiation
ASC were isolated from subcutaneous, visceral, and inguinal fat pads of male adult Sprague–Dawley rats as described previously (Kingham et al. 2007). After mechanical dissociation with a sterile blade, the fat pads were joined and enzymatically digested for 90 min at 37°C with 0.2 % of collagenase type I (Life technologies, UK). Debris were eliminated by filtering through a 100-μm cell strainer (BD bioscience, UK), and the resulting cell suspension was pelleted by 5 min of centrifugation at 900 rpm. Cells were resuspended in alpha modified Eagle’s medium (αMEM; Sigma-Aldrich, UK), containing 1 % P-S (Sigma-Aldrich, UK) and 10 % FBS (Biosera, UK), and plated onto 75 cm2 flasks. Daily washes were performed for 3 days following the harvesting in order to remove the non-adherent cells. When confluent, cultures were detached with trypsin-EDTA (Life technologies, UK), split and re-plated. To induce glial differentiation, passage 1 or passage 2 cultures of ASC were treated as previously described (Kingham et al. 2007; Faroni et al. 2012). In detail, cultures were incubated for 24 h in stem cell growth media containing 1 mM β-mercaptoethanol (Sigma-Aldrich, UK), and this was followed by 3 days of cell-pre-conditioning in growth media supplemented with 35 ng ml−1 all-trans-retinoic acid (Sigma-Aldrich, UK). The medium was then replaced with stem cell differentiation medium containing 5 ng ml−1 platelet-derived growth factor (PDGF; Sera Laboratories International, UK), 10 ng ml−1 basic fibroblast growth factor (bFGF; Sera Laboratories International, UK), 14 μM fsk and 126 ng ml−1 GGF-2 (Acorda Therapeutics Inc., USA). The cells were incubated for 2 weeks under these conditions, passaged with trypsin/EDTA when needed, and fresh medium was added approximately every 72 h. Successful differentiation into a glial phenotype was confirmed by immunocytochemical assessment of glial markers, as previously reported (Faroni et al. 2011; Faroni et al. 2012).
Baclofen Treatments and Samples Collections
For pharmacological treatments, nSC and dASC cultures were seeded in multi-6-well plates (Corning Life Sciences, USA) and incubated in their respective media. Once confluent, cells were rinsed and supplemented with fresh media containing 5 % FBS. After overnight incubation, cells were treated with 100 μm baclofen (Sigma-Aldrich, UK), controls were treated with drug vehicle. After 2 or 24 h, the cell layer was rinsed with ice cold phosphate buffer solution (PBS) and scraped in lysis buffer containing 100 mM 1,4-piperazinediethanesulfonic acid (PIPES), 5 mM MgCl2, 20 % (v/v) glycerol, 0.5 % (v/v) Triton X-100, 5 mM ethylene glycol tetraacetic acid (EGTA), and a cocktail of protease and phosphatase inhibitors (Sigma-Aldrich, UK). After 30 min of incubation on ice and a freeze-thaw cycle, protein concentration was determined with the Bio-Rad detergent-compatible protein assay kit (Bio-Rad Laboratories, UK). The whole cell lysates were used for western blot analysis of intracellular BDNF and NGF as described below. The tissue culture media collected from the cells at the 24 h time point was snap frozen in silicon coated tubes for enzyme-linked immunosorbant assay (ELISA) analisys of the released neurotrophic factors (see below).
Western Blot Analysis for Neurotrophins Expression
Protein (30 μg) from each sample was boiled in reducing Laemmli’s buffer and separated by gel electrophoresis at 120 V on 15 % (w/v) sodium dodecyl sulphate-polyacrylamide gels. Resolved proteins were transferred onto a nitrocellulose membrane by overnight electroblotting at 4°C and 30 mA (constant current). Ponceau red staining of the membranes was carried out to confirm successful transfer. After 1 h of blocking in a Tris-buffered saline (TBS)-Tween solution (10 mM Tris pH 7.5, 100 mM NaCl, 0.1 % (v/v) Tween) containing 5 % (w/v) of non-fat dry milk, the membranes were incubated with a polyclonal primary antibody raised in rabbit against BDNF (1:250, Santa Cruz Biotechnology Inc., USA). After overnight incubation at 4°C, membranes were washed with TBS-Tween and incubated with an anti-rabbit horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h (1:2,000, Cell Signalling Technologies, UK) for chemiluminescence detection. Blots were stripped for 30 min at room temperature with a glycine solution (100 mM, pH 2.9; Sigma-Aldrich, UK), re-blocked with TBS-Tween containing 5 % (w/v) of non-fat dry milk and incubated overnight at 4°C with a polyclonal rabbit anti-NGF antibody (1:500, Santa Cruz Biotechnology Inc., USA). Incubation with secondary antibody and chemiluminescence detection was performed as per the BDNF. Finally, after a further stripping step, the membranes were incubated with a primary antibody directed against β-tubulin (1:3,000, Abcam, UK), followed by incubation with the secondary HRP-conjugated antibody and antigen detection. After digital acquisition of the films, the intensity of the signals was analysed by densitometry using ImageJ 64 imaging software (National Institutes of Health NIH, USA).
RNA Preparation for Real-Time RT Polymerase Chain Reaction and Analysis of mRNA Levels
For RNA analyses, cells were collected after 24 h of treatments and stored in a RNA stabilisation agent (RNAlater, QIAGEN, UK). Total RNA was isolated by single-step guanidinium isothiocyanate/phenol extraction using PureZol RNA isolation reagent (Bio-Rad Laboratories, Italy) according to the manufacturer’s instructions and quantified by spectrophotometric analysis. Following total RNA extraction, the samples were processed for real-time polymerase chain reaction (PCR) to assess mRNA levels. An aliquot of each sample was treated with DNase to avoid DNA contamination.
RNA was analysed by TaqMan qRT-PCR instrument (CFX384 real time system, Bio-Rad Laboratories, Italy) using the iScript one-step RT-PCR kit for probes (Bio-Rad Laboratories, Italy). Samples were run in 384-well formats in triplicate as simplexed reactions with a normalizing internal control (18S). Probe and primer sequences of BDNF (forward primer: AAGTCTGCATTACATTCCTCGA and reverse primer: GTTTTCTGAAAGAGGGACAGTTTAT), NGF (forward primer: AAGGACGCAGCTTTCTATCC and reverse primer: CTATCTGTGTACGGTTCTGCC) and 18S (forward primer: GTAACCCGTTGAACCCCATT and reverse primer: CCATCCAATCGGTAGTAGCG) were purchased from Eurofins MWG-Operon (Germany).
Thermal cycling was initiated with an incubation at 50°C for 10 min (RNA retrotranscription) and then at 95°C for 5 min (TaqMan polymerase activation). After this initial step, 39 cycles of PCR were performed. Each PCR cycle consisted of heating the samples at 95°C for 10 s to enable the melting process and then for 30 s at 60°C for the annealing and extension reactions. A comparative cycle threshold (Ct) method was used to calculate the relative target gene expression.
ELISA for BDNF and NGF
The snap frozen media was thawed on ice and concentrated by centrifugation for 30′ at 13 rpm and 4°C using Amicon® Ultra centrifugal filters (molecular weight cut-off 3kDa, Chemicon, UK). Media from cells treated 24 h with baclofen, and controls were analysed by ELISA using the ChemiKine™ BDNF sandwich ELISA kit (Chemicon, UK) or the RayBio® rat beta-NGF ELISA kit (RayBiotech Inc., USA). All samples were assayed in duplicate following the protocols suggested by the manufacturers. Final absorbance was read at 450 nm with an Asys UVM-340 microplate reader/spectrophotometer (Biochrom Ltd., United Kingdom). Recombinant proteins provided in the kits were used to produce standard curves and to calculate the amount of neurotrophic factors released.
Statistical Analysis
Statistical significance for the western blot and ELISA studies was estimated by unpaired two-tailed Student’s t test using GraphPad Prism 5 (GraphPad Software Inc., USA). In Western blot studies, densitometry data of BDNF and NGF were normalised for the levels of β-tubulin used as a loading control and the data were expressed as percentage vs. controls ± standard error of the mean (SEM). In both cases, levels of significance were expressed as P values versus controls (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Stem Cell Differentiation into SC-Like Phenotype
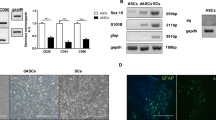
ASC were successfully isolated from digested fat pads of adult rats, exploiting their ability to adhere to tissue culture plastic. Undifferentiated cultures (uASC) at passage 1 and 2 showed the typical fibroblast-like flat phenotype (Fig. 1a). After the glial differentiation protocol, dASC showed a spindle-shaped, SC-like phenotype (Fig. 1b), similar to primary cultures of nSC used as positive controls (Fig. 1c).
Glial differentiation of ASC. Phase contrast image of uASC cultures (a) adhering to tissue culture plastic and showing fibroblast-like morphology. Following differentiation into glial phenotype dASC cultures (b) underwent through a morphological change and displayed the spindle-like shape typical of nSC cultures (c). Scale bar 100 μm
Effect of Baclofen Treatments on NGF Expression and Secretion
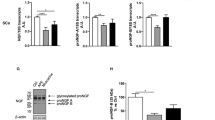
Using western blot analysis, we evaluated the expression of the neurotrophins NGF and BDNF in nSC and dASC treated with the specific GABA-B agonist baclofen (Fig. 2a). Following 2 h of exposure to 100 μm baclofen, the expression level of NGF was significantly higher in nSC compared to untreated controls (212.0 ± 17.0 % vs. controls, **p < 0.01, Fig. 2b). Moreover, 24 h of baclofen treatments significantly increased the expression of NGF in nSC (121.2 ± 4.303 % vs. controls, **p < 0.01, Fig. 2b). Similarly, dASC cultures treated with 100 μm baclofen for 2 or 24 h showed significantly higher levels of NGF compared to the untreated controls. In particular, expression levels were 120.0 ± 2.0 and 129.7 ± 4.8 % vs. controls in the cells treated for 2 or 24 h with baclofen, respectively (***p < 0.001, Fig. 2c). For quantification of western blot analysis, levels of NGF expression were normalised for the housekeeping gene β-tubulin, used as a loading control, and expressed as percentage vs. non-treated controls ± SEM. Recombinant NGF (Raybiotech Inc., USA) was used as a positive control.
Western blot analysis of NGF expression following GABA-B receptor stimulation: representative blots of nSC and dASC treated with baclofen (BAC) for 2 or 24 h (a). Controls (CTR) were treated with vehicle. nSC treated for 2 or 24 h with baclofen showed significantly higher levels of intracellular NGF (b, **p < 0.01). Similarly, NGF expression was significantly higher in dASC treated with baclofen for 2 or 24 h (c, ***p < 0.001). NGF expression data was normalised for the expression of the housekeeping gene β-tubulin, and expressed as percentage vs. untreated controls ± SEM. Recombinant beta-NGF was used as a positive control
To investigate whether the increased intracellular levels were due to the synthesis of NGF, mRNA levels for NGF were assessed by RT-PCR. In both nSC and dASC, NGF mRNA levels were not affected by baclofen treatment (nSC 111 ± 37 % vs. controls p = 0.84, dASC 60 ± 24 % vs. controls p = 0.25).
In order to assess the amount of secreted NGF, we analysed the culture media of the cells treated for 24 h using an ELISA kit for rat beta-NGF. NGF concentration in the cell culture supernatant of baclofen-treated nSC was significantly lower compared to the untreated controls (Fig. 3a, *p < 0.05). By contrast, 24 h of exposure to baclofen significantly increased the amount of NGF released by dASC compared to untreated cells (Fig. 3b, *p < 0.05).
ELISA for NGF: Cell culture supernatants of cells treated for 24 h were analysed by ELISA for NGF. Culture supernatants from baclofen-treated nSC showed significantly lower concentrations of NGF compared to untreated controls (a, *p < 0.05). In dASC, baclofen treatments significantly increased the extracellular concentration of NGF (b, *p < 0.05). Data was expressed as NGF concentration (pg/ml) ± SEM. BAC = baclofen, CTR = controls
Effect of Baclofen Treatments on BDNF Expression and Secretion
Using the same approaches followed for NGF, the expression of BDNF in cell cultures and in culture media was also analysed following baclofen treatments of nSC and ASC. Western blot analysis of nSC cultures showed an increase in BDNF after 2 or 24 h of exposure to baclofen, confirming the same trend observed with NGF expression (Fig. 4a). After 2 h of exposure to baclofen, nSC showed higher level of BDNF if compared to controls; however, the difference was not statistically significant. BDNF expression after 24 h of baclofen treatments was significantly increased compared to untreated controls (153.6 ± 14.8 % vs. controls, *p < 0.05, Fig. 4b). Similarly, baclofen treatments increased the expression of BDNF in dASC after 2 or 24 h (Fig. 4a). In particular, a significant increase in intracellular BDNF was observed in dASC exposed for 2 h to baclofen compared to untreated controls (142.8 ± 6.8 % vs. controls, **p < 0.01, Fig. 4c). Likewise, BDNF expression was also significantly increased after 24 h of treatments with baclofen in dASC (119.3 ± 2.8 % vs. controls, **p < 0.01, Fig. 4c). All BDNF expression data were normalised for the housekeeping gene β-tubulin and expressed as percentage vs. untreated controls ± SEM. Human recombinant BDNF (Chemicon, UK) was used as a positive control.
Western blot analysis of BDNF expression following GABA-B receptor stimulation: representative blots of nSC and dASC treated with baclofen for 2 or 24 h (a). Controls were treated with vehicle. 2 or 24 h of baclofen exposure caused increased BDNF expression in nSC (b). BDNF expression increase after 24 h of treatments was statistically significant (b, *p < 0.05). Similarly, dASC treated with baclofen for 2 or 24 h showed increased BDNF expression compared to untreated controls (c, **p < 0.01). BDNF expression data was normalised for housekeeping gene β-tubulin, and expressed as percentage vs. untreated controls ± SEM. Human recombinant BDNF was used as a positive control. BAC = baclofen, CTR = controls
RT-PCR was carried out also for BDNF to verify whether protein increase is due to synthesis of neurotrophin. In nSC treated with baclofen for 24 h, the levels of BDNF mRNA was similar to the untreated controls (119 ± 13 % vs. controls, p = 0.56). By contrast, in dASC, 24 h of exposure to baclofen strongly increased BDNF mRNA levels compared to the untreated controls (592 ± 72 % vs. controls, **p < 0.05).
BDNF released in the tissue culture media was quantified by ELISA analyses. nSC exposed for 24 h to baclofen released a significantly lower amount of BDNF in the media compared to untreated cells (*p < 0.05, Fig. 5a). Likewise, BDNF levels were lower in supernatants of dASC treated for 24 h with baclofen (*p < 0.05, Fig. 5b).
ELISA for BDNF: Cell culture supernatants of cells treated for 24 h were analysed by ELISA for BDNF. Culture media from baclofen treated nSC showed significantly lower concentrations of BDNF compared to untreated controls (a, *p < 0.05). Similarly, baclofen treated dASC released a significantly lower amount of BDNF in the culture supernatant (b, *p < 0.05). Data was expressed as BDNF concentration (pg/ml) ± SEM. BAC = baclofen, CTR = controls
Discussion
The findings reported in this paper demonstrated that the pharmacological modulation of GABA-B receptors with the specific agonist baclofen is able to regulate the expression and the secretion of the neurotrophic factors NGF and BDNF in SC and SC-like adult stem cells cultures derived from the adipose tissue. This is the first evidence indicating a role of GABA-B receptors in modulating the expression and release of neurotrophins in the PNS and represents a significant addition to previous work addressed to the study of GABA receptors in SC-like stem cells (Faroni et al. 2011; Faroni et al. 2012). BDNF and NGF produced by SC play crucial roles in neuronal survival and in the regenerative process that follows peripheral nerve injury (Fu and Gordon 1997; Terenghi 1999; Gordon 2009). This trophic role on nerve regeneration could be exploited by tissue engineering with the creation on bioartificial nerve conduits, containing transplanted SC, able to deliver neurotrophins and to support the growth of the injured axons, improving the functional outcome (Mosahebi et al. 2001; Radtke and Vogt 2009; Madduri and Gander 2010). However, obtaining an adequate number of transplantable SC would require a significant time of cell culturing and could imply donor site morbidity (Tohill and Terenghi 2004; Terenghi et al. 2009). An attractive alternative source of transplantable cells for regenerative medicine consists in the use of mesenchymal stem cells as SC replacement (Wiberg and Terenghi 2003). Bone marrow stromal cells were the first stem cell type to be successfully differentiated into SC to support nerve regeneration (Dezawa et al. 2001), followed by several studies addressing similar experimental settings with different stem cell populations from various origins (Keilhoff and Fansa 2011). Using a similar protocol, our group was the first to successfully differentiate adipose-derived stem cells into a SC phenotype (Kingham et al. 2007) and to show their potential of promoting nerve regeneration in vivo (di Summa et al. 2010; di Summa et al. 2011). Both undifferentiated (Zhao et al. 2009; Sowa et al. 2012) and differentiated (Kaewkhaw et al. 2011; Reid et al. 2011; Tse et al. 2011) ASC express neurotrophins, such as BDNF and NGF, which are responsible for their neurotrophic effects determining neuronal survival and neurite outgrowth. dASC and SC have been shown to possess the same molecular markers (that is GFAP, S100, p75) (Kingham et al. 2007; Faroni et al. 2011; Tomita et al. 2012). Functionally, dASC like SC are able to form myelin structures both in vivo and in vitro (Xu et al. 2008; Mantovani et al. 2010; Tomita et al. 2012) and to improve nerve regeneration in vivo (di Summa et al. 2010; Zhang et al. 2010; di Summa et al. 2011; Tomita et al. 2012). Although all these studies have addressed their similarities, to our knowledge, there is no literature available that is specifically focused on the differences between SC and dASC. However, clear differences of synthesis and secretion of neurotrophins have been found in our study, as shown below.
We have recently demonstrated the expression of GABA receptors on ASC and their function in modulating stem cell proliferation induced by forskolin (Faroni et al. 2011; Faroni et al. 2012). At least in the CNS, GABA receptors have been shown to be involved in the modulation of neurotrophins expression and in neuronal development (Heese et al. 2000; Obrietan et al. 2002); nevertheless, the mechanisms underlying such actions are not completely understood. Interestingly, GABA-B receptor stimulation with the specific agonist baclofen triggers BDNF expression and secretion in hippocampal neurons (Ghorbel et al. 2005; Fiorentino et al. 2009). A mechanism for this GABA-B mediated BDNF up-regulation has recently been suggested (Kuczewski et al. 2011). Moreover, there are also several evidences of BDNF and NGF up-regulation following treatments with GABA-B receptors antagonists, but the mechanisms underlying this action have not been clarified (Heese et al. 2000). Finally, also GABA-A receptor activation has been reported to up-regulate (Obrietan et al. 2002; Porcher et al. 2011) or down-regulate (Zafra et al. 1991; Giusi et al. 2009) neurotrophins expression levels.
We therein demonstrated that the stimulation of GABA-B receptors is able to increase neurotrophins levels in SC and dASC. All the treatments were performed in media deprived of forskolin, growth factors and with a reduced amount of serum, since they are known to interfere with neurotrophins expression levels (Matsuoka et al. 1991; Zafra et al. 1991; Meyer et al. 1992). Interestingly, both BDNF and NGF intracellular protein levels were increased after 2 and 24 h of exposure to the specific GABA-B agonist baclofen. Nevertheless, the amount of BDNF protein released by SC and dASC in the tissue culture media was reduced by baclofen treatments. This observation might also imply that the intracellular increase of BDNF might be due to inhibition of BDNF secretion and not only to increased protein synthesis. For this reason, we performed mRNA analyses to assess the levels of synthesis of BDNF. Interestingly, mRNA transcripts for BDNF were found upregulated after 24 h of treatments with baclofen in dASC, suggesting that the increase in intracellular BDNF protein may indeed be related to BDNF synthesis and not only to inhibition of BDNF release. However, in SC, BDNF mRNA levels did not seem to vary following baclofen stimulation, suggesting that the intracellular increase might be due mainly to inhibition of neurotrophin release. The decrease in BDNF release following baclofen treatments in SC and dASC is in contrast with the reported increased secretion observed in hippocampal neuron cultures (Fiorentino et al. 2009). However, in vivo observations in distinct hippocampal areas showed an inhibitory action by baclofen on BDNF (Khundakar and Zetterstrom 2011), suggesting that an intact in vivo system is required for such action. We might speculate that other cells, such as the glial cells supporting neurons in vivo, might play a role in determining the differences with the in vitro system and in decreasing the levels of BDNF in the intact in vivo system.
Similarly to what was observed for BDNF, NGF intracellular levels are also increased in SC and SC-like stem cells after GABA-B receptor stimulation. Moreover, as observed for BDNF, NGF secreted levels in SC were reduced by baclofen treatments as shown by ELISA assay of the tissue culture media. By contrast, 24 h of exposure to baclofen increased the levels of secreted NGF in dASC. The analysis of mRNA levels for NGF showed that the synthesis of RNA does not seem to be affected by baclofen treatments in both dASC and nSC. In SC, this might indicate that the increased intracellular levels of NGF may be due to inhibition of release as observed for BDNF. Whereas in dASC, where both intracellular and released NGF proteins were increased, the fact that mRNA levels were unchanged might be due to other mechanisms involving NGF metabolic kinetics, RNA stabilisation, neurotrophin degradation, and recycling (Matsuoka et al. 1991; Meyer et al. 1992). It could be hypothesized that the time course of NGF expression and release is different in dASC compared to SC. There are several examples of a variety of cytokines, peptide growth factors, and other compounds affecting the levels of NGF in different ways depending on the cell type stimulated. For example, the same pharmacological stimulations have been shown to modulate the expression and kinetics of NGF in different ways in astrocytes, hippocampal neurons, fibroblast or SC (Matsuoka et al. 1991; Zafra et al. 1991; Meyer et al. 1992; Zafra et al. 1992).
Neurotrophic factors, and in particular NGF, have been reported to have biphasic expression patterns in SC cultures and other cell types (Matsuoka et al. 1991; Zafra et al. 1991). A similar biphasic expression pattern for NGF is also observed in vivo after injury (Meyer et al. 1992). In particular, NGF levels in the nerve region immediately proximal to the lesion and in the distal stump rapidly and massively increase after nerve lesion with a first peak in the first 24 h (Heumann et al. 1987a; Heumann et al. 1987b), followed by a second slow increase which last several weeks (Lindholm et al. 1987; Matsuoka et al. 1991). This biphasic expression pattern is not observed for BDNF, which in contrast shows a slow monophasic increase that reach maximal levels at 4 weeks post transection and is only restricted to the distal segment (Meyer et al. 1992). Unfortunately such long time of cell culture and pharmacological stimulation are not possible in vitro. The distinctly different time course and spatial pattern of NGF and BDNF expression after nerve transection (Meyer et al. 1992) suggests a complementary role of the two neurotrophins in aiding nerve regrowth (Ide 1996; Terenghi 1999). A different time course in the maturation of NGF should not be totally excluded. Indeed, neurotrophins are initially synthesized as precursors or pro-neurotrophins, which are then cleaved intracellularly to produce the mature proteins (Chao 2003). Regarding the analyses of NGF and BDNF, all the previous work performed to assess expression and release in dASC compared with SC was made in basal conditions. Unfortunately, no studies of neurotrophins expression and release following pharmacological stimulation were reported previously in our in vitro model. Indeed, ours is the first study by which the expression and release of BDNF and NGF in SC and dASC have been performed and compared after pharmacological treatments with baclofen.
Alterations in neurotrophins levels have profound effects on a wide variety of phenomena, including myelination, regeneration, pain, aggression, depression and substance abuse (Chao 2003). Regarding peripheral nerve regeneration, both the biological timing and the expression levels of the neurotrophic factors are critical in promoting axon regeneration (Gordon 2009). Although increased levels of neurotrophins are beneficial for the regeneration process, excessively high concentrations of neurotrophic factors that mediate their effects via p75 rather than trk receptors, such as BDNF, inhibit axon growth and may even promote neuronal death (Boyd and Gordon 2001; Boyd and Gordon 2002; Gordon 2009). For this reason, finely tuning the expression level of these factors is very important, and the possibility of modulating such levels with pharmacological ligands represent an attractive approach.
Peripheral nerve injuries constitute a notable social and economical issue affecting 2.8 % of trauma patients (Heumann et al. 1987b), whose quality of life is impaired due to the poor functional recovery after surgical treatments (Wiberg and Terenghi 2003). In this context, the delivery of neurotrophic factors through transplanted stem cells represent an attractive clinical alternative to improve the functional outcome. To our knowledge, this report provides the first evidence of the involvement of GABA-B receptors in the regulation of neurotrophins expression and release in the peripheral nervous system. Although further studies are needed to fully understand the role of GABA in modulating stem cell physiology, their differentiation and neurotrophic potential, the presence of GABA-B receptors, which are able to tune neurotrophins expression levels in adult stem cells, could represent a potential pharmacological tool to improve nerve regeneration.
References
Boyd JG, Gordon T (2001) The neurotrophin receptors, Trkb and P75, differentially regulate motor axonal regeneration. J Neurobiol 49(4):314–325
Boyd JG, Gordon T (2002) A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci 15(4):613–626
Brockes JP, Fields KL, Raff MC (1979) Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res 165(1):105–118
Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4(4):299–309
Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H (2001) Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci 14(11):1771–1776
di Summa PG, Kingham PJ, Raffoul W et al (2010) Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg 63(9):1544–1552
di Summa PG, Kalbermatten DF, Pralong E et al (2011) Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience 181:278–291
Faroni A, Mantovani C, Shawcross SG et al (2011) Schwann-like adult stem cells derived from bone marrow and adipose tissue express gamma-aminobutyric acid type B receptors. J Neurosci Res 89(9):1351–1362
Faroni A, Terenghi G, Magnaghi V (2012) Expression of functional gamma-aminobutyric acid type A receptors in Schwann-like adult stem cells. J Mol Neurosci. doi:10.1007/s12031-011-9698-9
Fiorentino H, Kuczewski N, Diabira D et al (2009) Gaba(B) receptor activation triggers BDNF release and promotes the maturation of gabaergic synapses. J Neurosci 29(37):11650–11661
Fu SY, Gordon T (1997) The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol 14(1–2):67–116
Ghorbel MT, Becker KG, Henley JM (2005) Profile of changes in gene expression in cultured hippocampal neurones evoked by the gabab receptor agonist baclofen. Physiol Genomics 22(1):93–98
Giusi G, Facciolo RM, Rende M et al (2009) Distinct alpha subunits of the GABAA receptor are responsible for early hippocampal silent neuron-related activities. Hippocampus 19(11):1103–1114
Gordon T (2009) The role of neurotrophic factors in nerve regeneration. Neurosurg Focus 26(2):E3
Heese K, Otten U, Mathivet P et al (2000) Gaba(B) receptor antagonists elevate both mRNA and protein levels of the neurotrophins nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) but not neurotrophin-3 (Nt-3) in brain and spinal cord of rats. Neuropharmacology 39(3):449–462
Heumann R, Korsching S, Bandtlow C, Thoenen H (1987a) Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol 104(6):1623–1631
Heumann R, Lindholm D, Bandtlow C et al (1987b) Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci USA 84(23):8735–8739
Ide C (1996) Peripheral nerve regeneration. Neurosci Res 25(2):101–121
Jiang L, Zhu JK, Liu XL et al (2008) Differentiation of rat adipose tissue-derived stem cells into Schwann-like cells in vitro. Neuroreport 19(10):1015–1019
Kaewkhaw R, Scutt AM, Haycock JW (2011) Anatomical site influences the differentiation of adipose-derived stem cells for Schwann-cell phenotype and function. Glia 59(5):734–749
Kalbermatten DF, Schaakxs D, Kingham PJ, Wiberg M (2011) Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res 344(2):251–260
Keilhoff G, Fansa H (2011) Mesenchymal stem cells for peripheral nerve regeneration—a real hope or just an empty promise? Exp Neurol 232(2):110–113
Khundakar AA, Zetterstrom TS (2011) Effects of GABAB ligands alone and in combination with paroxetine on hippocampal BDNF gene expression. Eur J Pharmacol 671(1–3):33–38
Kingham PJ, Kalbermatten DF, Mahay D et al (2007) Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol 207(2):267–274
Kuczewski N, Fuchs C, Ferrand N et al (2011) Mechanism of GABAB receptor-induced BDNF secretion and promotion of GABAA receptor membrane expression. J Neurochem 118(4):533–545
Lindholm D, Heumann R, Meyer M, Thoenen H (1987) Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 330(6149):658–659
Locke M, Windsor J, Dunbar PR (2009) Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ J Surg 79(4):235–244
Lopatina T, Kalinina N, Karagyaur M et al (2011) Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One 6(3):e17899
Madduri S, Gander B (2010) Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J Peripher Nerv Syst 15(2):93–103
Magnaghi V, Cavarretta I, Galbiati M, Martini L, Melcangi RC (2001) Neuroactive steroids and peripheral myelin proteins. Brain Res Brain Res Rev 37(1–3):360–371
Magnaghi V, Ballabio M, Cavarretta IT et al (2004) GABAB receptors in Schwann cells influence proliferation and myelin protein expression. Eur J Neurosci 19(10):2641–2649
Magnaghi V, Ballabio M, Camozzi F et al (2008) Altered peripheral myelination in mice lacking GABAB receptors. Mol Cell Neurosci 37(3):599–609
Magnaghi V, Parducz A, Frasca A et al (2010) GABA synthesis in Schwann cells is induced by the neuroactive steroid allopregnanolone. J Neurochem 112(4):980–990
Makwana M, Raivich G (2005) Molecular mechanisms in successful peripheral regeneration. FEBS J 272(11):2628–2638
Mantovani C, Mahay D, Kingham M et al (2010) Bone marrow- and adipose-derived stem cells show expression of myelin mRNAs and proteins. Regen Med 5(3):403–410
Matsuoka I, Meyer M, Thoenen H (1991) Cell-type-specific regulation of nerve growth factor (NGF) synthesis in non-neuronal cells: comparison of Schwann cells with other cell types. J Neurosci 11(10):3165–3177
Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H (1992) Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol 119(1):45–54
Mosahebi A, Woodward B, Wiberg M, Martin R, Terenghi G (2001) Retroviral labeling of Schwann cells: in vitro characterization and in vivo transplantation to improve peripheral nerve regeneration. Glia 34(1):8–17
Obrietan K, Gao XB, Van Den Pol AN (2002) Excitatory actions of GABA increase BDNF expression via a MAPK-Creb-dependent mechanism—a positive feedback circuit in developing neurons. J Neurophysiol 88(2):1005–1015
Porcher C, Hatchett C, Longbottom RE et al (2011) Positive feedback regulation between {Gamma}-aminobutyric acid type a (GABAA) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons. J Biol Chem 286(24):21667–21677
Radtke C, Vogt PM (2009) Peripheral nerve regeneration: a current perspective. Eplasty 9:e47
Reid AJ, Sun M, Wiberg M et al (2011) Nerve repair with adipose-derived stem cells protects dorsal root ganglia neurons from apoptosis. Neuroscience 199:515–522
Reynolds ML, Woolf CJ (1993) Reciprocal Schwann cell–axon interactions. Curr Opin Neurobiol 3(5):683–693
Sernagor E, Chabrol F, Bony G, Cancedda L (2010) GABAergic control of neurite outgrowth and remodeling during development and adult neurogenesis: general rules and differences in diverse systems. Front Cell Neurosci 4:11
Snider WD, Zhou FQ, Zhong J, Markus A (2002) Signaling the pathway to regeneration. Neuron 35(1):13–16
Sowa Y, Imura T, Numajiri T, Nishino K, Fushiki S (2012) Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev. doi:10.1089/scd.2011.0403
Terenghi G (1999) Peripheral nerve regeneration and neurotrophic factors. J Anat 194(Pt 1):1–14
Terenghi G, Wiberg M, Kingham PJ (2009) Chapter 21: use of stem cells for improving nerve regeneration. Int Rev Neurobiol 87:393–403
Tohill M, Terenghi G (2004) Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol Appl Biochem 40(Pt 1):17–24
Tomita K, Madura T, Mantovani C, Terenghi G (2012) Differentiated adipose-derived stem cells promote myelination and enhance functional recovery in a rat model of chronic denervation. J Neurosci Res. doi:10.1002/jnr.23002
Tse KH, Kingham PJ, Novikov LN, Wiberg M (2011) Adipose Tissue and bone marrow-derived stem cells react similarly in an ischaemia-like microenvironment. J Tissue Eng Regen Med. doi:10.1002/term.452
Wiberg M, Terenghi G (2003) Will it be possible to produce peripheral nerves? Surg Technol Int 11:303–310
Xu Y, Liu L, Li Y et al (2008) Myelin-forming ability of Schwann cell-like cells induced from rat adipose-derived stem cells in vitro. Brain Res 1239:49–55
Yoshimura H, Muneta T, Nimura A et al (2007) Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 327(3):449–462
Zafra F, Castren E, Thoenen H, Lindholm D (1991) Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci USA 88(22):10037–10041
Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H (1992) Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci 12(12):4793–4799
Zhang Y, Luo H, Zhang Z et al (2010) A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials 31(20):5312–5324
Zhao L, Wei X, Ma Z et al (2009) Adipose stromal cells-conditional medium protected glutamate-induced CGNS neuronal death by BDNF. Neurosci Lett 452(3):238–240
Zuk PA, Zhu M, Ashjian P et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295
Acknowledgments
The authors are grateful to Acorda Therapeutics, USA, for kindly supplying GGF-2 for the continuation of this work. We also thank the Hargreaves and Ball Trust and Fondazione San Paolo di Torino (grant no. 2229/2008 to V.M.) for financial support. Alessandro Faroni was funded by a BBSRC DTG fellowship and by the University of Milan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faroni, A., Calabrese, F., Riva, M.A. et al. Baclofen Modulates the Expression and Release of Neurotrophins in Schwann-Like Adipose Stem Cells. J Mol Neurosci 49, 233–243 (2013). https://doi.org/10.1007/s12031-012-9813-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-012-9813-6