Abstract
Spinal and Bulbar Muscular Atrophy (SBMA), also known as Kennedy's disease, is a rare adult-onset lower motor neuron disorder with a classic X-linked inheritance pattern. It is caused by the abnormal expansion of the CAG-repeat tract in the androgen receptor gene. Despite important progress in the understanding of the molecular pathogenesis and the availability of a broad set of model organisms, successful translation of these insights into clinical interventions remains elusive. Here we review the available information on clinical trials in SBMA and discuss the challenges and pitfalls that impede therapy development. Two important factors are the variability of the complex neuro-endocrinological phenotype and the comparatively low incidence of the disease that renders recruitment for clinical trials demanding. We propose that these challenges can be and need to be overcome by fostering closer collaborations between clinical research centers, the patient communities and the industry and non-industry sponsors of clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
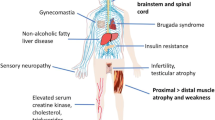
Spinal and bulbar muscular atrophy (SBMA), also known as Kennedy’s disease, is a rare adult-onset lower motor neuron disorder with a classic X-linked inheritance pattern. It is caused by the abnormal expansion of a CAG repeat tract in the androgen receptor gene (La Spada et al. 1991). The symptoms and disease course of SBMA are discussed elsewhere in this issue. Briefly, the condition is characterized clinically by adult-onset, slowly progressive weakness, atrophy, and fasciculations of the bulbar and limb skeletal musculature (Fratta et al. 2014). Dorsal root ganglia are also affected resulting in mild sensory involvement. Of note, the full clinical picture only develops in men, who typically also show signs of androgen insensitivity, namely gynecomastia and reduced fertility. Women are thought to be generally unaffected but may show electrophysiological or laboratory test abnormalities and report cramps (Fischbeck 2012). Thus, clinically SBMA is a motoneuron disease, and genetically, it belongs to the group of repeat expansion disorders, more specifically to the polyglutamine disorders. The common over-arching goal of all SBMA research is to enable the rational development of therapies against this relentless and potentially severely disabling disease. In addition, as SBMA shares salient features with other neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS) and Huntington’s disease (HD), there is reasonable hope that inroads into finding a cure for this comparatively rare disease can be leveraged into progress against some of the more common neurodegenerative diseases. Conversely, insights from other diseases can and should inform the strategies for finding a cure for SBMA.
Clinical Trials
The gold standard for evaluating therapeutic efficacy is a double-blind randomized clinical trial. According to the definition of the National Institutes of Health, a clinical trial is a prospective biomedical or behavioral research study that is designed to answer specific questions about biomedical or behavioral interventions (http://grants.nih.gov/grants/policy/hs/glossary.htm). In keeping with this definition, clinical trials usually proceed through the following phases:
-
Phase I. Study tests a new biomedical intervention in a small group of people (e.g., 20–80) for the first time to evaluate safety (e.g., determine a safe dosage range and identify side effects).
-
Phase II. Study where the biomedical or behavioral intervention is conducted in a larger group of people (up to several hundred) to determine efficacy and further evaluate safety.
-
Phase III. Study to determine efficacy of the biomedical or behavioral intervention in large groups of people (from several hundred to several thousand) by comparing the intervention to other standard or experimental interventions as well as to monitor adverse effects and to collect information that will allow the interventions to be used safely.
-
Phase IV. Studies conducted after the intervention has been marketed. These studies are designed to monitor the effectiveness of the approved intervention in the general population and to collect information about any adverse effects associated with widespread use.
The transitions through the different phases (until phase III, that is) require increasing resources in terms of infrastructure and participants. Thus, as discussed by Borowsky and Sampaio in the context of HD (Borowsky and Sampaio 2014), the decision to move up from one step to the next needs to be well justified. The outcome of a trial can be either positive (the null hypothesis is rejected), negative (the null hypothesis is confirmed), or inconclusive. From a scientific standpoint, the latter is clearly the least desirable outcome, as no new insights are gained. A “negative” trial either fails to show a therapeutic benefit or sometimes even demonstrates deleterious effects of an intervention. While from a clinician or patient standpoint this is obviously unsatisfactory, such outcomes should not be considered failures because they nevertheless represent important advances toward future successes.
The goal of a clinical trial is always to answer a prespecified hypothesis about the efficacy of an intervention. However, adequately designed and well-executed trials will allow one to obtain highly valuable clinical information with relevance beyond the actual clinical trial. Also, it should be noted that the infrastructure that is put in place to conduct a clinical trial can be perpetuated to establish a patient registry that then, in turn, can facilitate future clinical trials. Under the right circumstances, this can result in a virtuous cycle leading to a continuously enhanced understanding of the disease. This, in turn, would ideally contribute to steadily improved clinical trials, which, in turn, will energize the patient community and help to recruit patient cohorts available for further clinical trials (Fig. 1). This interaction offers an important opportunity for synergies between industry sponsors and other stakeholders such as patient organizations and government funding agencies. It also mandates that all clinical trials, regardless of their outcome, are published and the results are closely scrutinized for lessons to be learned beyond the mere testing of the main hypothesis.
Schematic of a possible virtuous cycle that can be developed from the synergistic interactions of clinical trials, the patient community, and study centers. Ideally, this will lead to continually improved clinical trials that will energize the patient community and lead to larger and better characterized patient cohorts
The molecular basis of SBMA is comparatively well understood. This is mainly a result of early breakthroughs in the understanding of the genetic basis of the disease and the successful translation into transgenic animal models. It is now established that SBMA is caused by the abnormal expansion of an unstable CAG repeat in the coding region of the androgen receptor (AR) gene (La Spada et al. 1991) which is translated into a N-terminal polyglutamine tract in the AR protein. The normal CAG repeat length in the AR gene ranges from 9 to 34 repeats, and repeats >38 in length produce the fully penetrant disease phenotype. The identification of this novel mutation as the cause of SBMA paved the way for studies in vitro and in vivo model organisms and helped identifying a range of potential therapeutic targets (for excellent reviews, see (Banno 2012; Fischbeck 2012; Rocchi and Pennuto 2013), and this issue). Prompted by highly informative animal trials, androgen reduction was singled out as a promising therapeutic strategy and led to several clinical trials that have focused on hormonal interventions in SBMA (Katsuno et al. 2010; Fernández-Rhodes et al. 2011). Katsuno and colleagues tested the therapeutic potential leuprorelin, a gonadotropin-lowering drug that reduces testicular testosterone production (Banno et al. 2009; Katsuno et al. 2010). Fernandes-Rhodes investigated the effect of the more motoneuron-selective anti-androgen dutasteride, a blocker of the enzymatic conversion of testosterone to dihydrotestosterone (Fernández-Rhodes et al. 2011). The other trials were aimed at improving muscle function either through exercise or anabolics (clenbuterol) (Preisler et al. 2009; Querin et al. 2013). While all trials failed to demonstrate a clear therapeutic benefit for the respective intervention in SBMA, several important lessons have emerged.
Past Trials
Through a review of the literature and internet databases, most importantly clinicaltrials.gov, we have identified seven clinical trials that have been completed and published over the past two decades in SBMA. The key points of these trials are summarized in Table 1.
Leuprorelin Trials
Leuprorelin is a luteinizing hormone-releasing hormone (LHRH) agonist that suppresses the release of gonadotrophins, thus reducing the level of testosterone produced by the testes.
A first study on male transgenic SBMA mice demonstrated the efficacy of leuprorelin in the inhibition of pathogenic AR accumulation by preventing its ligand-dependent nuclear translocation, which resulted in improvement of motor function in mice (Katsuno et al. 2003).
Later, the effectiveness of leuprorelin in reducing the nuclear accumulation of mutated AR, as assessed by 1C2 antibody staining of expanded PolyQ, was confirmed in human scrotal skin cells (Banno et al. 2006). This study was based on the previous finding that nuclear AR inclusions were detectable not only in motor neurons but also in cells of the scrotal skin and other visceral organs (Li et al. 1998). Five SBMA patients received subcutaneous injections of 3.75 mg leuprorelin every 4 weeks for 6 months; scrotal skin biopsy was done at 0, 4, and 12 weeks after initial treatment, and CK and testosterone levels were measured at months 1, 2, 3, and 6. Quantitative analysis showed a significant decrease in AR accumulation both 4 and 12 weeks after the start of leuprorelin treatment and a decrease of serum testosterone and CK in 6 months. Furthermore, the mutated AR accumulation in the scrotal skin of 13 untreated SBMA patients showed a direct correlation with CAG repeat length and an inverse correlation with the ALS functional scores (ALS-FRS). These results suggested scrotal skin biopsy as a potential biomarker of SBMA and supported further studies to determine the efficacy of leuprorelin in preventing disease progression (Banno et al. 2006).
A few years later, 50 SBMA patients were recruited in a randomized, placebo-controlled trial lasting 48 weeks; then, an open-label follow-up was performed in 34 patients for additional 96 weeks (Banno et al. 2009). Patients were evaluated every 4 weeks for the first 48 weeks and every 12 weeks in the successive follow-up. Subcutaneous leuprorelin was administered at a dose of 3.75 mg every 4 weeks during the first period and then at a dose of 11.25 mg every 12 weeks. The primary outcome measure was the revised ALS functional rating scale (ALSFRS-R), and secondary outcome measures included, among others, cricopharyngeal opening duration visualized by videofluorography, the frequency of 1C2-positive cells in scrotal skin biopsies, lung function values, and laboratory tests (see table for more details). After 48 weeks, there was no significant difference in ALSFRS-R total score between the two groups, whereas this was significantly better in patients who received leuprorelin for 144 or 96 weeks than in those who received no therapy throughout the trial. These results suggested a long-term action of leuprorelin and supported the need of a longer follow up in clinical trials, because of the slow progression of the disease. Furthermore, there was a tendency for the swallowing subscores to improve in the leuprorelin group during the first phase of the study, confirmed by the 96-week follow-up data, and the cricopharyngeal opening duration was prolonged in leuprorelin group compared to placebo group, supporting the hypothesis that the drug can indeed reduce deterioration of swallowing functions. Finally, the data confirmed the previous results showing a reduced AR accumulation in cells of the scrotal epithelium in the leuprorelin group.
A larger study was next performed to assess the effects of leuprorelin on swallowing functions and disease progression (Katsuno et al. 2010). A randomized, double-blind, placebo-controlled, multicenter trial enrolled 199 SBMA patients: 100 patients received subcutaneous leuprorelin at the dose of 11.25 mg every 12 weeks, and 99 took placebo. Pharyngeal barium residue at 48 weeks was the selected primary endpoint, because of its direct association with aspiration and its frequent finding in SBMA patients. Secondary outcome measures included other temporal parameters of videofluorography, such as stage transition duration, duration of maximum laryngeal elevation and of cricopharyngeal opening. Pharyngeal barium residue decreased between baseline and week 48 in the leuprorelin group in comparison to the placebo group, suggesting that leuprorelin might improve swallowing function, but this difference was not significant. In addition, no significant difference was detected also in the other temporal parameters of videofluorography, in contrast with the previous observation that leuprorelin treatment extended the duration of cricopharyngeal opening (Banno et al. 2009). Among the other secondary outcome measures, there were significant differences in favor of the treatment group for the mean change in frequency of 1C2-positive cells in scrotal skin biopsies, in serum CK concentration, and in amyotrophic lateral sclerosis assessment questionnaire 5 (ALSAQ-5) score, suggesting that leuprorelin reduces the pathogenic AR accumulation and serum CK level in SBMA patients.
The primary endpoint of the study failed to show drug efficacy. However, the subgroup of SBMA patients with disease duration less 10 years showed that leuprorelin improved swallowing function, suggesting that disease duration might have influenced results. Selection of candidate patients is important in such trials as treatment is less likely to be effective in the late disease phases, when neurodegeneration is too severe. This highlights a common challenge of the transition from a phase II to a phase III study in rare disorders.
Dutasteride Trial
Fernández-Rhodes and colleagues evaluated the efficacy of the 5α-reductase inhibitor dutasteride in 50 SBMA patients. The rationale for this study was a more selective approach to androgen reduction compared to other hormonal therapies, based on the different tissue expression of 5α-reductase and allowing a decrease of dihydrotestosterone (DHT) toxic effects in motor neuron while saving the beneficial effect of testosterone in muscle. Indeed 5α-reductase, which catalyzes the conversion of testosterone to DHT directly in the target cells, is highly expressed in motor neurons, where DHT is the primary ligand for AR, while in the muscle, this role is accomplished by testosterone. The study was a randomized, placebo-controlled clinical trial with treatment duration of 24 months. The primary outcome measure was quantitative muscle assessment (QMA). Secondary outcome measures included the bulbar rating scale, manual muscle testing, adult myopathy assessment tool (AMAT), 2-min timed walking, self-assessed quality of life, electromyography and nerve conduction studies, and biochemical profiles. Next, when results of the leuprorelin trial were available, barium swallow and pulmonary function studies were added. Strength based on the QMA decreased by 4.5 % in the placebo group whereas it increased by 1.3 % in the dutasteride group. However, such difference in the primary endpoint was not statistically significant; also not significant were the differences in the secondary outcomes, except physical quality of life and number of falls, which showed benefit of dutasteride over placebo. Therefore, the trial failed to prove an efficacy of dutasteride in the treatment of SBMA. However, the study was underpowered because of the slow progression in muscle weakness in the placebo group with the consequent need for more time or a larger number of patients to detect changes in disease progression.
Clenbuterol Trial
Clenbuterol is a β2-adrenoceptor agonist usually used to treat asthma. Its chronic administration at high doses produces an increase in skeletal muscle mass and a concomitant decrease in body fat. Indeed, β2-adrenoceptor is the predominant subtype in skeletal muscle, and its activation has an anabolic effect, through the activation of PI3K/Akt signaling (Lynch and Ryall 2008). Therefore, Querin and colleagues performed a pilot trial to test efficacy and tolerability of clenbuterol in 20 SBMA patients (Querin et al. 2013). Treatment consisted of oral administration of 0.04 mg clenbuterol per day for 12 months. The primary outcome was the 6-min walk test (6MWT), and secondary outcomes included manual testing of muscle strength (Medical Research Council (MRC) scores), forced vital capacity (fVC), and the ALSFRS-R. The study’s findings were suggestive of decreased disease progression with significant improvement of mean 6MWT and fVC values after 12 months of treatment; no changes in the other outcome measures of this pilot trial were detected.
Exercise Trials
Preisler and colleagues evaluated the effect of aerobic training in eight SBMA patients, given the muscle anabolic action of androgen and based on previous positive results of treatment in other muscle diseases (Sveen et al. 2007; Preisler et al. 2009). Training consisted of 30-min sessions on a stationary cycle ergometer for 12 weeks with a gradual increase of frequency from two sessions in weeks 1 and 2, to three in weeks 3 and 4, and then five sessions per week in the remaining 8 weeks. Changes in maximum oxygen uptake (VO2max), maximal work capacity (Wmax), and activities of daily living (ADL) were primary endpoints. Secondary endpoints were changes in muscle morphology and citrate synthase (CS) activity at muscle biopsy, body composition evaluated by using a full-body DEXA scan, EMG, strength measurements with hand-held dynamometry, and lung function assessed by measuring peak flow, forced vital capacity, and forced expiratory volume. Wmax increased by 18 % and CS activity by 35 % after 12 weeks of treatment, but no significant changes of VO2max, ADL, or other outcome measure were detected. The authors concluded that aerobic training has no efficacy in SBMA (Preisler et al. 2009).
More recently, Shrader and colleagues tested the efficacy of functional exercises in a series of 50 SBMA patients (Shrader et al. 2015). The program included different exercises such as trunk sit back, sit-to-stand, standing squats, standing lunge, double limb heel raise, and wall push-up, and maximal capacity for each exercise was assessed by the number of repetitions in 60 s. SBMA patients were randomly assigned to either an intervention group (n = 24) with a 12-week functional exercise program or a control group (n = 26) that performed only a stretching program. The AMAT served as primary outcome measure. Secondary measures included balance and muscle strength measurement, laboratory tests, and a quality of life questionnaire (see table). The trial provided no evidence in favor of efficacy of the functional exercise program in SBMA as no significant difference between the two groups was detected in primary and secondary outcome measures. However, a post hoc subgroup analysis revealed an increase of functional AMAT subscore in individuals with low baseline function in intervention group compared to the control group, suggesting possible efficacy of the treatment on more severely affected patients. The authors underlined the need for further clinical trials considering longer duration and selecting the appropriate functional assessment as outcome measures, with higher intensity exercises and a more targeted study population.
Ongoing Trials
At the time of this writing, the clinicaltrials.gov website (www.clinicaltrials.gov) identifies two ongoing interventional trials in SBMA. One is a trial (NCT02024932) of the effect of high-intensity training in patients with SBMA. This timely topic and the rationale for this trial are discussed elsewhere in this special issue. The other trial (NCT02024932) is a double-blind placebo-controlled phase I/II (safety, tolerability, and efficacy) trial of an experimental compound BVS857 sponsored by the Swiss pharmaceutical company Novartis. Trial NCT02024932 is estimated to be completed by Feb 2016.
Lessons Learned and Future Directions
SBMA has several features that in principle should facilitate the implementation of clinical trials. First, it is a genetically defined, fully penetrant disease, and as such, diagnosis is straightforward. Second, the pathomechanisms of SBMA are intensively studied in model systems (Banno 2012; Fischbeck 2012; Rocchi and Pennuto 2013). At first glance, the outcomes of the therapeutic trials completed in SBMA so far may appear sobering. To date, none of the interventions that were tested have translated into approved therapeutic or preventive options for SBMA patients. However, the insights derived from these trials are very useful, and they should inform the next steps forward.
Indisputably, the understanding of the molecular pathogenesis and thus the mechanistic basis for therapeutic trials in SBMA is remarkably strong (Banno 2012; Fischbeck 2012; Rocchi and Pennuto 2013). The downstream effects of the CAG-repeat expansion in the AR represent relatively clear-cut therapeutic targets. Excellent in vivo and in vitro models are available and have contributed to the prioritization among these targets. For instance, findings in mouse models of SBMA have shown that muscle-specific overexpression of the anabolic hormone insulin-like growth factor 1 (IGF-1) is therapeutic with regards to both functional outcomes and survival (Palazzolo et al. 2009; Papanikolaou and Ellerby 2009). Also, gene silencing methods that are approaching clinical testing in HD and familial ALS are also promising in preclinical studies of SBMA (Sahashi et al. 2015).
The translation of mechanistic insights into clinical trials remains unsatisfactory, and clearly, a reasonable understanding of disease mechanisms is not enough to guarantee successful clinical trials. While the clinicaltrials.gov website lists 9 interventional trials (including 2 open or not yet recruiting) for SBMA, the same database catalogs 866 such trails (132 open or not yet recruiting) for HD and 626 (175) for ALS. This discrepancy may reflect specific challenges that deserve special attention in order to be overcome.
With an estimated prevalence of 1–2 per 100,000, SBMA is clearly a rare disorder, even when compared to HD, which has a prevalence of above 10 per 100,000 or ALS, which has a prevalence of roughly 5 per 100,000 worldwide (Katsuno et al. 2012; Bates et al. 2015; Chio et al. 2013). Already setting up cohorts that are adequately sized for full-fledged clinical trials, i.e., comprising up to several hundreds of patients, may seem a daunting task. Yet, reinforced efforts in this direction are clearly justified. The above prevalence suggests that there are about 5000 SBMA patients living among in the 500 million inhabitants of the European Union. The only way to fully realize this untapped potential is to connect different SBMA clinics and to thus create a network of international collaborations. A powerful example of this approach is the creation of the European Huntington Disease Network (EHDN). It was established in 2004 and has since enrolled more than 10,000 of the estimated 35,000 HD patients in Europe in its patient registry (www.euro-hd.net). It remains to be determined whether the lower prevalence of SBMA will make it easier or more difficult to achieve such a remarkable capture rate.
Moreover, the phenotype and disease course of SBMA can be quite variable (Atsuta 2006). The combination of neuromuscular and endocrinological symptoms gives rise to a complex phenotype, and patient-reported onset of symptoms can vary by several decades. The confounding effect of the clinical heterogeneity is accentuated by the lack of sensitive and standardized outcome measures. Health-related or biomedical outcomes, according to the NIH glossary definition (http://grants.nih.gov/grants/policy/hs/glossary.htm), are “prespecified goal(s) or condition(s) that reflect the effect of one or more interventions on human subjects’ biomedical or behavioral status or quality of life.” In the case of SBMA, these outcomes can be either dry biomarkers, e.g., functional scales, performance tasks, electrophysiological, test or imaging read-outs, or they are so-called wet biomarkers such as serum or cerebrospinal fluid parameters (Pennuto et al. 2015). In part because of its low incidence and prevalence, most of these outcome measures have not been specifically validated in SBMA. It is therefore unfortunate, but not surprising, that no two of the completed SBMA trials have relied on the same primary outcomes. This lack of harmonization significantly compromises the comparability of study results. Again, close international collaborations ideally under the umbrella of a European coordination organization would offer a powerful way to overcome these limitations.
Efforts in these directions are being carried out. Studies on outcome measures led to the development of a more specific and hopefully responsive scale, such as the SBMA functional rating scale, derived from the ALS-FRS (Hashizume et al. 2015). Quantitative muscle MRI is a promising responsive paraclinical measures in neuromuscular disorders (Willis et al. 2013) and has been incorporated into the ongoing BVS857 study. A European NeuroMuscular Workshop on SBMA took place in March 2015, and an agreement was reached among several centers to use a common protocol to follow patients toward the goal of building an International SBMA Registry (Pennuto et al. 2015).
In summary, while the situation for preclinical therapy development in SBMA is quite encouraging with a clear concept of the pathogenesis and a powerful lineup of model organisms, the relative lack of clinical tools hampers the successful translation of therapeutic strategies. Experience from related diseases, e.g., HD, shows that multi-center networks can overcome many of these limitations.
References
Atsuta N (2006) Natural history of spinal and bulbar muscular atrophy (SBMA): a study of 223 Japanese patients. Brain 129:1446–1455. doi:10.1093/brain/awl096
Banno H (2012) Molecular pathophysiology and disease-modifying therapies for spinal and bulbar muscular atrophy. Arch Neurol 69:436. doi:10.1001/archneurol.2011.2308
Banno H, Adachi H, Katsuno M et al (2006) Mutant androgen receptor accumulation in spinal and bulbar muscular atrophy scrotal skin: a pathogenic marker. Ann Neurol 59:520–526. doi:10.1002/ana.20735
Banno H, Katsuno M, Suzuki K et al (2009) Phase 2 trial of leuprorelin in patients with spinal and bulbar muscular atrophy. Ann Neurol 65:140–150. doi:10.1002/ana.21540
Bates GP, Dorsey R, Gusella JF et al (2015) Huntington disease. Nat Rev Dis Primers 15005–21. doi:10.1038/nrdp.2015.5
Borowsky B, Sampaio C (2014) Experimental therapeutics: moving forward in clinical trials. In: Bates GP, Tabrizi SJ, Jones L (eds) Huntington's disease, 4th edn. Oxford University Press, Oxford, p 462–502
Chio A, Logroscino G, Traynor BJ et al (2013) Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology 41:118–130. doi:10.1159/000351153
Fernández-Rhodes LE BSNADK, MD MJW et al (2011) Efficacy and safety of dutasteride in patients with spinal and bulbar muscular atrophy: a randomised placebo-controlled trial. Lancet Neurol 10:140–147. doi:10.1016/S1474-4422(10)70321-5
Fischbeck KH (2012) Developing treatment for spinal and bulbar muscular atrophy. Prog Neurobiol 99:257–261. doi:10.1016/j.pneurobio.2012.05.012
Fratta P, Nirmalananthan N, Masset L et al (2014) Correlation of clinical and molecular features in spinal bulbar muscular atrophy. Neurology 82:2077–2084. doi:10.1212/WNL.0000000000000507
Hashizume A, Katsuno M, Suzuki K et al (2015) A functional scale for spinal and bulbar muscular atrophy: cross-sectional and longitudinal study. Neuromuscul Disord 25:554–562. doi:10.1016/j.nmd.2015.03.008
Katsuno M, Adachi H, Doyu M et al (2003) Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat Med 9:768–773. doi:10.1038/nm878
Katsuno M, Banno H, Suzuki K et al (2010) Efficacy and safety of leuprorelin in patients with spinal and bulbar muscular atrophy (JASMITT study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 9:875–884. doi:10.1016/S1474-4422(10)70182-4
Katsuno M, Tanaka F, Adachi H et al (2012) Pathogenesis and therapy of spinal and bulbar muscular atrophy (SBMA). Prog Neurobiol 99:246–256. doi:10.1016/j.pneurobio.2012.05.007
La Spada AR, Wilson EM, Lubahn DB et al (1991) Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 352:77–79. doi:10.1038/352077a0
Li M, Nakagomi Y, Kobayashi Y et al (1998) Nonneural nuclear inclusions of androgen receptor protein in spinal and bulbar muscular atrophy. Am J Pathol 153:695–701. doi:10.1016/S0002-9440(10)65612-X
Lynch GS, Ryall JG (2008) Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 88:729–767. doi:10.1152/physrev.00028.2007
Palazzolo I, Stack C, Kong L et al (2009) Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron 63:316–328. doi:10.1016/j.neuron.2009.07.019
Papanikolaou T, Ellerby LM (2009) IGF-1: elixir for motor neuron diseases. Neuron 63:277–278. doi:10.1016/j.neuron.2009.07.024
Pennuto M, Greensmith L, Pradat P-F et al (2015) 210th ENMC International Workshop: research and clinical management of patients with spinal and bulbar muscular atrophy, 27–29 March, 2015, Naarden, The Netherlands. Neuromuscul Disord 25:802–812. doi:10.1016/j.nmd.2015.06.462
Preisler N, Andersen G, Thøgersen F et al (2009) Effect of aerobic training in patients with spinal and bulbar muscular atrophy (Kennedy disease). Neurology 72:317–323. doi:10.1212/01.wnl.0000341274.61236.02
Querin G, D’Ascenzo C, Peterle E et al (2013) Pilot trial of clenbuterol in spinal and bulbar muscular atrophy. Neurology 80:2095–2098. doi:10.1212/WNL.0b013e318295d766
Rocchi A, Pennuto M (2013) New routes to therapy for spinal and bulbar muscular atrophy. J Mol Neurosci. doi:10.1007/s12031-013-9978-7
Sahashi K, Katsuno M, Hung G et al (2015) Silencing neuronal mutant androgen receptor in a mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet 24:5985–5994. doi:10.1093/hmg/ddv300
Shrader JA, Kats I, Kokkinis A et al (2015) A randomized controlled trial of exercise in spinal and bulbar muscular atrophy. Ann Clin Transl Neurol 2:739–747. doi:10.1002/acn3.208
Sveen M-L, Jeppesen TD, Hauerslev S et al (2007) Endurance training: an effective and safe treatment for patients with LGMD2I. Neurology 68:59–61. doi:10.1212/01.wnl.0000250358.32199.24
Willis TA, Hollingsworth KG, Coombs A et al (2013) Quantitative muscle MRI as an assessment tool for monitoring disease progression in LGMD2I: a multicentre longitudinal study. PLoS One 8:e70993–7. doi:10.1371/journal.pone.0070993
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Weydt, P., Sagnelli, A., Rosenbohm, A. et al. Clinical Trials in Spinal and Bulbar Muscular Atrophy—Past, Present, and Future. J Mol Neurosci 58, 379–387 (2016). https://doi.org/10.1007/s12031-015-0682-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0682-7