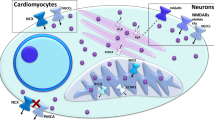
Abstract
Inducers of mitochondrial biogenesis are widely under investigation for use in a novel therapeutic approach in neurodegenerative disorders. The ability of Gemfibrozil, a fibrate, is investigated for the first time to modulate mitochondrial pro-survival factors involved in the mitochondrial biogenesis signaling pathway, including peroxisome proliferator-activated receptor coactivator-1α (PGC-1α), nuclear respiratory factor (NRF-1), and mitochondrial transcription factor A (TFAM) in the brain. Gemfibozil is clinically administered to control hyperlipidemia. It secondarily prevents cardiovascular events such as cardiac arrest in susceptible patients. In this study, pretreatment of animals with gemfibrozil prior to ischemia–reperfusion (I/R) resulted in a sexually dimorphic outcome. While the expression of NRF-1 and TFAM were induced in gemfibrozil-pretreated met-estrous females, they were suppressed in males. Gemfibrozil also proved to be neuroprotective in met-estrous females, as it inhibited caspase-dependent apoptosis while in males it led to hippocampal neurodegeneration via activation of both the caspase-dependent and caspase-independent apoptosis. In the mitogen-activated protein kinase (MAPKs) pathway, gemfibrozil pretreatment induced the expression of extracellular signal-regulated kinases (ERK1/2) in met-estrous females and reduced it in males. These findings correlatively point to the sexual-dimorphic effects of gemfibrozil in global cerebral I/R context by affecting important factors involved in the mitochondrial biogenesis, MAPKs, and apoptotic cell death pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mitochondrial biogenesis is activated in different pathological conditions including critical illness (Carre et al. 2010), hypoxia (Gutsaeva et al. 2008), hypoxic–ischemic brain injury (Yin et al. 2008), oxidant injury (Rasbach and Schnellmann 2007), as well as focal (Chen et al. 2001), and global cerebral ischemia (Chen et al. 2010a), as a defensive and compensatory response to the insult. Induction of mitochondrial biogenesis has been proposed as a therapeutic approach in some pathological conditions, such as brain ischemic stroke (Lagouge et al. 2006; Dong et al. 2007; Wenz 2009).
Fibrates such as benzafibrate and fenofibrate are a group of commonly used lipid-lowering agents and have been found to induce mitochondrial biogenesis in skeletal muscle and within the liver (Nagai et al. 2002; Wenz et al. 2008). However, the potential for fibrates to induce mitochondrial biogenesis within the brain has not yet been investigated. Currently, research has focused on fibrates to investigate their potential to protect against various cerebral disorders. It has been demonstrated that fibrates, as peroxisome proliferator-activated receptor (PPAR)-α agonists, induce both preventive and acute neuroprotection through either cerebral or vascular mechanisms (Bordet et al. 2006). It has also been reported that phenofibrate, a PPAR-α activator, protects against cerebral injury by antioxidant and anti-inflammatory mechanisms (Deplanque et al. 2003; Xu et al. 2007; Wang et al. 2010). In this regard, there are limited data supporting or opposing such ability for another widely used fibrate, gemfibrozil. Guo and collaborators have reported that prophylactic use of gemfibrozil in middle cerebral artery occlusion (MCAO) model of ischemic stroke has led to both beneficial and deleterious results (Guo et al. 2009), indicating that further investigations may be meritorious to unravel whether prophylactic application of gemfibrozil against cerebral ischemia is beneficial.
PGC-1α has been identified as a major regulator of mitochondrial biogenesis in vivo (Wu et al. 1999). PGC-1α interacts and coactivates different transcription factors such as NRF-1 and NRF-2 (Wu et al. 1999; Baar 2004), as well as estrogen-related receptor-α (ER-α) (St-Pierre et al. 2006), and PPARs including PPAR-α (Vega et al. 2000; Baar 2004). NRFs are known to regulate the expression of most nuclear genes involved in mitochondrial biogenesis such as mitochondrial transcription factor A (TFAM). TFAM directly regulates the replication and transcription of mtDNA, as well as genes encoding subunits of respiratory complexes (Wu et al. 1999; Nadal-Casellas et al. 2010). PGC-1α, NRF-1, and TFAM are considered as mitochondrial pro-survival factors involved in the mitochondrial biogenesis.
Many diseases of CNS display sexual dimorphism, with a predilection for one gender (Sacco 2001; Van Den Eeden et al. 2003). In addition, gender has been found to influence treatment outcome (Hurn and Macrae 2000; Hariz et al. 2003). Such differences may be attributed to circulating sex hormones (Azcoitia et al. 2003), as well as the inherent gender differences (Du et al. 2004). Gender dimorphism has been previously noted in mitochondrial oxidative metabolism within the liver of rats (Justo et al. 2005; Nadal-Casellas et al. 2010). Additionally, it has been found that sex hormones influence the expression of mitochondrial biogenesis-signaling factors (Rodríguez-Cuenca et al. 2007). However to date, mitochondrial pro-survival factors involved in the mitochondrial biogenesis signaling pathway have not been studied in the male and female brains, specifically in the cerebral ischemia–reperfusion (I/R) context, which is a sexually dimorphic brain pathology.
Sex-dependent differences have also been reported within ischemia-induced cell death pathways (Nuñez et al. 2001). Caspase-independent apoptosis seems to be the major neural cell death pathway in response to ischemia within males whereas, in females, it is the caspase-dependent apoptosis (McCullough et al. 2005; Lang and McCullough 2008).
The MAPK pathway including c-Jun N-terminal kinases (JNK), ERK1/2, and p38, upstream of mitochondrial biogenesis and cell death pathways, are reported to be stimulated following ischemia (Irving and Bamford 2002; Wright 2007; Lira et al. 2010). When activated, MAPKs coordinate a broad range of intracellular activities from metabolism, motility, mitosis, inflammation, differentiation, cell survival, and even cell death (Roux and Blenis 2004). Recent papers support the hypothesis that neuronal apoptosis and cerebral ischemia induce the robust activation of MAPK cascades. Although extracellular signal-regulated kinases pathways promote cell survival and proliferation and c-Jun N-terminal protein kinases/p38 pathways induce apoptosis in general, the roles of MAPK cascades in neuronal death and survival seem to be complicated and altered by the type of cells and the magnitude and timing of insults (Nozaki et al. 2001). Intriguingly, gender-related differences in the activation of MAPKs have also been found, and these may contribute to the observed sexual dimorphism of brain disorders as well (Zhang 2002).
The present study, therefore, aimed to investigate for the first time the ability of gemfibrozil to induce major mitochondrial pro-survival factors involved in the mitochondrial biogenesis-signaling pathway in the male and female hippocampus exposed to global cerebral I/R. Gemfibrozil is administered routinely to control hyperlipidemia, and it prevents cardiovascular events in susceptible patients. Due to the absence of sufficient evidences regarding the neuroprotective/neurodegenerative effects of gemfibrozil, the present study also examined its therapeutic potential in global cerebral I/R, mainly concentrating on the mitochondrial apoptotic cell death and the upstream MAPKs signaling proteins within the hippocampus of both sexes.
Materials and Methods
Animals
Six-month-old male and female Wistar rats, with body weight ranging from 270 to 310 g, were housed in standard cages under controlled temperature (22 ± 2 °C), humidity, and a 12 h light/dark cycle (light on 07:00–19:00), with food and water provided ad libitum. Female rats were selected in the met-estrous phase of the estrous cycle using vaginal smears, previously described by Marcondes et al. (2002). Experimentation was approved by the Ethics Committee of Shahid Beheshti Medical University in accordance with National Institutes of Health guide for the care and use of laboratory animals (NIH publications no.80-23, revised 1978). All efforts were made to minimize animal suffering, and to reduce the number of animals used.
Experimental Groups
Animals of both genders were randomly divided into three experimental groups: sham, ischemic, and treatment (n = 12/group). The rats within the treatment groups were pretreated with gemfibrozil (Sigma; 30 mg/kg p.o.) once daily for 7 days through a feeding needle (Guo et al. 2009). One hour after the final dose, animals were subjected to 10 min of ischemia using the four-vessel occlusion model (4VO), as previously described by Pulsinelli and Brierley ( 1979). In ischemic groups, animals were pretreated with vehicle (5 ml/kg of 0.5 % carboxymethyl cellulose), then subjected to ischemia. Animals in the sham groups were managed according to the protocol of 4VO model and underwent anesthesia and surgery without occluding blood vessels. Additional animals, both male and female, were utilized for the apoptosis-inducing factor (AIF) assessment in the nuclear fraction for each experimental group (n = 5). Females were also subjected to ischemia and sacrifice when they were at their met-estrous. The reason that female rats were selected in the met-estrous phase of their cycle was to diminish the variation in data and to reduce the probable intervention of circulating estradiol in female experimental groups.
Abbreviations used in graphs for experimental groups are SM for sham male, IM for ischemic male, TM for treatment male, SF sham female, IF ischemic female, TF treatment female.
A few number of animals of both sexes were also specified to receive “gemfibrozil only treatment.” However, their results regarding the measured parameters in this study were not significantly different from their respective sham groups (data not shown).
Surgery
Rats underwent transient forebrain global ischemia as described by Pulsinelli and Brierley (1979). This model is a clinically relevant one, approved to model the ischemic–hypoxic damage within the brain following cardiac arrests. Briefly, on the first day, rats were anesthetized by chloral hydrate (400 mg/kg). A sterile string was loosely placed around each common carotid artery (CCA) without interrupting carotid blood flow, and the incision was sutured. Both vertebral arteries were permanently electrocoagulated. EEG electrodes were fixed bilaterally at the skull on the parietal cortex. On the second day, under chloral hydrate anesthesia, both CCA were occluded for 10 min. The 4VO rats were only included if electroencephalogram (EEG) was flattening during ischemia (Diler et al. 2002). Seventy-two-hour reperfusion was initiated by opening the carotid clamps after 10 min of ischemia. Sham surgery involved exposure of common carotid and vertebral arteries. Rectal temperature was monitored (Citizen-513w) and kept at 37 °C by surface heating and cooling during surgery.
Sacrifice and Tissue Preparation
After 72-h reperfusion, each group (n = 12) was split into two subgroups. The animals within the first subgroup (n = 6) were perfused transcardially with phosphate-buffered saline (PBS) (pH 7.4), followed by 4 % paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) under chloral hydrate anesthesia (400 mg/kg i.p.). The brains were then removed and post-fixed in 4 % paraformaldehyde for 24 h and subsequently embedded in paraffin for histopathologic studies. In the second subgroup, animals (n = 6) were killed by CO2 asphyxiation. Rats were decapitated, brains removed, and CA1 subfield of the hippocampus isolated on ice and frozen in liquid nitrogen and stored at −80 °C for Western blot analysis. The same procedure was performed for animals specified for evaluation of nuclear AIF expression.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End-Labeling (TUNEL)
Whole brain tissue embedded in paraffin was used for further histopathologic preparations. Coronal sections (4–5 μm thickness) of hippocampal formation were prepared, and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) was performed using the in situ cell death detection kit, POD (Roche Applied Science, Germany). Tissue sections were deparaffinized in xylene, rehydrated, and immersed in 3 % hydrogen peroxide to block the endogenous peroxidase activity. After rinsing with PBS, sections were treated with proteinase K solution at 37 °C for 30 min to enhance the staining, incubated for 60 min at 37 °C with 50 μl of TUNEL reaction mixture, and then incubated for 30 min at 37 °C with 50 μl of converter-POD. Sections were rinsed in PBS, then incubated for 10 min at 15–25 °C with 50 μl of DAB substrate solution and rinsed again with PBS. Counter staining was achieved with 0.5 % methyl green. Tissue was incubated in DNase solution for 10 min at 15–25 °C for positive staining. Sections were then dehydrated and coverslipped for analysis under light microscopy. Negative controls were performed by omission of the enzyme solution step. To obtain the mean percentage of apoptotic cells to normal cells within the CA1 subfield of the hippocampus, the number of TUNEL-positive pyramidal neurons was counted on three adjacent ×400 microscopic images.
Preparation of Total Protein Extracts
The CA1 region of hippocampi were dissected on ice in ice-cold 125 mmol/L Tris–HCl, pH 7.4, containing 320 mmol/L sucrose, 2 mmol/L sodium orthovanadate, 20 mmol/L sodium diphosphate decahydrate, 20 mmol/L dl-a-glycerophosphate, 0.1 mmol/L phenylmethylsulfonyl fluoride, and 5 mg/mL each of antipain, aprotinin, and leupeptin (homogenization buffer). Total protein extract was collected by centrifugation at 13,000×g for 5 min. The samples were stored at −80 °C until needed for Western blot analysis (Niimura et al. 2006).
Preparation of Nuclear Protein Extracts for Assessment of Nuclear AIF
Tissues were homogenized with 300 ml lysis buffer [10 mmol/L N-2-hydroxyethylpiperazine-N_-2-ethanesulfonic acid (pH 7.9), 1 mmol/L EDTA, 1 mmol/L EGTA, 10 mmol/L KCl, 1 mmol/L dithiothreitol, 0.5 mmol/L phenylmethylsulfonyl fluoride, 0.1 mg/ml aprotinin, 1 mg/ml leupeptin, 1 mg/ml Na-p-tosyll-lysine-chloromethyl ketone, 5 mmol/L NaF, 1 mmol/L NaVO4, 0.5 mmol/L sucrose, and 10 mmol/L Na2MoO4]. After 15 min, Nonidet P-40 (Roche, Mannheim, Germany) was added to reach a concentration of 0.5 %. The tubes were gently vortexed for 15 s, and nuclei were collected by centrifugation at 8,000×g for 5 min. The pellets were resuspended in 100 ml buffer supplemented with 20 % glycerol and 0.4 mol/L KCl and gently shaken for 30 min at 4 °C. Nuclear protein extracts were obtained by centrifugation at 13,000×g for 5 min, and aliquots of the supernatant were stored at −80 °C. All steps were carried out at 4 °C (Garcia-Bueno et al. 2008).
Western Blotting
Western blotting was used to measure the protein expression of PGC-1α (ABCAM; 1 μg/ml), NRF-1 (Santa Cruz; 1/1000) and TFAM (BioVision; 0.5 μg/ml) as indicators of mitochondrial biogenesis. The expressions of cleaved caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP)-1, as well as nuclear AIF (Cell Signaling Technology; 1/1,000) were measured as markers of caspase-dependent and caspase-independent apoptosis, respectively. Phospho-p38 MAP kinase, p38 MAP kinase, phospho-JNK (Cell Signaling Technology; 1/1,000), JNK, phospho-ERK1/2, and ERK1/2 (ABCAM; 1/1,000) were used for evaluation of MAPK signaling pathway in hippocampi, as previously published by Jalalvand et al. (2008).
Briefly, the supernatant was collected and assayed for protein concentration using the Bradford method (Bradford 1976). Standard plots were generated using bovine serum albumin. Lysates equivalent to 30 μg of protein were resolved on SDS–10 % polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Porablot, Macherey–Nagel, Germany). In consequent steps, horseradish peroxidase-conjugated secondary antibodies were used as follows: rabbit and mouse IgG-HRP-linked antibodies (Cell Signaling; 1/10,000). Blots were revealed by ECL advanced kit (Amersham Biosciences). To normalize for protein content, blots were stripped and then probed with anti β-actin and anti-lamin B antibodies (Santa Cruz Biotechnology; 0.5 μg/ml and 1/1000, respectively). The density of bands was quantified using NIH Image J, and the ratio to β-actin or lamin B was calculated.
Statistical Analysis
The number of neurons within the hippocampus was analyzed using a non-parametric method, Kruskal–Wallis test. Western blot data was analyzed by a one-way analysis of variance (ANOVA) followed by Tukey’s HSD for multiple comparisons, using SPSS 16.0 package programs. Data are expressed as mean ± SEM, and statistical significance was set at P < 0.05.
Results
Histopathological Evaluation
The CA1 region of hippocampus is considered a region of high susceptibility to global cerebral injury. Using the TUNEL staining methodology, we were able to detect apoptotic nuclei within our experimental groups, shown in Fig. 1. No evidence of TUNEL reactivity, hence no apoptotic cells were found in the CA1 region of the hippocampus within our sham animals of both male and met-estrous female groups (Fig. 1a, d).
Representative hippocampi sections stained with TUNEL. a, d, b show hippocampal CA1 field of male and female sham groups, and male ischemic group, respectively. Male ischemic group stained negative for TUNEL-positive neurons (b). Neurons were moderately TUNEL-stained in gemfibrozil-pretreated male ischemic group (arrows) (c). The large number of TUNEL-positive neurons detected in female ischemic group (arrows) (e), were significantly decreased in their respective gemfibrozil-pretreated group (f). Magnification, ×400. The graph represents the quantitative percentage of TUNEL-positive neurons in the hippocampal CA1 region of animals in each experimental group (g). Values are mean ± SEM; Kruskal–Wallis test; *P < 0.05; ***P < 0.001. Abbreviations used in graphs for experimental groups: SM sham male, IM ischemic male, TM treatment male, SF sham female, IF ischemic female, TF treatment female
Surprisingly, there was no difference in the percentage of apoptotic nuclei within the CA1 region of the hippocampus between the ischemic male and respective sham groups (Fig. 1b). However, a significant number of apoptotic nuclei were detected by TUNEL positivity within male rats pretreated with gemfibrozil before I/R injury (P < 0.05) (Fig. 1c).
On the other hand, within the ischemic met-estrous female group, there was a significant increase in the number of TUNEL-positive neurons in the CA1 region of the hippocampus (Fig. 1e) (P < 0.001). Interestingly, by pretreatment with gemfibrozil, the number of TUNEL-positive neurons was reduced significantly in met-estrous female rats compared with the met-estrous female ischemic control group (P < 0.001) (Fig. 1f).
The quantitative results of the TUNEL test are represented in Fig. 1g. These results suggest that males were resistant to 10 min of global cerebral I/R injury, whilst met-estrous females showed widespread neurodegeneration in the hippocampal CA1 region under the same conditions. Interestingly, gemfibrozil pretreatment resulted in a sexually dimorphic outcome, providing neuroprotection within met-estrous females but resulting in neurodegeneration in males.
Mitochondrial Pro-survival Factors Involved in the Mitochondrial Biogenesis Signaling Pathway
PGC-1α Expression
PGC-1α is considered as a master regulator of mitochondrial biogenesis and a potent antioxidant (Wu et al. 1999). We found the expression level of PGC-1α to be significantly higher in female sham groups in comparison to the male sham groups. However, the overall pattern of expression of PGC-1 α in the experimental groups of both genders was similar.
As shown in Fig. 2, I/R caused a significant increase in the expression of PGC-1α compared with the sham groups of both sexes. This increase was higher in met-estrous females compared with males (P < 0.001 and P < 0.01, respectively). Administration of gemfibrozil was associated with a non-significant drop of PGC-1α expression in both sexes; however, its expression was higher than that in the respective sham groups.
Western blot analysis to measure the expression of PGC-1α in the hippocampus tissues derived at 72 h after 10 min global cerebral ischemia in male and female experimental groups. a Immunoblot bands of PGC-1α and β-actin. b The densities of corresponding bands were measured, and the ratio to β-actin was calculated and represented as arbitrary units on the graph for each experimental group (n = 6). Bars indicate the mean ± SEM; one-way ANOVA; *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations used in graphs for experimental groups: SM sham male, IM ischemic male, TM treatment male, SF sham female, IF ischemic female, TF treatment female
NRF-1 Expression
NRF-1, another major player in the mitochondrial signaling pathway, showed a similar pattern of expression to PGC-1α in response to I/R. We found that NRF-1 expression was induced in I/R groups compared with the respective sham groups (P < 0.01 and P < 0.001 in males and females, respectively). Interestingly, gemfibrozil pretreatment resulted in a converse pattern of NRF-1 expression in both genders. In males, NRF-1 expression reduced significantly (p < 0.01) compared with the ischemic groups whilst, within met-estrous females, NRF-1 expression increased considerably (P < 0.01) (Fig. 3).
Western blot analysis to measure the expression of NRF-1 in the hippocampus tissues derived at 72 h after 10 min global cerebral ischemia in male and female experimental groups. a Immunoblot bands of NRF-1 and β-actin. b The densities of corresponding bands were measured, and the ratio to β-actin was calculated and represented as arbitrary units on the graph for each experimental group (n = 6). Bars indicate the mean ± SEM; one-way ANOVA; **P < 0.01; ***P < 0.001. Abbreviations used in graphs for experimental groups: SM sham male, IM ischemic male, TM treatment male, SF sham female, IF ischemic female, TF treatment female
TFAM Expression
TFAM expression was significantly different between the sexes in our experimental groups (Fig. 4). Within the male rats, we observed a significantly higher level of TFAM expression in the sham group compared with female sham (P < 0.001). TFAM expression was significantly decreased within ischemic male rats in comparison to met-estrous females (P < 0.05). In contrast, within the met-estrous female ischemic group, TFAM expression was increased to a significant extent compared with the respective sham group (P < 0.01).
Western blot analysis to measure the expression of TFAM in the hippocampus tissues derived at 72 h after 10 min global cerebral ischemia in male and female experimental groups. a Immunoblot bands of TFAM and β-actin. b The densities of corresponding bands were measured, and the ratio to β-actin was calculated and represented as arbitrary units on the graph for each experimental group (n = 6). Bars indicate the mean ± SEM; one-way ANOVA; *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations used in graphs for experimental groups: SM sham male, IM ischemic male, TM treatment male, SF sham female, IF ischemic female, TF treatment female
In addition, TFAM expression was further increased in the gemfibrozil-pretreated met-estrous females in comparison to both ischemic (P < 0.01) and sham groups (P < 0.001). Conversely, gemfibrozil pretreatment in males resulted in a further decrease in TFAM expression compared with both male ischemic (P < 0.05) and sham groups (P < 0.001). Taken together, these results suggest that gemfibrozil pretreatment provides protection to met-estrous females partially through induction of NRF-1 and TFAM whilst, in males, gemfibrozil pretreatment was associated with neurodegeneration, probably via repression of the same factors of the mitochondrial biogenesis signaling pathway.
Caspase-Dependent Apoptosis
Cleaved Form of Caspase-3
Caspase-3 is an executive caspase in the caspase-dependent apoptotic cell death (Jänicke et al. 1998). As shown in Fig. 5, the results regarding the expression of cleaved form of caspase-3 in the experimental groups of this study were in accordance with the TUNEL assay results, represented in Fig 1. While there were no significant differences between male ischemic and sham groups, within met-estrous females, the expression of cleaved caspase-3 was significantly increased in response to I/R compared with the sham group (P < 0.05). In contrast, pretreatment of rats with gemfibrozil resulted in a significant induction of activated caspase-3 in males (P < 0.01), but an extensive decrease within met-estrous females compared with both ischemic (P < 0.001) and sham groups (P < 0.01). These results further support the observed sexual-dimorphic outcome of gemfibrozil pretreatment.
Western blot analysis to measure the expression of cleaved caspase-3 in the hippocampus tissues derived at 72 h after 10 min global cerebral ischemia in male and female experimental groups. a Immunoblot bands of cleaved caspase-3 and β-actin. Changes in the expression of 17 kDa fragment of cleaved caspase-3 are considered. b The densities of corresponding bands were measured, and the ratio to β-actin was calculated and represented as arbitrary units on the graph for each experimental group (n = 6). Bars indicate the mean ± SEM; one-way ANOVA; *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations used in graphs for experimental groups: SM sham male, IM ischemic male, TM treatment male, SF sham female, IF ischemic female, TF treatment female
Cleaved PARP-1
PARP-1 is cleaved by activated caspase-3 in the caspase-dependent apoptotic pathway into 24- and 89-kDa fragments (Soldani and Scovassi 2002). The results of cleaved PARP-1 (89 kDa fragment) followed almost the same pattern of expression as the cleaved caspase-3 (Fig. 6). These results further confirmed the activation of caspase-dependent apoptosis in the met-estrous female ischemic group, and the beneficial role of gemfibrozil pretreatment via inhibition of this pathway. Interestingly, while gemfibrozil pretreatment protected met-estrous females of the present study, it worsened the males’ condition through activation of caspase-dependent apoptosis.
Western blot analysis to measure the expression of cleaved PARP-1 in the hippocampus tissues derived at 72 h after 10 min global cerebral ischemia in male and female experimental groups. a Immunoblot bands of cleaved PARP-1 and β-actin. Changes in the expression of 89 kDa fragment of cleaved PARP-1 are considered. b The densities of corresponding bands were measured, and the ratio to β-actin was calculated and represented as arbitrary units on the graph for each experimental group (n = 6). Bars indicate the mean ± SEM; one-way ANOVA; **P < 0.01; ***P < 0.001. Abbreviations used in graphs for experimental groups: SM sham male, IM ischemic male, TM treatment male, SF sham female, IF ischemic female, TF treatment female
Caspase-Independent Apoptosis
Nuclear AIF Expression
Nuclear translocation of AIF is a sign of activated caspase-independent apoptotic cell death (Matsumori et al. 2005; Carre et al. 2010). Therefore, in this study, we measured the nuclear expression of AIF using Western blot analysis. As represented in Fig. 7, nuclear expression of AIF in the male ischemic group was not affected by comparison to sham groups, however, AIF expression was induced significantly in ischemic met-estrous female rats compared with their respective sham group (P < 0.01). These results support the activation of caspase-independent apoptotic pathway in ischemic conditions within met-estrous female rats.
Western blot analysis to measure the expression of AIF in nuclear fraction of hippocampus tissues derived at 72 h after 10 min global cerebral ischemia in male and female rats. a Immunoblot bands of AIF and Lamin B. b The densities of corresponding bands were measured, and the ratio to Lamin B was calculated and represented as arbitrary units on the graph for each experimental group (n = 5). Bars indicate the mean ± SEM; one-way ANOVA; *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations used in graphs for experimental groups: SM sham male, IM ischemic male, TM treatment male, SF sham female, IF ischemic female, TF treatment female
The expression of nuclear AIF in gemfibrozil-pretreated males was consistent with our previous observations of neurodegeneration, such that it enhanced apoptosis compared with both male sham (P < 0.05) and ischemic groups (P < 0.05).
On the other hand, results of nuclear AIF expression within met-estrous female gemfibrozil pretreated groups contradicted our previous findings such that, by treating met-estrous female rats with gemfibrozil, we observed an enhancement of nuclear AIF expression in comparison to both the ischemic (P < 0.05) and sham groups (P < 0.05).
MAPKs Signaling Pathway
To further unravel the molecular mechanisms of the sexually dimorphic outcome of gemfibrozil pretreatment, we examined the expression of p38 MAPK, ERK1/2, and JNK and their phosphorylated states using Western blot analysis. Although controversial, it has been shown that activation of p38 MAPK and JNK mostly trigger apoptotic cell death (Sugino et al. 2000; Ma et al. 1999). On the other hand, ERK1/2 activation has been demonstrated to have anti-apoptotic properties in I/R conditions.
P38 MAPK Activation
The ratio of phosphorylated (p)-p38/p38 followed a similar pattern of expression in experimental groups of both sexes. P38 MAPK expression was induced in the ischemic groups compared with sham, however, we observed a decreased expression within gemfibrozil-pretreated males and met-estrous females (P < 0.05 and P < 0.001, respectively) compared with their respective ischemic groups. It was noticeable that the same pattern of expression in the experimental groups of both genders was associated with two different outcomes: neurodegeneration in males and neuroprotection in met-estrous females (Fig. 8).
Western blot analysis to measure the expression of activated p38 (the ratio of phosphorylated (p)-p38 to p38) in hippocampus tissues derived at 72 h after 10 min global cerebral ischemia in male and female experimental groups. a Immunoblot bands of p-p38, p38, and β-actin. b The densities of corresponding bands were measured, and the ratio of activated p38 to β-actin was calculated and represented as arbitrary units on the graph for each experimental group (n = 6). Bars indicate the mean ± SEM; one-way ANOVA; *P < 0.05; ***P < 0.001. Abbreviations used in graphs for experimental groups: SM sham male, IM ischemic male, TM treatment male, SF sham female, IF ischemic female, TF treatment female
JNK Activation
The ratio of p-JNK/JNK was not affected in any male experimental groups, as shown in Fig. 9. However, dietary administration of gemfibrozil for 7 days prior to induction of global cerebral ischemia in females attenuated phosphorylated levels of this protein significantly (P < 0.05).
Western blot analysis to measure the expression of activated JNK (the ratio of phosphorylated (p)-JNK to JNK) in hippocampus tissues derived at 72 h after 10 min global cerebral ischemia in male and female experimental groups. a Immunoblot bands of p-JNK, JNK, and β-actin. b The densities of corresponding bands were measured, and the ratio of activated JNK to β-actin was calculated and represented as arbitrary units on the graph for each experimental group (n = 6). Bars indicate the mean ± SEM; One-way ANOVA; *P < 0.05. Abbreviations used in graphs for experimental groups: SM sham male, IM ischemic male, TM treatment male, SF sham female, IF ischemic female, TF treatment female
ERK1/2 Activation
The expression ratio of p-ERK1/2/ ERK1/2 further supported our observed sexual dimorphism between the sexes. In males, I/R injury led to a slight but not significant increase in this ratio. However, the ratio decreased significantly when gemfibrozil was used prior to I/R (P < 0.01). In contrast, within female rats, a significant reduction of the p-ERK1/2/ ERK1/2 ratio was detected compared with the respective sham groups (P < 0.05). In addition, this reduction was reversed significantly within the female gemfibrozil pretreated group (P < 0.001) (Fig. 10).
Western blot analysis to measure the expression of activated ERK1/2 (the ratio of phosphorylated (p)-ERK1/2 to ERK1/2) in hippocampus tissues derived at 72 h after 10 min global cerebral ischemia in male and female experimental groups. a Immunoblot bands of p-ERK1/2, ERK1/2, and β-actin. b The densities of corresponding bands were measured, and the ratio of activated ERK1/2 to β-actin was calculated and represented as arbitrary units on the graph for each experimental group (n = 6). Bars indicate the mean ± SEM; one-way ANOVA; *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations used in graphs for experimental groups: SM sham male, IM ischemic male, TM treatment male, SF sham female, IF ischemic female, TF treatment female
Discussion
The novel findings of this study are as follows:
-
1.
TUNEL assay confirmed that the CA1 region of the hippocampus was more susceptible to ischemia and showed extensive apoptotic cell death within met-estrous female rats after 10 min of ischemia compared with male rats, which were almost resistant to injury.
-
2.
Gemfibrozil pretreatment led to a sexually dimorphic outcome conferring protection to met-estrous females against global cerebral I/R insult, while detrimental within male rats resulting in hippocampal CA1 neurodegeneration.
-
3.
Gemfibrozil induced the expression of NRF-1 and TFAM; mitochondrial pro-survival factors in the mitochondrial biogenesis-signaling pathway within the hippocampus of met-estrous females, while repressing them within males.
-
4.
Gemfibrozil also modulated apoptotic cell death pathways, as well as JNK and ERK1/2, the upstream MAPKs, in a sexually dimorphic manner; in met-estrous females, it inhibited caspase-dependent apoptosis, while in males it induced both caspase-dependent and caspase-independent apoptosis.
Petito et al. found a significant percentage of apoptotic neurons in CA1 region of male rats after 10 min of 4VO I/R injury (Petito et al. 1997). We expected the same results in our male rats as well. But, in parallel to another similar study performed in our lab (Mohagheghi et al. 2012), we observed that despite flattening of EEG, as a confirmation of ischemia induction in these animals, there was not any noticeable morphologic damage in our male ischemic group at least at this time point. However, significant induction of PGC-1α and NRF-1 and reduction of TFAM, as well as modulation of cleaved PARP-1 and ERK1/2 detected in ischemic males compared with the sham indicated that molecular pathways were in fact influenced by I/R injury; however, their morphologic representation was not detectable, at least at this time point. Time course analyses are certainly required to provide evidences for such cause–effect relationships and to decide whether the observed results are just endpoints simply correlating with the damage or protection.
Significantly higher susceptibility of met-estrous females to the same I/R injury compared with males was in parallel to our previous study, and the report of Carswell et al. (1999), showing consistently that female stroke-prone spontaneously hypertensive rats which underwent ischemia during met-estrous developed larger infarcts than their respective males in MCAO model of stroke (Carswell et al. 1999). Currently in our lab, we are examining conditions that provide sublethal injury in female rats (shorter ischemia) and more severe injury in males (to induce substantial damage).
Mitochondrial biogenesis is enhanced in cells under different conditions such as during high energy demand (Wu et al. 2002; Handschin et al. 2003) and in response to increased oxidative stress (Wei et al. 2001; Onyango et al. 2010). According to recent evidences, mitochondrial biogenesis is altered by mitochondrial dysfunction in many pathological settings (Yin et al. 2008; Onyango et al. 2010), and its stimulation is suggested to be neuroprotective in conditions such as brain ischemic stroke (Lagouge et al. 2006; Dong et al. 2007; Wenz 2009).
Coordinated actions of nuclear and mitochondrial genomes and transcription factors, specifically PGC-1α, NRF-1, and TFAM regulate mitochondrial biogenesis (Wu et al. 1999). In our study, the expression of PGC-1α and NRF-1 in ischemic groups of both sexes were induced compared with their respective sham groups. Induction of PGC-1α in response to oxidative stress and ischemic insult has been demonstrated previously (Yin et al. 2008; Onyango et al. 2010). However, the expression of TFAM, the downstream protein in mitochondrial biogenesis signaling pathway, showed an appreciable sexually dimorphic behavior in sham and ischemic groups of both sexes. Justo et al. have reported sort of relationship between estradiol and TFAM level, such that protein levels of TFAM were four times greater in the liver of females rats selected randomly in their estrous cycle, compared with males (Justo et al. 2005). Estradiol level in met-estrous female rats is lower than when they are selected randomly (Carswell et al. 1999). Hence, with low levels of estradiol in female sham group of this study, a low level of TFAM expression is evident, which may explain the sensitivity of females to ischemia compared with males during the met-estrous period of the female reproductive cycle. This is the first report indicating that gemfibrozil pretreatment in met-estrous females induced NRF-1 and TFAM significantly compared with the sham and ischemic groups while, in males, it repressed their expression.
It was of interest that PGC-1α did not follow the same pattern of NRF-1 and TFAM expression in the mitochondrial biogenesis signaling pathway. Gustaeva et al. have claimed that mitochondrial biogenesis-signaling factors follow a time-dependent pattern of expression after hypoxia. mRNA expression of PGC-1α and NFR-1 in the mice brain subcortex increased after 6 h of hypoxia, remaining elevated at 24 h and returned to control level by 48 h. At the same context, TFAM mRNA expression increased at the end of hypoxia, remained elevated at 24 h, and returned to baseline by 48 h (Gutsaeva et al. 2008). In another report, induction of transient global ischemia in rats resulted in augmentation of PGC-1α expression at 1 h, as well as mitochondrial number at 4 h after transient global ischemia in the hippocampal CA1 subfield (Chen et al. 2010b).
Experiments of the present study were performed at 72 h after induction of global cerebral ischemia. Therefore, one assumption which obviously needs to be demonstrated is that gemfibrozil pretreatment may have influenced the expression of PGC-1α transiently in earlier time points. Subsequently, it has resulted in a more persistent modulation of NRF-1 and TFAM which were still detectable at the time point of our study. The other interpretation is that gemfibrozil pretreatment probably modulate mitochondrial biogenesis-signaling pathway in both sexes without affecting PGC-1α expression. It is reported that, in some cases, induction of mitochondrial biogenesis is not dependent on PGC-1α. For example, estradiol has been able to stimulate transcription of NRF-1 and increase mitochondrial biogenesis through a direct transcriptional effect mediated by ER-α in cancer cell lines (Mattingly et al. 2008). Also, regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors has not been dependent on PGC-1α, but NRF-1 is instead the main player (Chen et al. 2009).
In this study, we have just examined the expression pattern of three major mitochondrial pro-survival proteins involved in the mitochondrial biogenesis signaling pathway. So with these limited data, we cannot make a firm conclusion regarding mitochondrial biogenesis. Undoubtedly, looking at mitochondrial structure, dynamic or function using other approaches will be required to further confirm whether induction of NRF-1 and TFAM by gemfibrozil in met-estrous females and their suppression in males undergoing I/R injury has been connected to the induction or suppression of mitochondrial mass and number as well as other indicators of mitochondrial biogenesis.
Considering several studies indicating to a sex-dependent difference in apoptotic neural cell death pathways, notably in the cerebral ischemic context (Zhang et al. 2003; Park et al. 2005; Zhu et al. 2006), and in order to assess the therapeutic potential of gemfibrozil in such context, we also looked at apoptotic cell death in our experimental groups. Extensive susceptibility of met-estrous females revealed by the high percentage of TUNEL-positive neurons was further confirmed by the significantly higher expression of both cleaved caspase-3 and PARP-1, as well as nuclear AIF in ischemic females compared with sham, suggesting activation of both caspase-dependent and caspase-independent apoptosis in their hippocampus.
Parallel to the TUNEL assay results, pretreatment of groups with gemfibrozil led to induction of caspase-dependent and –independent apoptosis in males after I/R insult whilst, in met-estrous females, gemfibrozil proved to be protective partially via inhibition of the caspase-dependent apoptotic pathway. Since caspase-dependent and caspase-independent pathways are almost interrelated (Cregan et al. 2004), the significant induction of AIF observed in gemfibrozil-pretreated met-estrous females may result from inhibition of the caspase-dependent pathway, leading to the induction of the caspase-independent pathway. It is reported previously that caspase-dependent apoptotic pathway is the major pathway leading to cell death in females. Therefore, it seems that, in gemfibrozil-pretreated females of our study, ischemia was not detrimental due to gemfibrozil interference of caspase-independent pathway (Lang and McCullough 2008).
MAPKs pathway including JNK, ERK1/2, and p38, at the upstream of both mitochondrial biogenesis and cell death pathways, are reported to be stimulated following ischemia (Irving and Bamford 2002; Wright 2007; Lira et al. 2010). Extracellular signal-regulated kinases pathways promote cell survival and proliferation, and c-Jun N-terminal protein kinases/p38 pathways induce apoptosis in general. However, the roles of MAPK cascades in neuronal death and survival seem to be complicated and altered by the type of cells and the magnitude and timing of insults (Nozaki et al. 2001).
P38 is suggested to regulate the expression of PGC-1a, stabilizing this protein and increasing its ability to function as a coactivator in the mitochondrial biogenesis signaling pathway. In fact, mitochondrial biogenesis following oxidant injury is reported to be mediated by p38 activation of PGC1-a (Rasbach and Schnellmann 2007).
There is another study providing evidence that MAPKs including ERK promote mitochondrial biogenesis in part through PGC-1β expression (Gao et al. 2011). JNK and ERK are also demonstrated to contribute in the Nrf-2 mediated induction of mitochondrial biogenesis and the subsequent neuroprotective effects exerted in the context of Parkinson’s disease (Tufekci et al. 2011).
Based on studies suggesting gender-related differences in MAPKs activation and their possible contribution to sexual dimorphisms in the brain (Zhang 2002), we also sought to investigate how MAPKs are affected in the context of the present study.
While the expression of JNK was not affected in most of experimental groups, its significant reduction in gemfibrozil-pretreated met-estrous females was consistent with the protection observed in this group. JNK is reported to be involved in neuronal apoptosis (Sugino et al. 2000). The expression of activated p38 followed a similar pattern within the met-estrous female and male groups. Activated p38 is demonstrated to be associated with apoptosis (Sugino et al. 2000). While alteration of activated p38 expression in female experimental groups was consistent with the protective effects of gemfibrozil pretreatment in females, this was not the case with males. Such difference may be due to p38 not taking part in the observed neuroprotection/degeneration, or activated p38 may have activated opposite pathways in both genders. The precise molecular mechanism involved in conferring either protection or degeneration with the same pattern of activated p38 expression within males and females need to be further investigated.
Our findings regarding the expression of ERK1/2 in the experimental groups of both sexes were also consistent with the known role of ERK1/2 in promoting cell survival (Irving and Bamford 2002; Nozaki et al. 2001). Such sex-dependent expression of ERK1/2 in this study is in accordance with reports indicating that ERK1/2 activation is affected by estrogen and that ERK inhibitors have removed neuroprotection of estrogens (Belayev et al. 1996; Lebesgue et al. 2009).
Interestingly, the molecules investigated here in the global cerebral I/R context, including NRF-1 and TFAM, the mitochondrial pro-survival factors in the mitochondrial biogenesis signaling pathway, caspase-3 and PARP-1 in the apoptotic cell death pathway, as well as ERK1/2 and JNK in the MAPKs signaling pathway, in accordance with the TUNEL assay, all indicate to the sexually dimorphic effects of gemfibrozil, being neuroprotective in met-estrous females while neurotoxic in males. Undoubtedly, further investigations are necessary to clarify the exact causal and mechanistic links between the mitochondrial pro-survival factors, as well as the apoptotic cell death and MAPKs signaling pathways detected here.
In 1976, Dr. Kurtz and his colleagues performed an extensive toxicological study on gemfibrozil, mainly focusing on the liver. They reported remarkable mitochondrial enlargement with variable distortion of their normal cristae in hepatocytes. The alterations of liver weights and the hepatocellular hypertrophy in response to gemfibrozil consumption were found to be more prominent in males than females (Kurtz et al. 1976). Another study provides interesting evidence indicating that individual fibrates induce mitochondrial dysfunction via different molecular mechanisms. Gemfibrozil is suggested to induce respiratory function impairment due to opening of the mitochondrial transition pore. It remains to be clarified to what extent direct mitochondrial actions contribute to the beneficial and the adverse effects associated with clinical fibrate administration (Brunmair et al. 2004).
There are a few studies indicating that pharmacokinetic parameters of gemfibrozil including its absorption, metabolism, and excretion are not affected by gender in human and animals (Cayen 1985; Knauf et al. 1990; Dix et al. 1999; Borges et al. 2005). In the present study, we did not perform a pharmacokinetic analysis to determine whether the differential sex-dependent effects of gemfibrozil were related to different effects of gemfibrozil in males versus females, or to its pharmacokinetic issues. However, due to extremely limited information which exists on the absorption, distribution, metabolism, and excretion of gemfibrozil, this issue awaits to be examined.
Considering that fibrates and other PPAR-α activators have been proved to affect metabolism of steroid hormones (Xu et al. 2001a, b, c; Fan et al. 2004; Lebesgue et al. 2009) and the close interrelation which exist between ER-α, PGC-1α, and PPAR-α in the mitochondrial biogenesis signaling pathway (Huss et al. 2004), in our lab, we have started investigating whether the sex-dependent outcome of gemfibrozil pretreatment in global cerebral I/R context regarding mitochondrial biogenesis signaling pathway, cell death, and JNK and ERK1/2 of MAPKs is mediated via estrogen- and testosterone-dependent mechanisms. Figure 11 summarizes the molecular changes detected in the context of present study.
The graphical abstract represents major changes of the studied molecules affected by global cerebral ischemia–reperfusion and gemfibrozil pretreatment, resulting in the final outcome of male neurotoxicity, female neuroprotection in the CA1 region of hippocampus; plus sign induction; minus sign reduction
Abbreviations
- AIF:
-
Apoptosis-inducing factor
- DAB:
-
Diaminobenzidine
- ER-α:
-
Estrogen-related receptor-α
- ERK1/2:
-
Extracellular signal-regulated kinases
- I/R:
-
Ischemia–reperfusion
- JNK:
-
C-Jun N-terminal kinases
- MAPK:
-
Mitogen-activated protein kinase
- MCAO:
-
Middle cerebral artery occlusion
- mtDNA:
-
Mitochondrial DNA
- NRF:
-
Nuclear respiratory factor
- PARP:
-
Poly (ADP-ribose) polymerase
- PBS:
-
Phosphate-buffered saline
- PGC-1α:
-
Peroxisome proliferator-activated receptor coactivator-1α
- TFAM:
-
Mitochondrial transcription factor A
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling
- 4VO:
-
Four-vessel occlusion model
References
Azcoitia I, DonCarlos LL, Garcia Segura LM (2003) Are gonadal steroid hormones involved in disorders of brain aging. Aging Cell 2:31–37
Baar K (2004) Involvement of PPARg co-activator-1, nuclear respiratory factors 1 and 2, and PPARa in the adaptive response to endurance exercise. Proc Nutr Soc 63:269–274
Belayev L, Ginsberg MD, Alonso OF, Singer JT, Zhao W, Busto R (1996) Bilateral ischemic tolerance of rat hippocampus induced by prior unilateral transient focal ischemia: relationship to c-fos mRNA expression. Neuroreport 8:55
Bordet R, Ouk T, Petrault O et al (2006) PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans 34:1341
Borges NC, Mendes GD, Barrientos-Astigarraga RE, Zappi E, Mendes FD, De Nucci G (2005) Comparative bioavailability study with two gemfibrozil tablet formulations in healthy volunteers. Arzneimittelforschung 55:38–386
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brunmair B, Lest A, Staniek K et al (2004) Fenofibrate impairs rat mitochondrial function by inhibition of respiratory complex I. J Pharmacol Exp Ther 311:109–114
Carre JE, Orban JC, Re L et al (2010) Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med 182:745–751
Carswell HVO, Anderson NH, Clark JS (1999) Genetic and gender influences on sensitivity to focal cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension 33:681–685
Cayen MN (1985) Disposition, metabolism and pharmacokinetics of hyperlipidemic agents in laboratory animals and man. Pharmacotherapy 29:157
Chen H, Hu CJ, He YY, Yang DI, Xu J, Hsu CY (2001) Reduction and restoration of mitochondrial DNA content after focal cerebral ischemia/reperfusion. Stroke 32:2382–2387
Chen JQ, Cammarata PR, Baines CP, Yager JD (2009) Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim Biophys Acta 1793:1540–1570
Chen SD, Lin TK, Lin JW et al (2010a) Activation of calcium/calmodulin dependent protein kinase IV and peroxisome proliferator activated receptor coactivator 1 signaling pathway protects against neuronal injury and promotes mitochondrial biogenesis in the hippocampal CA1 subfield after transient global ischemia. J Neurosci Res 88(14):3144–3154
Chen SD, Lin TK, Yang DI et al (2010b) Protective effects of peroxisome proliferator activated receptors coactivator 1 against neuronal cell death in the hippocampal CA1 subfield after transient global ischemia. J Neurosci Res 88:605–613
Cregan SP, Dawson VL, Slack RS (2004) Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 23:2785–2796
Deplanque D, Gelé P, Pétrault O et al (2003) Peroxisome proliferator-activated receptor- activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci 23:6264–6271
Diler A, Ziylan Y, Uzum G, Lefauconnier J, Seylaz J, Pinard E (2002) Passage of spermidine across the blood–brain barrier in short recirculation periods following global cerebral ischemia: effects of mild hyperthermia. Neurosci Res 43:335–342
Dix KJ, Coleman DP, Jeffcoat AR (1999) Comparative metabolism and disposition of gemfibrozil in male and female Sprague–Dawley rats and Syrian golden hamsters. Drug Metab Dispos 27:138146
Dong W, Gao D, Zhang X (2007) Mitochondria biogenesis induced by resveratrol against brain ischemic stroke. Med Hypotheses 69:700–701
Du L, Bayir H, Lai Y (2004) Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem 279:38563–38570
Fan LQ, You L, Brown-Borg H, Brown S, Edwards RJ, Corton JC (2004) Regulation of phase I and phase II steroid metabolism enzymes by PPAR [alpha] activators. Toxicol 204:109–121
Gao M, Wang J, Lu N, Fang F, Liu J, Wong CW (2011) Mitogen-activated protein kinase kinases promote mitochondrial biogenesis in part through inducing peroxisome proliferator-activated receptor [gamma] coactivator-1 [beta] expression. Biochim Biophys Acta 1813(6):1239–1244
Garcia-Bueno B, Madrigal JLM, Pérez-Nievas BG, Leza JC (2008) Stress mediators regulate brain prostaglandin synthesis and peroxisome proliferator-activated receptor-activation after stress in rats. Endocrinol 149:1969–1978
Guo Q, Wang G, Liu X, Namura S (2009) Effects of gemfibrozil on outcome after permanent middle cerebral artery occlusion in mice. Brain Res 1279:121–130
Gutsaeva DR, Carraway MS, Suliman HB et al (2008) Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci 28:2015–2024
Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM (2003) An autoregulatory loop controls peroxisome proliferator-activated receptor coactivator 1 expression in muscle. Proc Nat Acad Sci 100:7111–7116
Hariz GM, Lindberg M, Hariz MI, Tommy Bergenheim A (2003) Gender differences in disability and health related quality of life in patients with Parkinson's disease treated with stereotactic surgery. Acta Neurol Scand 108:28–37
Hurn PD, Macrae IM (2000) Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab 20:631–652
Huss JM, Torra IP, Staels B, Giguere V, Kelly DP (2004) Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 24:9079–9091
Irving EA, Bamford M (2002) Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab 22:631–647
Jalalvand E, Javan M, Haeri-Rohani A, Ahmadiani A (2008) Stress- and non-stress-mediated mechanisms are involved in pain-induced apoptosis in hippocampus and dorsal lumbar spinal cord in rats. Neurosci 157:446–452
Jänicke RU, Sprengart ML, Wati MR, Porter AG (1998) Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 273:9357–9360
Justo R, Boada J, Frontera M, Oliver J, Bermúdez J, Gianotti M (2005) Gender dimorphism in rat liver mitochondrial oxidative metabolism and biogenesis. Am J Physiol Cell Physiol 289C372-8
Knauf H, Kölle EU, Mutschler E (1990) Gemfibrozil absorption and elimination in kidney and liver disease. Klin Wochenschr 68:692–698
Kurtz S, Fitzgerald J, Fisken R, Schardein J, Reutner T, Lucas J (1976) Toxicological studies on gemfibrozil. Proc R Soc Med 69:15–23
Lagouge M, Argmann C, Gerhart-Hines Z (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 [alpha]. Cell 127:1109–1122
Lang J, McCullough L (2008) Pathways to ischemic neuronal cell death: are sex differences relevant. J Transl Med 6:33
Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM (2009) Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids 74:555–561
Lira VA, Benton CR, Yan Z, Bonen A (2010) PGC-1 regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab 299:E14561
Ma XL, Kumar S, Gao F et al (1999) Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation 99:1685–1691
Marcondes F, Bianchi F, Tanno A (2002) Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62:609–614
Matsumori Y, Hong SM, Aoyama K et al (2005) Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab 25:899–910
Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM (2008) Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol 22:609–622
McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn P (2005) Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab 25:502–512
Mohagheghi F, Khalaj L, Ahmadiani A, Rahmani B (2012) Gemfibrozil pretreatment affecting antioxidant defense system and inflammatory, but not nrf-2 signaling pathways resulted in female neuroprotection and male neurotoxicity in the rat models of global cerebral ischemia–reperfusion. Neurotox Res. doi:10.1007/s12640-012-9338-3
Nadal-Casellas A, Amengual-Cladera E, Proenza A, Lladó I, Gianotti M (2010) Long-term high-fat-diet feeding impairs mitochondrial biogenesis in liver of male and female rats. Cell Physiol Biochem 26:291–302
Nagai Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kashiwagi A (2002) Amelioration of high fructose-induced metabolic derangements by activation of PPAR. Am J Physiol Endocrinol Metab 282:E1180–E1190
Niimura M, Takagi N, Takagi K et al (2006) Prevention of apoptosis-inducing factor translocation is a possible mechanism for protective effects of hepatocyte growth factor against neuronal cell death in the hippocampus after transient forebrain ischemia. J Cereb Blood Flow Metab 26:1354–1365
Nozaki K, Nishimura M, Hashimoto N (2001) Mitogen-activated protein kinases and cerebral ischemia. Mol Neurobiol 23:1–19
Nuñez JL, Lauschke DM, Juraska JM (2001) Cell death in the development of the posterior cortex in male and female rats. J Comp Neurol 436:32–41
Onyango IG, Lu J, Rodova M, Lezi E, Crafter AB, Swerdlow RH (2010) Regulation of neuron mitochondrial biogenesis and relevance to brain health. Biochim Biophys Acta 1802:228–234
Park EM, Cho S, Frys KA et al (2005) Inducible nitric oxide synthase contributes to gender differences in ischemic brain injury. J Cereb Blood Flow Metab 26:392–401
Petito CK, Torres-Munoz J, Roberts B, Olarte JP, Nowak TS, Pulsinelli WA (1997) DNA fragmentation follows delayed neuronal death in CA1 neurons exposed to transient global ischemia in the rat. J Cereb Blood Flow Metab 17:967–976
Pulsinelli WA, Brierley JB (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 10:267–272
Rasbach KA, Schnellmann RG (2007) Signaling of mitochondrial biogenesis following oxidant injury. J Biol Chem 282:2355–2362
Rodríguez-Cuenca S, Monjo M, Gianotti M, Proenza AM, Roca P (2007) Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17-estradiol, testosterone, and progesterone. Am J Physiol Endocrinol Metab 292:E340–E346
Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68:320–344
Sacco RL (2001) Newer risk factors for stroke. Neurol 57:S31–S34
Soldani C, Scovassi A (2002) Poly (ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis 7:321–328
St-Pierre J, Drori S, Uldry M et al (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408
Sugino T, Nozaki K, Takagi Y et al (2000) Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J Neurosci 20:4506–4514
Tufekci KU, Civi Bayin E, Genc S, Genc K (2011) The Nrf2/ARE pathway: a promising target to counteract mitochondrial dysfunction in Parkinson’s disease. Parkin Dis 2011:314082
Van Den Eeden SK, Tanner CM, Bernstein AL et al (2003) Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 157:1015–1022
Vega RB, Huss JM, Kelly DP (2000) The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20:1868–1876
Wang G, Liu X, Guo Q, Namura S (2010) Chronic treatment with fibrates elevates superoxide dismutase in adult mouse brain microvessels. Brain Res 1359:247–255
Wei YH, Lee CF, Lee HC (2001) Increases of mitochondrial mass and mitochondrial genome in association with enhanced oxidative stress in human cells harboring 4,977 BP deleted mitochondrial DNA. Ann N Y Acad Sci 928:97–112
Wenz T (2009) PGC 1 activation as a therapeutic approach in mitochondrial disease. IUBMB Life 61:1051–1062
Wenz T, Diaz F, Spiegelman BM, Moraes CT (2008) Activation of the PPAR/PGC-1 [alpha] pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab 8:249–256
Wright DCWDC (2007) Mechanisms of calcium-induced mitochondrial biogenesis and GLUT4 synthesis. Appl Physiol Nutr Metab 32:840–845
Wu H, Kanatous SB, Thurmond FA et al (2002) Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296:349–352
Wu Z, Puigserver P, Andersson U (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124
Xu J, Racke MK, Drew PD (2007) Peroxisome proliferator activated receptor agonist fenofibrate regulates IL 12 family cytokine expression in the CNS: relevance to multiple sclerosis. J Neurochem 103:1801–1810
Xu LL, Su YP, Labiche R (2001a) Quantitative expression profile of androgen regulated genes in prostate cancer cells and identification of prostate specific genes. Intl J Cancer 92:322–328
Xu S, Zhu BT, Cai MX, Conney AH (2001b) Stimulatory effect of clofibrate on the action of estradiol in the mammary gland but not in the uterus of rats. J Pharmacol Exp Ther 297:50–56
Xu S, Zhu BT, Turan V et al (2001c) PPAR -dependent induction of liver microsomal esterification of estradiol and testosterone by a prototypical peroxisome proliferator. Endocrinol 142:3554–3557
Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J (2008) Rapidly increased neuronal mitochondrial biogenesis after hypoxic–ischemic brain injury. Stroke 39:3057–3063
Zhang L (2002) Sex-related differences in MAPKs activation in rat astrocytes: effects of estrogen on cell death. Mol Brain Res 103:1–11
Zhang L, Li PP, Feng X, Barker JL, Smith SV, Rubinow DR (2003) Sex-related differences in neuronal cell survival and signaling in rats. Neurosci Lett 337:65–68
Zhu C, Xu F, Wang X et al (2006) Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia–ischaemia. J Neurochem 9:1016–1027
Acknowledgment
We thank the research council of Shahid Beheshti University of Medical Sciences for the funding of this project. The authors declare that there are no competing and conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohagheghi, F., Ahmadiani, A., Rahmani, B. et al. Gemfibrozil Pretreatment Resulted in a Sexually Dimorphic Outcome in the Rat Models of Global Cerebral Ischemia–Reperfusion via Modulation of Mitochondrial Pro-survival and Apoptotic Cell Death Factors as well as MAPKs. J Mol Neurosci 50, 379–393 (2013). https://doi.org/10.1007/s12031-012-9932-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-012-9932-0