Abstract
Inhibition of RhoA/Rock could promote axon growth and alleviate optic nerve injury. However, the role of RhoA/Rock in the traumatic retinal nerve in vivo was not completely clear. In this study, we established a rabbit model of traumatic retinal nerve injury, and primary retinal ganglion cells (RGCs) were isolated and cultured under hypoxia-hypoglycemia condition that was mock to the microenvironment in the injured retinas in vivo. The Rock inhibitor fasudil was used to treat primary RGCs and ear vein injected into the model rabbits in vivo. RhoA/Rock signaling was activated in the injured optic nerve in rabbits. Western blotting analysis showed that RhoA/Rock signaling in the retina was activated during the traumatic optic neuropathy. Data on gene expression examination and Annexin V/PI dual staining combined with flow cytometry analysis displayed that fasudil injection reduced expression of Rho/Rock and apoptotic genes, as well as the apoptosis of RGCs in traumatic retinal nerve injury in vitro and in vivo. Moreover, fasudil injection reduced expression of Rho/Rock and apoptotic genes, as well as the apoptosis of RGCs in the rabbits with traumatic retinal nerve injury in vivo. In conclusion, fasudil treatment could significantly reduce the apoptosis of RGCs and relieved retinal nerve injury in vitro and in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic optic neuropathy is an indirect injury to the retinal nerve caused by external force through bone or eye movement. Forty to 50 % of the cases of optic nerve injury developed to be blindness (Wilhelm 2009). This is a main cause of traumatic visual defect. A previous study indicated that the visual defect after optic nerve injury is caused by primary and secondary injuries caused apoptosis of retinal ganglion cells (RGCs) (Stone 2013). The primary injuries mainly included concussion, tear, distraction, and compression of the optic nerve. The histopathological events caused by primary injuries were mainly circulation disorders, inflammatory reactions, and electrochemical disorders, etc. (Li et al. 2015; Giacci et al. 2014).
RGCs are intrinsically important components of the retinal nerve and are responsible for the communication between the eye and the brain (Steketee et al. 2014). Their axons extend to the optic nerve papilla, pass through the sieve plate, and form optic nerve. On one hand, the apoptosis of RGCs directly resulted in defects of optic nerve (Fernandes et al. 2014). On the other hand, when the RGC axons were disrupted, the supply of target-derived neurotrophic factors would be blocked. And then, glial cells (especially microglia) were activated releasing a large number of inflammatory factors and toxic substances to aggravate the injury of optic nerve (Benowitz et al. 2015). Therefore, to slow down or inhibit the degeneration of RGCs after optic nerve injury is important for the therapy of optic nerve injury and the recovery of visual function.
Compared with the peripheral nerve, the regeneration ability of the central nervous system (CNS) is relatively weak (Cho et al. 2005). After central nerve injury, the microenvironment in the nervous tissues was changed, and several axon growth inhibitory factors were induced, including myelin-associated glycoprotein (MAG), Nogo myelin-associated glycoprotein (Nogo-A), myelin-oligodendrocyte glycoprotein (MOG), etc. (Hasegawa et al. 2005; Hannila et al. 2013; Vajda et al. 2014). Studies revealed that Nogo-A and its receptor NgR were upregulated in retinal nerve injury and inhibited RGC axon growth (Vajda et al. 2014; Pernet and Schwab 2012). Rho is a famous subfamily of Ras oncogene super family of low molecular weight GTPases (Strutt et al. 1997). They have been involved in many physiological activities including cell migration, adhesion, cytokinesis, proliferation, differentiation, and apoptosis (Takeda et al. 2006; Wang et al. 2013; Andalib et al. 2013). According to cutting-edge researches, Rho and their associated protein kinase Rock played a key role in the alternation of the microenvironment and lead to the growth inhibition of neuron axons after injury (Fujita and Yamashita 2014). In the injured area, RhoA/Rock was robustly activated. Some studies indicated that inhibition of RhoA/Rock could promote axon growth (Gu et al. 2013; Tan et al. 2012). Moreover, Rock inhibitors have been applied in the inhibition of leukocyte adhesion and migration, regeneration of neuron axon, and protection of neurons (Yang et al. 2013; Wang et al. 2012). However, the role of RhoA/Rock in the traumatic retinal nerve in vivo was still not completely clear.
In this study, rabbit model of traumatic retinal nerve injury was established, and primary RGCs were isolated and cultured. The role of Rock inhibitor fasudil on the apoptosis of RGCs was explored in vitro and in vivo.
Materials and Methods
Preparation of the Model of Traumatic Optic Neuropathy
Two hundred adult male New Zealand rabbits (clean grade) were numbered and weighed. After anesthesized with 3 % phenobarbital, their optic nerve around the nerve bone pipe (or ring) was separated under an operation microscope (ZC-X-4B, Zhuochuang Medical Tech Co., LTd., Zhengjiang, China). Yasargil Aneurgsm clip (65742) then was stretched into the bone pipe (or ring) to clamp the nerve. After 30 s, the clip was removed. The pupil size and light reflex of the rabbits were measured to evaluate the model establishment. As the right side direct light reflex disappeared and the Marcus-Gunn’s pupil with indirect light reflex appeared, we confirmed the model was established successfully.
Sampling and Histological Examination
After anesthetized with 4 % phenobarbital, the optic nerves of the rabbit eyes were exposed. Remove the cornea and then take on the entire retina (the cross stitch structure). Gently snip off the laser-disc (white) in the center of the retina. The retina and laser-disc were respectively fixed with 3 % formaldehyde and then sectioned (4 μm) post-paraffin embedded. Then, the sections were stained with hematoxylin-eosin method (H&E) and observed under a microscope (400×).
For the detection of degree of inflammatory infiltration in the tissue, an automatic counting software image pro-plus (IPP) was applied. Inflammatory cells in each area were selected, and the parameters of each inflammatory cell was set according to the instructions of the software. The inflammatory cells were counted and statistically analysed.
Transmission Electron Microscopy (TEM)
The retinal ganglions were fixed with 3 % glutaraldehyde. Then, the tissues were post-fixed with 1 % osmium tetroxide at 4 °C for 2.5 h. After a graded series of dehydration, they were embedded in epoxy resin at room temperature for 4 h and sectioned (10 nm). Ultrathin sections were obtained using a RMC MT6000 XL ultra-microtome and stained with uranyl acetate and lead citrate. Samples were then examined under an electron microscope (JEM-1200EX, JEOL, Tokyo, Japan) at an accelerating voltage of 75 kV.
Culture of Retinal Ganglion Cells
RGCs were isolated with the method described in a previous study (Vecino et al. 2014). The cells were cultured in high glucose (4.5 mg/mL) B27-supplemented Neurobasal-A medium (Invitrogen) containing L-glutamine and gentamicin for 6 days at 37 °C in a humidified atmosphere containing 5 % CO2. Medium was changed every 3 days, and no growth factors were added to the culture medium. Three independent experiments were repeated.
Hypoxia-Hypoglycemia and Fasudil Treatment
After culture for 24 h, the medium was replaced with low-glucose B27-Neurobasal-A medium (1 mg/mL) supplemented with 300-μM CoCl2 (to simulate a hypoxic environment in vitro). RGCs cultured in high-glucose B27-supplemented Neurobasal-A medium with 300-μM DMSO were regarded as the control (normal control).
The cells under hypoxia-hypoglycemia (HO-HG) condition were treated with fasudil at the dosages of 0, 25, 50, 75, and 100 μM. After incubation for 48 h, the cells were collected for the following detection.
Cell Viability and Apoptosis Detection In Vitro
The cell viability assay was performed with the tetrazolium salt colorimetric assay (MTT) method (Promega, Madison, Wisconsin) according to the manufacturer’s instructions. After stained with 0.1 % crystal violet for 20 min, the absorption was detected under excitation of 550 nm.
Cell apoptosis was detected with Annexin V/PI dual staining combined with flow cytometry. After transfection for 48 h, the medium was removed, and pre-cooled PBS was used to wash the cells twice. Removing the supernatant, the cells were suspended with 300 μL of 1× binding buffer (at the density of 5 × 105 /mL), 5-μL Annexin V FITC, and 5-μL PI was added into the suspension, and then the mixture was incubated on ice for 10 min in dark. The 300 μL of cold binding buffer was added in each sample, and then the samples were analyzed with flow cytometry.
Real-Time Quantitative PCR
Following treatment, total RNA of the cells was extracted with Trizol reagent (Invitrogen), and its quantity and integrity were then checked. Real-time qPCR reactions were carried out in a final volume of 25 μL, using SYBR Premix Ex Taq (TaKaRa), with 0.4 mM of each primer and 200 ng of the cDNA template. Primers applied in the reactions are as follows (F: forward; R: reverse): RhoA (F: 5′- CCG TGC ATC TTG CAG TAC ATC T -3′, 5′- CTA CCC CAT TTC GCC CAA GT -3′); Rock1 (F: 5′- ATG AGT TTA TTC CTA CAC TCT ACC ACT TTC-3′, R: 5′- TAA CAT GGC ATC TTC GAC ACT CTA G-3′); Rock2 (F: 5′- CTG GTG GTC TGT GGG TGT TT-3′, 5′- CTA CCC CAT TTC GCC CAA GT-3′); MAG (F: 5′- AGT AGG GTA CTG CAT AAA AGG -3′, R: 5′-GCA GAA ATC CAC CAC CAT GAA AC-3′); NgR (F: 5′-CTT ATG CTAT AAC ACG CT-3′, R: 5′-TAG TCC ACG TCA CTC AT-3′); Caspase-3 (F: 5′- ATG GAG AAC AAT AAA ACC T-3′, R: 5′- CTA GTG ATA AAA GTA GAG TTC -3′); BDNF (F: 5′-ATG GGA CTC TGG AGA GCC TGA A-3′, 5′-CGC CAG CCA ATT CTC TTT TTG C-3′); Bcl-2 (F: 5′-ATT GTG GCC TTC TTT GAG TTC G-3′, 5′-CAT CCC AGC CTC CGT TAT CC-3′); GAPDH (F: 5′- ACC ATC TTC CAG GAG CGA GA -3′, 5′- GGT TCA CGC CCA TCA CAA AC -3′). Each individual sample was run in triplicate wells. PCR amplification cycles were performed using the iQTM5 Multicolor Real-Time PCR Detection System (Bio-Rad) and SYBR Premix Ex Taq II kit (Invitrogen). The reactions were initially denatured at 95 °C for 3 min followed by 40 cycles of 95 °C for 10 s, 56 °C for 20 s, 72 °C for 20 s. The change in transcript abundance of all tested genes was calculated using the 2-ΔΔCt method. All messenger RNA (mRNA) levels were normalized to GAPDH.
Western Blotting
Cells were lysed in lysis buffer (Beyotime) supplemented with 1-mM PMSF. The protein concentration was determined using the BCA protein assay (Tiangen). Twenty micrograms of protein in each sample were separated by 12 % SDS-PAGE and electro-transferred to PVDF membranes (Millipore) for immunoblotting analysis. The following primary antibodies were used: anti-AMPK α2 (1:300, ab3760, Abcam), anti-CREB (1:300, ab32515, Abcam), anti-CREB phosphor S133 (1:300, ab32096, Abcam), anti-SIRT1 (1:300, ab32441, Abcam), anti-BDNF (1:200, ab6201, Abcam), anti-Bcl-2 (1:400, ab117115, Abcam), and anti-β-actin (1:800, Santa Cruz), which was used as the internal reference. After incubation with the appropriate HRP-conjugate secondary antibody, proteins were detected using a ChemiDoc XRS imaging system and analysis software Quantity One (Bio-Rad).
Fasudil Injection
Thirty-two normal rabbits (no-injury control group) and 96 model rabbits were enrolled in the injection experiment. The model rabbits were divided into three groups on average: injury fasudil injection group, injected with fasudil at the dosage of 6 mg/kg; injury-control group, injected with equivalent saline; injury-dexamethasone injection group (injury-DXM), injected with DXM at the dosage of 1 mg/kg. The fasudil was initially injected 6 days before the retinal injury day and then injected into the rabbits once every 3 days.
Immunohistochemical Staining
Following deparaffinization of the sections, immunohistochemical staining was performed with streptavidin-biotinylated peroxidase to detect the expression of Rock, RhoA, Caspase-3, and Bcl-2. A rabbit polyclonal antibody against rat Rock, RhoA, Caspase-3, and Bcl-2 (diluted 1:100 to 1:500, Cell Signaling Tech) was used as a primary antibody.
Terminal Deoxynucleotidyl Transferase dUTP Nickend Labeling (TUNEL) Assay
The TUNEL assay was performed to detect internucleosomal DNA fragmentation. The staining was performed as described elsewhere (Ohno et al. 2012). The number (/0.2-mm length of each area) of the TUNEL-positive cells were counted by two authors in a blinded fashion. A confocal microscope was used for the measurements. The apoptotic index was expressed as the percentage of TUNEL-positive cells out of the total counted cells.
Statistical Analysis
All data were obtained from at least three independent experiments. Values are expressed as mean ± SEM. Statistics were calculated using SPSS 19.0. Multiple comparisons were assessed by one-way ANOVA followed by Dunnett’s tests. Differences between groups were considered statistically significant if P < 0.05.
Results
Rho/Rock Signaling was Activated in the Injured Optic Nerve in Rabbits
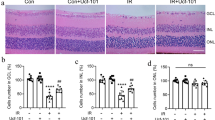
To explore the role of Rho/Rock in the traumatic optic neuropathy, model rabbits with traumatic optic neuropathy were established. Their right retinal nerve was injured and the left side was regarded as the control. At time points of day 3, day 7, day 14, and day 21 post-trauma, the retinal ganglions were sampled and detected with H&E staining and TEM approaches. The H&E staining for no-injury retinal ganglions showed that retinal morphology and RGC distribution were normal (100×), the cells in the core layer distributed uniformly (400×), and there were only a few inflammatory cells were observed (0 d of Fig. 1a). With the extension of injury time, RGCs gradually reduced, and the distribution of layers and the core layer cells gradually became disorder (100×). Under higher magnification, we could observe that the number of the core layer cells gradually lacked accompanied with the increase of inflammatory cell infiltration (3 d to 21 d of Fig. 1a). In particular, core layer cells sharply reduced, and inflammatory cell infiltration robustly increased at day 14 and day 21 (14 d and 21 d of Fig. 1a). TEM assay depicted that with the extension of injury time, myelin distribution was gradually disorder, the gap between the membrane and the myelin sheath was gradually widened, the nerve fibers gradually decreased, and the bubble-like change of mitochondria was gradually growing (Fig. 1b). At day 21, the axons were severely separated, and the structures of mitochondria, microtubules, and actin filaments were severely damaged in the axoplasma (21 d of Fig. 1b). Subsequently, the expression levels of RhoA, Rock1, and Rock2 were detected with Western blotting. The results showed that expression of them did not change in the control side but was gradually increased in the injured side (Fig. 1c). These data above suggested that RhoA/Rock signaling was activated during the retinal nerve injury and was likely to play a key role in traumatic optic neuropathy.
RhoA/Rock was being upregulated during traumatic retinal nerve injury of rabbits. a H&E staining for the retinal nerve from traumatic retinal nerve injury of rabbits. The amplification of upper panel is 40× and that of the lower is 400×; the length of the bars is respectively 0.8 and 2 cm. The retinas were removed from non-traumatic and retina-traumatic side at day 3, day 7, day 14, and day 21, respectively. The retinas were paraffin embedded and stained with H&E approach. b TEM detection for the structure of RGC myelin sheath (10000×). The bar represents 2 cm. c RhoA/Rock was being increased during traumatic retinal nerve injury. Total protein of the retina was extracted from non-traumatic and retina-traumatic side at day 3, day 7, day 14, and day 21, respectively. The protein levels of RhoA, Rock1, and Rock2 were detected with Western blotting
Fasudil Treatment Greatly Reduced Apoptosis of Rabbit RGCs Under Hypoxia-Hypoglycemia Condition In Vitro
To verify the role of RhoA/Rock signaling in retinal nerve injury in vitro, primary RGCs were isolated, HO-HG culture condition was used to simulate the environment surrounding RGCs in vivo after retinal nerve injury, and RhoA/Rock inhibitor fasudil was applied to treat the cells. MTT analysis showed that fasudil treatment at the dosage of 25, 50, 75, and 100 μM had a significant effect on suppressing apoptosis of RGCs, among which the effect of 75-μM fasudil treatment had a most significant effect (Fig. 2a). Therefore, 75 μM was the dosage used in the following experiment in vitro. Annexin V/PI dual staining combined with flow cytometry analysis for cell apoptosis showed that fasudil treatment reduced the apoptosis of the RGCs by nearly 40 % to the HO-HG control (Fig. 2b). Simultaneously, expression of key genes in regulation of RGC apoptosis was detected with real-time qPCR and Western blotting. The results displayed that as a specific inhibitor of RhoA/Rock, fasudil treatment significantly reduced the expression of RhoA, Rock1, and Rock2 (Fig. 3a–c, i) but did not change the expression of their upstream genes MAG and NgR compared to the HO-HG control (Fig. 3d, e, i). As a typical apoptosis inducer, caspase-3 expression was also largely reduced by fasudil treatment, while apoptosis suppressors BDNF and Bcl-2 were significantly increased (Fig. 3f–i). These data indicated that RhoA/Rock had an important impact on RGC apoptosis, and its inhibitor fasudil could alleviate RGC injury in vitro.
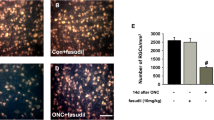
Fasudil treatment reduced HO-HG-induced apoptosis of primary rabbit RGCs. Different dosages of fasudil ranging from 0 to 100 μM were used to treat primary rabbit RGCs under HO-HG condition. After incubation for 48 h, the cell viability was detected with MTT method and Annexin V/PI dual staining combined with flow cytometry. a MTT analysis for viability of primary rabbit RGCs treated or not treated with fasudil. b Annexin V/PI dual staining combined with flow cytometry assay for the apoptosis of primary RGCs treated or not treated with 75 μM fasudil. c Fsdl treatment reduced the proportion of apoptotic cells after injury (statistical analysis for panel b). # P < 0.05 vs HO-HG-control,*P < 0.05 vs both normal-control and HO-HG-control, **P < 0.01 vs both normal-control and HO-HG-control
Fasudil treatment reduced expression of RhoA/Rock1/2 and apoptotic genes in primary rabbit HO-HG RGCs. The primary rabbit RGCs were treated or not treated with 75 μM fasudil under HO-HG or normal condition. After incubation for 48 h, total protein of the retina was extracted and the mRNA levels of the following genes were detected with real-time qPCR: RhoA, Rock1, Rock2, MAG, NgR, Caspase-3, BDNF, and Bcl-2 (a–h, respectively). The protein levels of a–h were detected with Western blotting (i). # P < 0.05 vs HO-HG-control, *P < 0.05 vs both normal-control and HO-HG-control
Fasudil Injection Alleviated the Traumatic Retinal Nerve Injury In Vivo
Finally, to investigate the effect of fasudil on the alleviation of traumatic retinal nerve injury in vivo, fasudil was injected into the rabbit ear vein at the dosage of 6 mg/kg. Immunohistochemical staining assay was used to detect the expression of Rock, RhoA, Caspase-3, and Bcl-2 after injection. The results showed that, consistent with the results in vitro, expression of Rock, RhoA, and Caspase-3 were reduced by the fasudil injection compared with the injured rabbits with no injection (Fig. 4a–c), while Bcl-2 expression was markedly increased by the injection (Fig. 4d). TUNEL assay for apoptosis of RGCs after injury for 14 days in vivo also displayed that the fasudil injection distinctly alleviated the apoptosis of RGCs (Fig. 4e). These results indicated fasudil could alleviate the traumatic retinal nerve injury in vivo.
Fasudil injection alleviated trauma-induced apoptosis of rabbits RGCs in vivo. Normal or traumatic model rabbits were ear vein injected with 6 mg/kg fasudil and 1 mg/kg DXM or not injected. At day 14, their retinal never fibers were removed, paraffin embedded, and sectioned. a–d Immunohistochemical staining for Rock, RhoA, Caspase-3, and Bcl-2 in the retinal nerve fibers. e TUNEL assay for apoptosis of RGCs in the retinal never fibers and the apoptotic indexes were analyzed. # P < 0.05 vs injury-control, *P < 0.05 vs both no-injury-control and injury-control
Discussion
As for mammalian optic nerve injury, there are several typical time points when distinct physiological and pathological changes occur in the organisms: at 72 h, the retina displays hemorrhage, vacuole-like changes and the peak of capillary expansion; at day 7, optic nerve fiber and interstitial edema, axonal infarction and thrombosis emerge apparently; at day 14, a most optimal time point to observe the arrangement and structure of nerve fibers, the edema subsided and the glial cells robustly proliferate; at day 21, nerve fibers adhere and the retina begins to repair (Song et al. 2003). In this study, we first examined the histological, cellular, and subcellular changes at these four time points in the retinas of the model rabbits. The results showed a series of pathological changes during the injury accompanied with an increasing expression pattern of RhoA/Rock.
RhoA/Rock is a well-described signaling cascade transmitting inhibitory cues to the axon growth in CNS, which could be activated by the trimeric NgR/p75/Lingo1 receptor complex (Trifunovski et al. 2004). Accumulating evidence indicates that Rho/Rock plays an important role in a variety of cell functions, regulating and controlling the proliferation and differentiation of cells, movement and migration, and generation and death. Abnormal Rock activation was believed to have an important effect on adult mammalian CNS regeneration suppression following lesion, for its role in the integration of inhibitory signals for axon growth coming from several inhibitory receptors (Koch et al. 2014a). However, its role in the regulation of cell survival in CNS regeneration gradually aroused the attention of the researchers. A most recent study revealed that knockdown of RhoA promoted neurite outgrowth of RGCs grown on the inhibitory substrate chondroitin sulfate proteoglycan in vitro (Koch et al. 2014b). Rock inhibitors Y-39983 and Y-27632 were also proven to have a positive effect on axonal regeneration (Yang et al. 2013; Ichikawa et al. 2008; Tokushige et al. 2011; Hirata et al. 2008). In this study, we established a rabbit model with traumatic retinal nerve injury. Rock inhibitor fasudil was used to treat primary RGCs under HO-HG conditions and injected into the model. Our date showed that fasudil treatment blocked RhoA/Rock pathway and significantly reduced the apoptosis of RGCs and relieved retinal nerve injury.
It has been reported that NgR-MAG/NogoA was a main axis that suppressed axon regeneration upstream RhoA/Rock (Pernet and Schwab 2012). Response to local hypoxia-ischemia stress, NgR complex transducted the signals and then MAG/Nogo-A was activated, which led to the activation of Rock and RhoA. We detected a marked increase in the levels of MAG and NgR in the cultured primary RGCs under HO-HG condition in vitro. Moreover, when treated with fasudil, the levels of Rock and RhoA in the HO-HG RGCs were significantly reduced, while their upstream MAG and NgR expression was not changed.
For the treatment of optic nerve injury, researchers have carried on interventions with different drugs to animal models with optic nerve injury. The mechanisms of the interventions were generally to reduce the edema and inflammatory reaction, improve local blood circulation, prevent axonal injury, and promote injured axonal regeneration. Fasudil is a typical Rock inhibitor, which had an effect on reducing inflammatory cytokines, inhibiting inflammatory cell adhesion and invasion (Yamashita et al. 2007). and improving the microcirculation of increase local blood flow (Shin et al. 2007). Whether fasudil treatment could alleviate the secondary degeneration of RGCs was not clear. Our results here indicated that fasudil treatment did have a suppressive effect on apoptosis of rabbit primary RGCs in vitro. Moreover, in the rabbits with traumatic optic nerve injury, fasudil injection reduced Rock, RhoA and Caspase-3 expression and apoptosis of RGCs. These data indicated that fasudil could alleviate the secondary degeneration of RGCs.
In conclusion, we established a rabbit model of traumatic retinal nerve injury. Fasudil treatment significant reduced the apoptosis of RGCs and relieved retinal nerve injury in vitro and in vivo.
References
Andalib MN, Lee JS, Ha L, Dzenis Y, Lim JY (2013) The role of RhoA kinase (ROCK) in cell alignment on nanofibers. Acta Biomater 9:7737–7745
Benowitz LI, Andereggen L, Trakhtenberg EF, Yin Y (2015) Inflammation and optic nerve regeneration. Wiley-Blackwell, Hoboken, pp 189–204, Neuroinflammation
Cho KS, Yang L, Lu B, Ma HF, Huang X, Pekny M, Chen DF (2005) Re-establishing the regenerative potential of central nervous system axons in postnatal mice. J Cell Sci 118:863–872
Fernandes KA, Harder JM, John SW, Shrager P, Libby RT (2014) DLK-dependent signaling is important for somal but not axonal degeneration of retinal ganglion cells following axonal injury. Neurobiol Dis 69:108–116
Fujita Y, Yamashita T (2014) Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci 8:338
Giacci MK et al (2014) Differential effects of 670 and 830 nm red near infrared irradiation therapy: a comparative study of optic nerve injury, retinal degeneration, traumatic brain and spinal cord injury. PLoS One 9:e104565
Gu H, Yu SP, Gutekunst CA, Gross RE, Wei L (2013) Inhibition of the Rho signaling pathway improves neurite outgrowth and neuronal differentiation of mouse neural stem cells. Int J Physiol Pathophysiol Pharmacol 5:11–20
Hannila SS et al (2013) Secretory leukocyte protease inhibitor reverses inhibition by CNS myelin, promotes regeneration in the optic nerve, and suppresses expression of the transforming growth factor-β signaling protein Smad2. J Neurosci 33:5138–5151
Hasegawa T, Ohno K, Sano M, Omura T, Omura K, Nagano A, Sato K (2005) The differential expression patterns of messenger RNAs encoding Nogo-A and Nogo-receptor in the rat central nervous system. Mol Brain Res 133:119–130
Hirata A, Inatani M, Inomata Y, Yonemura N, Kawaji T, Honjo M, Tanihara H (2008) Y-27632, a Rho-associated protein kinase inhibitor, attenuates neuronal cell death after transient retinal ischemia. Graefes Arch Clin Exp Ophthalmol 246:51–59
Ichikawa M, Yoshida J, Saito K, Sagawa H, Tokita Y, Watanabe M (2008) Differential effects of two ROCK inhibitors, Fasudil and Y-27632, on optic nerve regeneration in adult cats. Brain Res 1201:23–33
Koch J, Tönges L, Barski E, Michel U, Bähr M, Lingor P (2014a) ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS. Cell Death Dis 5:e1225
Koch JC, Tönges L, Michel U, Bähr M, Lingor P (2014b) Viral vector-mediated downregulation of RhoA increases survival and axonal regeneration of retinal ganglion cells. Front Cell Neurosci 8:273
Li, H.Y., Chan, H.H., Chu, P.H., Chang, R.C. and So, K.F. (2015). Secondary degeneration after partial optic nerve injury and possible neuroprotective effects of Lycium barbarum (Wolfberry). In Lycium barbarum and human health, Springer, pp. 135–151
Ohno Y, Nakanishi T, Umigai N, Tsuruma K, Shimazawa M, Hara H (2012) Oral administration of crocetin prevents inner retinal damage induced by N-methyl-d-aspartate in mice. Eur J Pharmacol 690:84–89
Pernet V, Schwab ME (2012) The role of Nogo-A in axonal plasticity, regrowth and repair. Cell Tissue Res 349:97–104
Shin HK et al (2007) Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow Metab 27:998–1009
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20:1714–1722
Steketee MB et al (2014) Regulation of intrinsic axon growth ability at retinal ganglion cell growth cones. Invest Ophthalmol Vis Sci 55:4369–4377
Stone, J. (2013). Parallel processing in the visual system: the classification of retinal ganglion cells and its impact on the neurobiology of vision, In Springer Science & Business Media, Springer, 3–5
Strutt DI, Weber U, Mlodzik M (1997) The role of RhoA in tissue polarity and Frizzled signalling. Nature 387:292–295
Takeda N, Kondo M, Ito S, Ito Y, Shimokata K, Kume H (2006) Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. Am J Respir Cell Mol Biol 35:722–729
Tan H, Zhong Y, Shen X, Cheng Y, Jiao Q, Deng L (2012) Erythropoietin promotes axonal regeneration after optic nerve crush in vivo by inhibition of RhoA/ROCK signaling pathway. Neuropharmacology 63:1182–1190
Tokushige H, Waki M, Takayama Y, Tanihara H (2011) Effects of Y-39983, a selective Rho-associated protein kinase inhibitor, on blood flow in optic nerve head in rabbits and axonal regeneration of retinal ganglion cells in rats. Curr Eye Res 36:964–970
Trifunovski A, Josephson A, Ringman A, Brené S, Spenger C, Olson L (2004) Neuronal activity-induced regulation of Lingo-1. Neuroreport 15:2397–2400
Vajda F, Jordi N, Dalkara D, Joly S, Christ F, Tews B, Schwab M, Pernet V (2014) Cell type-specific Nogo-A gene ablation promotes axonal regeneration in the injured adult optic nerve. Cell Death Differ 22:323–335
Vecino E, Heller JP, Veiga-Crespo P, Martin KR, Fawcett JW (2014) Influence of extracellular matrix components on the expression of integrins and regeneration of adult retinal ganglion cells. PLoS One 10:e0125250
Wang YH, Wang DW, Wu N, Wang Y, Yin ZQ (2012) Alpha-crystallin promotes rat axonal regeneration through regulation of RhoA/Rock/Cofilin/MLC signaling pathways. J Mol Neurosci 46:138–144
Wang CH, Wu CC, Hsu SH, Liou JY, Li YW, Wu KK, Lai YK, Yen BL (2013) The role of RhoA kinase inhibition in human placenta-derived multipotent cells on neural phenotype and cell survival. Biomaterials 34:3223–3230
Wilhelm H (2009) Traumatic optic neuropathy. Laryngorhinootologie 88:194–203, quiz 204–7
Yamashita K, Kotani Y, Nakajima Y, Shimazawa M, Yoshimura SI, Nakashima S, Iwama T, Hara H (2007) Fasudil, a Rho kinase (ROCK) inhibitor, protects against ischemic neuronal damage in vitro and in vivo by acting directly on neurons. Brain Res 1154:215–224
Yang Z, Wang J, Liu X, Cheng Y, Deng L, Zhong Y (2013) Y-39983 downregulates RhoA/Rho-associated kinase expression during its promotion of axonal regeneration. Oncol Rep 29:1140–1146
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.81160153) and Xinjiang Medical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures described herein were approved by the Ethics Committee of the Second Affiliated Hospital of Xinjiang Medical University. The rabbits were monitored in their home cage in a stress-free environment where they were given food and water, ad libitum. After experimentation, the rabbits were rapidly euthanized to minimize their pains.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Yu, J., Luan, X., Lan, S. et al. Fasudil, a Rho-Associated Protein Kinase Inhibitor, Attenuates Traumatic Retinal Nerve Injury in Rabbits. J Mol Neurosci 58, 74–82 (2016). https://doi.org/10.1007/s12031-015-0691-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0691-6