Abstract
Microglia cells have been reported to mediate hypoxia-induced inflammation through the production of proinflammatory cytokines, including interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-6. Given the fact that the activation of the type 2 cannabinoid receptor (CB2R) provides antioxidative and anti-inflammatory results, it is suspected that its selective agonist, trans-caryophyllene (TC), may have protective effects against hypoxia-induced neuroinflammatory responses. In this study, TC was found to significantly inhibit hypoxia-induced cytotoxicity as well as the release of proinflammatory cytokines, including IL-1β, TNF-α, and IL-6, through activation of BV2 microglia following hypoxic exposure (1 % O2, 24 h). Furthermore, TC significantly inhibited hypoxia-induced generation of reactive oxygen species (ROS) in mitochondria as well as the activation of nuclear factor kappa B (NF-κB) in microglia. Importantly, TC’s effects on inhibiting the activation of NF-κB and the secretion of inflammatory cytokines can be abolished by muting the CB2R using small RNA interference. These observations indicate that TC suppresses the hypoxia-induced neuroinflammatory response through inhibition of NF-κB activation in microglia. Therefore, TC may be beneficial in preventing hypoxia-induced neuroinflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is one of the most common brain diseases and is linked with very high rates of morbidity and mortality. Because of its high demand for oxygen supply, the brain is extremely susceptible to hypoxia-induced damage (Shi et al. 2013; Carlson et al. 2008). Since hypoxia activates microglial cells, which are the resident macrophages of the central nervous system (CNS). It is generally accepted that inflammation plays a critical role in the disease progression of ischemic stroke and hypoxia (Chamorro and Hallenbeck 2006). Activated microglial cells provoke excessive secretion of proinflammatory cytokines, including interleukin-1 beta (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α) (Sivakumar et al. 2011). It is also known that proinflammatory cytokines secreted by microglia play a critical role in neuronal dysfunction and neuronal death in ischemic stroke (Yang et al. 2010) and Alzheimer’s disease (Heneka et al. 2010). Previous studies have demonstrated that enhanced production of reactive oxygen species (ROS), resulting from increased mitochondrial DNA damage in microglia, is involved in triggering exaggerated inflammatory responses (Nakanishi and Wu 2009). Multiple lines of evidence have revealed that hypoxia could drive microglia to generate ROS (Rathnasamy et al. 2011). Thus, modulating ROS production along with either these inflammatory cytokines or their regulatory pathways is a potential strategy for the treatment of hypoxia.
Cannabinoids have been attracting increasingly more interest throughout the past decade. The biological effects of cannabinoids are mainly mediated by two main subtypes of cannabinoid receptors: type 1 cannabinoid receptors (CB1Rs) and type 2 cannabinoid receptors (CB2Rs). CB2Rs have been reported to be expressed primarily in activated microglia and peripheral immune cells, thereby regulating antigen presentation, cytokine/chemokine production, and cell migration (Cabral and Griffin-Thomas 2009). Selective agonists and antagonists for these receptors have been identified in previous studies (Pertwee 1997). Considering the critical role of inflammation in ischemic and hypoxic pathophysiological processes, selective activation of CB2R by administration of CB2R agonists has drawn much attention as a potential therapeutic target for the treatment of cerebral ischemia (Pertwee 2009). Trans-Caryophyllene (TC) is a kind of bicyclic sesquiterpene, which has been reported to be a CB2R-selective agonist. Previous studies have demonstrated that TC is able to bind to CB2R, but not to CB1R, resulting in activation of the Gi/Go subtype of G proteins (Gertsch et al. 2008). It was also found that TC can reduce oxygen–glucose deprivation (OGD)-evoked cell death in neuroblastoma cells in vitro (Chang et al. 2007). Importantly, TC was also found to produce neuroprotective effects in in vitro and in vivo ischemic models (Choi et al. 2013). However, the anti-inflammatory effects of TC found in cases of hypoxia and its underlying mechanism have yet to be elucidated.
In the present study, TC was found to significantly inhibit hypoxia-induced cytotoxicity and the release of proinflammatory cytokines, including IL-1β, TNF-α, and IL-6, through activation of BV2 microglia following hypoxic exposure. Furthermore, TC significantly inhibited hypoxia-induced generation of ROS in mitochondria as well as the activation of nuclear factor kappa B (NF-κB) in microglia.
Materials and Methods
Cell Culture, Transfection, and Hypoxic Exposure
The murine BV2 cell line was cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10 % fetal bovine serum (FBS), 1 % penicillin, and streptomycin at 37 °C in a humidified incubator with 5 % CO2. Confluent cultures were passaged by trypsinization. BV2 microglia were plated overnight and then cultivated using a chamber under normoxia (20 % O2, 5 % CO2) or hypoxia (1 % O2, 5 % CO2, and 92 % N2) at 37 °C for 24 h in the presence or absence of TC (5 μM).
Small RNA Interference Transfection
BV2 cells were plated onto six-well plates and grown in DMEM with 10 % FBS for 24 h to achieve about 50 % confluency. The small interfering RNA (siRNA) transfection complex, formed by combining the transfection reagent Lipofectamine RNAiMAX (Invitrogen, USA) and 50 nM siRNA (Ctrl_Allstars_1 Negative Control or Mm_CNR2_9 target sequence: AAGGCCCAAGGTCCTCGGTTA) (QIAGEN, USA) was added into six-well plates with Opti-MEM. The cells were then incubated for 8 h in Opti-MEM, and the medium was replaced with DMEM with 10 % FBS and incubated for 48 h. The successful knockdown of CB2R was verified using western blot analysis.
Determination of Mitochondrial ROS
Mitochondrial ROS was measured using MitoSOX Red (Invitrogen, USA) according to the manufacturer’s instructions. MitoSOX Red is a live-cell permeant and is rapidly and selectively targeted to mitochondria. Once in the mitochondria, MitoSOX Red reagent is oxidized by superoxide and exhibits red fluorescence (with exCitation at 510 nm and emission at 580 nm). Briefly, the cultured BV2 microglia cells were exposed to normoxia or hypoxia in the presence or absence of TC (5 μM). The cells were collected at 24 h after treatment and then incubated with 5 mM MitoSOX Red in Hank’s balanced salt solution (HBSS) containing for 10 min at 37 °C. Images were recorded by a fluorescence microscope. Cells from five fields in each group were randomly selected for statistical analysis. The level of intracellular ROS was indexed by the average fluorescence intensity of each individual cell analyzed by Image-Pro Plus software.
Cell Viability Measurement
After various treatments, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay was used to determine cell viability. Briefly, 1 mg/mL MTT was added into the serum-free medium and incubated for 4 h in a CO2 incubator at 37 °C. The resultant insoluble formazan crystals were dissolved by dimethyl sulfoxide (DMSO). Absorbance recorded at 570 nm by a microtiter plate reader was used to index cell viability.
Cytokine Assays
The cultured BV2 microglia cells (density of 5 × 105 cells/mL) were subjected to normoxia (20 % O2, 5 % CO2) or hypoxia (1 % O2, 5 % CO2, and 92 % N2) at 37 °C for 24 h in the presence or absence of TC (5 μM). Cytokine levels were determined using a commercial ELISA kit. ELISA kits for IL-1β, IL-6, and TNF-α were from Cayman Chemical (Ann Arbor, MI, USA). Absorbance was examined at 450 nm using a microplate reader.
Western Blot Analysis
After having been washed three times with PBS, BV2 cells were lysed in cell lysis buffer (Cell Signaling, USA) containing protease inhibitor cocktail tablets (Roche, Germany). Proteins were separated using 10 % SDS-PAGE and then transferred onto Immobilon PVDF membranes (Millipore). After having been blocked in 5 % nonfat milk in Tris-buffered saline Tween (TBST) (100 mM Tris, pH 8.0, 150 mM NaCl, and 0.1 % Tween 20) for 1 h at room temperature, membranes were incubated with primary antibodies at RT for 3 h. After being washed three times with TBST, membranes were incubated with a secondary antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology, USA) for 1 h at RT. Immunoreactive bands were visualized using the ECL detection system (Pierce, USA).
Luciferase Reporter Assays
BV2 cells were transfected with pNF-κB-Luc using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. Transfected cells were exposed to hypoxia. After 24 h, the cells were lysed, and luciferase activity was determined using a commercial luciferase kit (Promega, USA) and a TD-20/20 luminometer (Turner Designs, USA). Luciferase activity was normalized by protein concentrations, which served as an internal control.
Immunofluorescence
BV2 cells were fixed in 4 % paraformaldehyde for 10 min at RT, followed by permeabilization with 0.4 % Triton X-100 for 15 min on ice. After being blocked with 5 % BSA and 2.5 % FBS in PBST, cells were then incubated with the primary antibody anti-NF-κB for 2 h at RT, followed by Alexa 594-conjugated secondary antibodies for 1 h at RT. Fluorescence signals were captured using a fluorescence microscope.
Statistical Analysis
Data are represented as mean ± S.E.M. Statistical significance was determined by one-way analysis of variance (ANOVA). p < 0.05 was considered as the minimum level of statistical significance.
Results
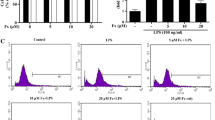
The effects of TC on the cell viability of BV2 microglia were first investigated using an MTT assay. Administration of TC, with final concentrations of 0.5 and 5 μM, did not lead to a significant change in cell viability of BV2 cells. However, mean cell viability was significantly reduced after treatment with TC using the final concentration of 50 μM (Fig. 1a). Therefore, we administered TC at a concentration of 5 μM in order to examine its effects on hypoxia-induced reduction of microglial cell viability. As shown in Fig. 1b, hypoxic exposure (1 % O2, 24 h) significantly reduced mean cell viability of BV2 microglia. Treatment with TC (5 μM) significantly restored the hypoxia-induced reduction of microglial cell viability.
Effects of trans-caryophyllene (TC) on hypoxia-induced cytotoxicity in cultured microglia. a Cell viability in BV2 microglia in the presence of TC with each of three different concentrations (*p < 0.01 vs. normoxia, n = 5). b Cell viability of BV2 microglia exposed to normoxia (20 % O2) or hypoxia (1 % O2) in the presence or absence of TC (5 μM) for 24 h (*p < 0.01 vs. normoxia; #p < 0.01 vs. hypoxia, n = 5)
Oxidative stress occurs during the hypoxic process. Previous studies have demonstrated that mitochondria in microglial cells were found to be most susceptible to oxidative damage (Nakanishi and Wu 2009). Thus, we examined hypoxia-induced mitochondrial oxidant generation in microglia using MitoSOX Red probe, a mitochondrially targeted hydroethidine derivative. As shown in Fig. 2a, b, results show that levels of mitochondrial ROS were significantly increased in BV2 microglia at 24 h after hypoxia; however, TC significantly inhibited this hypoxia-induced mitochondrial ROS increase, as was seen in the mean fluorescent intensity of MitoSOX Red probes in microglia.
Effects of TC on mitochondria-generated reactive oxygen species (ROS). a Fluorescent images of MitoSOX Red fluorescence signals in BV2 microglia exposed to normoxia (20 % O2) or hypoxia (1 % O2) in the presence or absence of TC (5 μM) for 24 h. b Quantitative analyses of MitoSOX Red fluorescence signal intensity (*p < 0.01 vs. normoxia; #p < 0.01 vs. hypoxia, n = 5)
We next examined the effects of TC on hypoxia-induced secretion of proinflammatory cytokines by microglia. Levels of IL-1β, TNF-α, and IL-6 secreted by BV2 microglia into the culture medium were measured by ELISA after hypoxic exposure (1 % O2, 24 h). Test results demonstrated that hypoxia led to a significant increase in concentrations of IL-1β, TNF-α, and IL-6 in the culture medium. Importantly, administration of TC significantly inhibited hypoxia-induced secretion of IL-1β, TNF-α, and IL-6 by microglia (Fig. 3).
Inhibitory effects of TC on hypoxia-induced proinflammatory cytokines secretion by cultured microglia. The mean concentration of IL-1β, TNF-α, and IL-6 in the culture medium of BV2 microglia exposed to normoxia (20 % O2) or hypoxia (1 % O2) in the presence or absence of TC (5 μM) for 24 h was measured using a commercial ELISA kit. a Mean concentration of IL-1β (*p < 0.01 vs. normoxia; #p < 0.01 vs. hypoxia, n = 5). b Mean concentration of TNF-α (*p < 0.01 vs. normoxia; #p < 0.01 vs. hypoxia, n = 5). c Mean concentration of IL-6 (*p < 0.01 vs. normoxia; #p < 0.01 vs. hypoxia, n = 5)
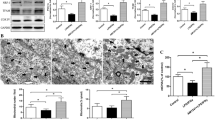
NF-κB has been considered as a critical transcriptional factor in the activation of several proinflammatory cytokines, including IL-1β, TNF-α, and IL-6. Normally, NF-κB can be found in the cytoplasm as a heterodimer of the p50 and p65 subunits. IκB inhibits NF-κB. Inflammatory cytokines are able to induce phosphorylation and subsequent degradation of IκB, thereby leading to the liberation of NF-κB heterodimers, which can then translocate to the nucleus, bind specific DNA sequences, and affect target gene expression. Here, we found that the expression of IκBα phosphorylation in BV2 microglia was significantly increased after hypoxia. However, TC treatment significantly inhibited hypoxia-induced phosphorylation of IκBα in microglia (Fig. 4a, b).
Inhibitory effects of TC on hypoxia-induced phosphorylation of IκBα in cultured microglia. a BV2 microglia exposed to normoxia (20 % O2) or hypoxia (1 % O2) in the presence or absence of TC (5 μM) for 24 h. Representative western blot bands of pIκBα, IκBα, and β-actin. b Quantitative analyses of immunoblots in a (*p < 0.01 vs. normoxia; #p < 0.01 vs. hypoxia, n = 5)
Immunocytochemistry experiments revealed that hypoxia-induced p65 nuclear translocation was blocked by the administration of TC, thereby confirming the inhibitory effects of TC on NF-κB activation (Fig. 5). As a next step, we assessed the inhibitory effects of TC on NF-κB by performing luciferase reporter assays. In BV2 cells transfected with NF-κB-Luc reporter plasmid, the presence of hypoxia drastically induced NF-κB luciferase activity. This induction was markedly suppressed by TC (Fig. 6).
NF-κB luciferase reporter assays. After indicated transfection, BV2 cells transfected with pNF-κB-Luc reporter were exposed to normoxia (20 % O2) or hypoxia (1 % O2) in the presence or absence of TC (5 μM) for 24 h before measuring luciferase activity (*p < 0.01 vs. normoxia; #p < 0.01 vs. hypoxia, n = 5)
In order to determine whether or not the effects of TC on hypoxia-induced microglia inflammation are dependent on activation of CB2R, we inhibited the expression of CB2R using CB2R siRNA. Successful suppression of CB2R is shown by western blot analysis in Fig. 7a. The effects of TC on the NF-κB-Luc reporter were abolished when BV2 cells were transfected with CB2R siRNA (Fig. 7b). Most importantly, ELISA results revealed that TC’s effects on the secretion of inflammatory cytokines can be abolished by muting the CB2 receptor (Fig. 8).
Inhibitory effects of TC on NF-κB transcriptional activity are dependent on CB2R. siCB2R indicates the CB2R siRNA group; NS indicates the nonspecific siRNA group. a Western blot analysis revealed the successful knockdown of CB2R (*p < 0.01 vs. NS group). b NF-κB luciferase reporter assays. After indicated transfection, BV2 cells transfected with pNF-κB-Luc reporter were exposed to normoxia (20 % O2) or hypoxia (1 % O2) in the presence or absence of TC (5 μM) for 24 h before measuring luciferase activity (*p < 0.01 vs. normoxia; #p < 0.01 vs. hypoxia; $p < 0.01 vs. hypoxia+TC group, n = 5)
Inhibitory effects of TC on hypoxia-induced proinflammatory cytokines secretion by cultured microglia are dependent on CB2R. siCB2R indicates the CB2R siRNA group; NS indicates the nonspecific siRNA group. The mean concentration of IL-1β, TNF-α, and IL-6 in the culture medium of BV2 microglia exposed to normoxia (20 % O2) or hypoxia (1 % O2) in the presence or absence of TC (5 μM) for 24 h was measured by ELISA. a Mean concentration of IL-1β. b Mean concentration of TNF-α. c Mean concentration of IL-6 (*p < 0.01 vs. normoxia; #p < 0.01 vs. hypoxia; $p < 0.01 vs. hypoxia+TC group, n = 5)
Discussion
Inflammation in the brain caused by activated microglia is a prominent pathological feature associated with several brain diseases. The major finding in this study is that TC, a CB2R-selective agonist, significantly inhibits hypoxia-induced activation of the NF-κB-dependent neuroinflammatory pathway in microglia. The nuclear factor NF-κB, a central regulator of inflammatory processes, encodes the genes of proinflammatory cytokines, including IL-1β, TNF-α, and IL-6 (Liu and Malik 2006). Proinflammatory cytokines secreted by activated microglia, such as IL-1β, IL-6, and TNF-α, are known to be important mediators in the process of inflammation. These proinflammatory mediators are thought to be responsible for some of the harmful effects of brain injuries and diseases. Activation of NF-κB is known to be involved in the pathological process of stroke. Previous studies have demonstrated that mice lacking the p50 subunit of NF-κB develop significantly smaller infarcts after transient focal ischemia (Nurmi et al. 2004), and antioxidants work to reduce infarct volume and improve behavior deficits (Qin et al. 2007). The work reported here suggests that TC could be used to inhibit proinflammatory cytokines released by microglia. Activation of CB2R has been proposed to have neuroprotective properties in neurological diseases. A recent study demonstrated that administration of JWH-133, an agonist of CB2R, significantly improved infarct outcome, as shown by a reduction in brain infarction and neurological impairment in a stroke model. This effect was reversed by the CB2R antagonist and was absent in CB2R knockout mice. Concomitantly, administration of JWH-133 also led to a lower level of intensity of Iba1+ microglia/macrophages along with reduced secretion of inflammatory cytokines (Zarruk et al. 2012). Consistently, TC was reported to decrease neuronal injury and mitochondrial depolarization caused by OGD/re-oxygenation (OGD/R) by way of its enhancement of AMPK and CREB phosphorylation (Choi et al. 2013).
It is also known that NF-κB activation is facilitated by conditions associated with an increased intracellular redox state (Toledano and Leonard 1991). In addition, accumulating evidence suggests an important role for oxidative stress in the regulation of neuroinflammation following stroke. In the present study, hypoxia-induced mitochondria-derived ROS generation was effectively inhibited by treatment with TC, suggesting a potential antioxidative stress capability of TC. Activation of CB2R has been reported to attenuate oxidative stress damage in different kinds of tissues, including brain (Aso et al. 2013), kidney (Mukhopadhyay et al. 2010), and liver tissues (Cao et al. 2013). TC, as a natural sesquiterpene, has demonstrated its antioxidant activity in previous studies (Salehi et al. 2012). It has been widely accepted that various intracellular signaling pathways are involved in the expression of inflammatory mediators. An early signal occurs in the cytoplasm which leads to IκB phosphorylation. This phosphorylation results in the degradation of IκB, thus allowing NF-κB translocation to the nucleus. Activation of CB2R in different tissues might result in coupling to several principal signaling pathways, including MAP kinase (e.g., ERK1/2 and p38), c-Jun N-terminal kinase, and PI3/Akt pathways (Montecucco et al. 2009). The underlying mechanism that allows TC to restore reduced levels of IκB under hypoxia needs to be elucidated in future studies.
Microglial cells are a major glial cell element of the CNS. It plays a critical role as resident immunocompetent, host defense, and tissue repair in the CNS and CNS diseases, such as multiple sclerosis, Parkinson’s disease, and Alzheimer’s disease (Nakajima and Kohsaka 2001). Microglia has two opposite functions in the brain. On one hand, microglia provides immunosurveillance. On the other hand, microglia can also be toxic (Kreutzberg 1996). Recent studies have classified microglia as an activated proinflammatory type M1 and a less inflammatory and neuroprotective type M2 involved in tissue repair and modeling of the extracellular matrix (Kigerl et al. 2009). Hypoxia shifted microglia to the proinflammatory M1 phenotype (Habib et al. 2013). After switch from a balanced phenotype towards an activated and secretion M1 status, secretion of inflammatory cytokines, such as TNF-α, IL-6, and IL-1β by microglia have been increased. The microglial population is heterogeneous. Inflammatory mediators and ROS induced by hypoxia lead to the death of microglia cells. Although the total number of microglia is reduced, more microglia in M1 status will lead to the upregulation of TNF-α, IL-6, and IL-1β. It has been reported that depletion of the proliferative microglia exacerbated injury in stroke by enhancing excitotoxic injury and further increasing the cytokine and chemokine production already induced by ischemia–reperfusion (Lalancette-Hébert et al. 2007). In contrast, injection of microglia into the ischemic brain ameliorated injury in stroke (Imai et al. 2007). Recently, the concept of microglial M1 and M2 phenotypes also has entered the field of stroke research. As the M2 subtype protected the hippocampal neurons against oxygen–glucose deprivation (Girard et al. 2013), our findings suggest that TC treatment might be able to promote proinflammatory M1 microglial phenotypes converting into anti-inflammatory M2 microglia.
Stroke, the most common form of hypoxia-ischemic brain injury, presents a major challenge in maintaining public health due to its high rate of incidence and its life-threatening nature. Oxidative stress and neuroinflammation are considered as the two major pathophysiological mechanisms involved in hypoxia-ischemic brain injuries (Lakhan et al. 2009). Activated microglial cells have been found in the brains of stroke patients. This plays a critical role in stroke progression (Gerhard et al. 2000). Furthermore, microglia may play a dual role, both participating in the brain’s host defense system as well as acting as phagocytes, which engulf tissue debris and dead cells. On the other hand, microglial cells have been pinpointed as the major cell population in the human brain that leads to NF-κB-dependent upregulation of proinflammatory molecules, such as TNF-α and IL-1β, during stroke. These factors can cause neuronal death and demyelination (Glezer et al. 2007). Thus, blockage of microglia activation has been considered as an effective strategy in preventing hypoxia-ischemic brain injury (Zawadzka et al. 2012). TC efficiently attenuates hypoxia-induced activation of the NF-κB-dependent neuroinflammatory pathway in microglia and, therefore, may be beneficial in the prevention and treatment of stroke.
References
Aso E, Juvés S, Maldonado R, Ferrer I (2013) CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AβPP/PS1 mice. J Alzheimers Dis 35:847–858
Cabral GA, Griffin-Thomas L (2009) Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med 11:e3
Cao Z, Mulvihill MM, Mukhopadhyay P, Xu H, Erdélyi K, Hao E, Holovac E, Haskó G, Cravatt BF, Nomura DK, Pacher P (2013) Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology 144:808–817
Carlson BW, Carlson JR, Neelon VJ, Hartman M (2008) Tailoring protocols to successfully recruit and retain older adults in a longitudinal study of sleep and cognition. Res Gerontol Nurs 1:232–237
Chamorro A, Hallenbeck J (2006) The harms and benefits of inflammatory and immune responses in vascular disease. Stroke 37:291–293
Chang HJ, Kim HJ, Chun HS (2007) Quantitative structure-activity relationship (QSAR) for neuroprotective activity of terpenoids. Life Sci 80:835–841
Choi IY, Ju C, Anthony Jalin AM, da Lee I, Prather PL, Kim WK (2013) Activation of cannabinoid CB2 receptor-mediated AMPK/CREB pathway reduces cerebral ischemic injury. Am J Pathol 182:928–939
Gerhard A, Neumaier B, Elitok E, Glatting G, Ries V, Tomczak R, Ludolph AC, Reske SN (2000) In vivo imaging of activated microglia using [11C]PK11195 and positron emission tomography in patients after ischemic stroke. NeuroReport 11:2957–2960
Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A (2008) Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A 105:9099–9104
Girard S, Brough D, Lopez-Castejon G, Giles J, Rothwell NJ, Allan SM (2013) Microglia and macrophages differentially modulate cell death after brain injury caused by oxygen-glucose deprivation in organotypic brain slices. Glia 61:813–824
Glezer I, Simard AR, Rivest S (2007) Neuroprotective role of the innate immune system by microglia. Neuroscience 147:867–883
Habib P, Slowik A, Zendedel A, Johann S, Dang J, Beyer C (2013) Regulation of hypoxia-induced inflammatory responses and M1–M2 phenotype switch of primary rat microglia by sex steroids. J Mol Neurosci
Heneka MT, O’Banion MK, Terwel D, Kummer MP (2010) Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm 117:919–947
Imai F, Suzuki H, Oda J, Ninomiya T, Ono K, Sano H, Sawada M (2007) Neuroprotective effect of exogenous microglia in global brain ischemia. J Cereb Blood Flow Metab 27:488–500
Kigerl KA, Gendel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG (2009) Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29:13435–13444
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7:97
Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J (2007) Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci 27:2596–2605
Liu SF, Malik AB (2006) NF-κB activation as a pathological mechanism of septic shock and inflammation. Am J Physiol Lung Cell Mol Physiol 290:L622–L645
Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, Steffens S (2009) CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol 46:612–620
Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Bátkai S, Gao B, Haskó G, Pacher P (2010) Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med 48:457–467
Nakajima K, Kohsaka S (2001) Microglia: activation and their significance in the central nervous system. J Biochem 130:169–175
Nakanishi H, Wu Z (2009) Microglia-aging: roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav Brain Res 201:1–7
Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J (2004) Nuclear factor-κB contributes to infarction after permanent focal ischemia. Stroke 35:987–991
Pertwee RG (1997) Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 74:129–180
Pertwee RG (2009) Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol 156:397–411
Qin ZH, Tao LY, Chen X (2007) Dual roles of NF-κB in cell survival and implications of NF-κB inhibitors in neuroprotective therapy. Acta Pharmacol Sin 28:1859–1872
Rathnasamy G, Ling EA, Kaur C (2011) Iron and iron regulatory proteins in amoeboid microglial cells are linked to oligodendrocyte death in hypoxic neonatal rat periventricular white matter through production of proinflammatory cytokines and reactive oxygen/nitrogen species. J Neurosci 31:17982–17995
Salehi P, Sonboli A, Khaligh P, Mirzajani F (2012) Essential oil composition and antioxidant activity of different extracts of Nepeta betonicifolia C.A. Meyer and Nepeta saccharata Bunge. Nat Prod Res 26:736–743
Shi H, Sheng B, Zhang F, Wu C, Zhang R, Zhu J, Xu K, Kuang Y, Jameson SC, Lin Z, Wang Y, Chen J, Jain MK, Atkins GB (2013) Kruppel-like factor 2 protects against ischemic stroke by regulating endothelial blood brain barrier function. Am J Physiol Heart Circ Physiol 304:H796–H805
Sivakumar V, Foulds WS, Luu CD, Ling EA, Kaur C (2011) Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J Pathol 224:245–260
Toledano MB, Leonard WJ (1991) Modulation of transcription factor NF-κB binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A 88:4328–4332
Yang Q, Yang ZF, Liu SB, Zhang XN, Hou Y, Li XQ, Wu YM, Wen AD, Zhao MG (2010) Neuroprotective effects of hydroxysafflor yellow A against excitotoxic neuronal death partially through down-regulation of NR2B-containing NMDA receptors. Neurochem Res 35:1353–1360
Zarruk JG, Fernández-López D, García-Yébenes I, García-Gutiérrez MS, Vivancos J, Nombela F, Torres M, Burguete MC, Manzanares J, Lizasoain I, Moro MA (2012) Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain macrophage/microglial activation concomitant to neuroprotection. Stroke 43:211–219
Zawadzka M, Dabrowski M, Gozdz A, Szadujkis B, Sliwa M, Lipko M, Kaminska B (2012) Early steps of microglial activation are directly affected by neuroprotectant FK506 in both in vitro inflammation and in rat model of stroke. J Mol Med (Berl) 90:1459–1471. doi:10.1007/s00109-012-0925-9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, K., Mou, X., Huang, J. et al. Trans-Caryophyllene Suppresses Hypoxia-Induced Neuroinflammatory Responses by Inhibiting NF-κB Activation in Microglia. J Mol Neurosci 54, 41–48 (2014). https://doi.org/10.1007/s12031-014-0243-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0243-5