Abstract
The neurotrophic effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on rat sensory neuronal cell line ND7/23 cells were investigated. PACAP caused a concentration-dependent increase in the number of neurite-bearing cells and the expression of the substance P precursor (PPT) mRNA in 24 h. The effects of PACAP were mimicked by vasoactive intestinal polypeptide with lower potency and dibutyryl-cyclic AMP, and inhibited by inhibitors of protein kinase A, ERK kinase or p38 kinase, KT5720, U0126, or SB203580, respectively. In a PPT promoter luciferase reporter assay, the increase of PPT mRNA was the result of an increase in PPT gene transcriptional activity by PACAP. The increasing effects of PACAP on PPT mRNA were similarly observed in primary cultured rat dorsal root ganglion cells. Thus, PACAP could induce differentiation-like phenomena in sensory neurons in a cAMP-, protein kinase A-, ERK kinase-, and p38 kinase-dependent manner. These results provide evidence of the neurotrophic action of PACAP, which may function to rescue damaged neurons or to switch the neuronal phenotype in injured or inflamed sensory neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a 38-amino acid peptide that belongs to the vasoactive intestinal popypeptide (VIP)/secretin family of bioactive peptides. PACAP is widely distributed in the body, particularly in the central and peripheral nervous systems, and binds specific receptors, PAC1, VPAC1, and VPAC2, resulting in the activation of adenylate cyclase, mitogen-activated protein kinases (MAPKs) and, in the case of PAC1, phospholipase C (Vaudry et al. 2009). VIP has a similar sequence to PACAP with 68 % identity and can bind PAC1 with much lower affinity and VPAC1 and VPAC2 with the same affinity as PACAP. PACAP acts as a neurotransmitter, a neuromodulator or a neurotrophic factor regulating neuronal survival after neuronal trauma, neurotransmitter phenotype, axon growth, and growth cone attraction through these receptors. PACAP is expressed in the embryonic rat dorsal root ganglion (DRG), regulates the differentiation of nascent DRG neurons (Nielsen et al. 2004), promotes the survival and neurite outgrowth of some DRG neurons, and regulates their expression of calcitonin gene-related peptide (Lioudyno et al. 1998). Exogenously applied PACAP reduces cell death in embryonic chick DRG neurons (Arimura et al. 1994). The expression of PACAP is upregulated in rat sensory neurons following sciatic nerve transection (Jongsma et al. 2000), following nerve compression injury (Pettersson et al. 2004), or following unilateral adjuvant-induced inflammation in rats (Jongsma et al. 2003). These numerous studies suggest its neurotrophic roles in the prevention of cell death and the promotion of neurogenesis or neuronal regeneration.
Substance P (SP), an undecapeptide, has been shown to act as a neurotransmitter in primary afferent sensory neurons (Fernandes et al. 2009). This molecule is released from central and peripheral nerve terminals by the activation of small diameter primary afferent sensory neurons. It relays noxious signals to the spinal cord in the central terminals while augmenting inflammatory responses in peripheral tissues (Richardson and Vasko 2002), where bioactive substances such as SP, released from sensory neurons and acting on target cells in the periphery, such as mast cells, immune cells, and vascular endothelial cells, produce inflammatory events such as vasodilation and plasma extravasation. Thus, neurogenic inflammation is characterized by redness, warmth, and swelling and, in turn, must induce the hypersensitivity of certain sensory neurons.
Hypersensitivity of sensory neurons is induced by alterations in the excitability of neurons resulting in not only the accumulation of peripheral neurogenic inflammation but also the relay of much more pain information centrally. Some neuronal events to alter excitability reflect the mechanisms involved in regulating biosynthesis and the release of neurotransmitters. Inflammation increases the amount of SP in rabbit synovial fluid (O’Byme et al. 1991) and the synthesis of SP in rat sensory neurons (Calza et al. 1998), which might be mediated by several inflammatory mediators. These mediators have been reported to evoke the release or facilitate the evoked release of SP (Inoue et al. 1999; Grider 2003) and must facilitate the biosynthesis of SP to induce facilitated release. In the soma of primary afferent sensory neurons in the spinal DRG, SP is synthesized through the transcription of three types of mRNAs encoding α-, β-, and γ-precursor preprotachykinins (PPTs) derived from one PPT-I (also referred to as PPT-A or TAC1) gene (Krause et al. 1987); however, the effect of inflammatory mediators on the biosynthesis of SP remains obscure because the cellular regulatory mechanisms of biosynthesis are under investigation. One of the reasons for this disadvantage in research is that most cell lines do not express endogenous SP. Recently, Calin-Jageman et al. (2006) found that PPT gene expression is regulated through a protein kinase A-dependent but cyclic AMP response element-binding protein-independent mechanism in rat insulinoma cell line RINm5F cells, which have been shown to express endogenous SP. However there are few reports about the regulatory mechanisms of the biosynthesis of SP in useful neuronal cell lines, which may be involved in different transcriptional methods with cell- or stimulus-specific characteristics.
ND7/23 cells are one of the cell lines derived by cell fusion of N18Tg2 mouse neuroblastoma and DRG neurons from neonatal rats (Wood et al. 1990). ND7/23 cells have been shown to have sensory neuron-like properties and differentiated by the inhibition of mitosis and the extension of several long processes in cAMP-containing media, similar to those produced by DRG neurons in vitro (Suburo et al. 1992). One report detected SP in ND7/23 by radioimmunoassay (Wood et al. 1990), but another could not detect SP even in a differentiation state, which might be due to the limits of immunocytochemical detection (Suburo et al. 1992). DRG neurons have the property of plasticity, a phenotype switch in a subpopulation of neurons so that they can change the level of SP, resulting from differentiation or changes in cellular conditions such as inflammation (Neumann et al. 1996). Thus, it is interesting to investigate the potential change in the expression of SP in ND7/23 cells to identify the biological regulatory mechanisms involved in the biosynthesis of SP.
In this study, we investigated whether PACAP increased SP expression in ND7/23 cells, which may differentiate to the neuronal phenotype, involving the reorganization of cellular components and the extensive network of long cellular processes, characteristics of neurons, by a protein kinase A-dependent pathway.
Materials and Methods
Cell Culture and Drug Treatment
ND7/23 cells were maintained at 37°C in a humidified atmosphere of 5 % CO2 and cultured in Dulbecco's modified eagle's medium (DMEM) supplemented with 10 % fetal bovine serum (Biosera, East Sussex, UK), 100 units/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Tokyo, Japan). Cells were passaged about twice a week. All the cells used in this study were used at a low passage number (<20). In experiments, ND7/23 cells were plated in 35-mm diameter dishes at a density of 105 cells for 1 day, and the serum concentration in the same medium was reduced to 0.5 % before drug treatment. PACAP (Peptide Inst. Inc., Osaka, Japan) or VIP (Peptide Inst. Inc.) was added at the indicated concentration to each dish, and the cells were then cultured for the indicated hours at 37°C under 5 % CO2. If the effects of inhibitors of several kinases were assayed, inhibitors were added 10 min before the addition of PACAP. KT5720 was purchased from Alomone Labs Ltd. (Jerusalem, Israel), U73122 and bisindolylmaleimide I were from Calbiochem (Darmstadt, Germany), and 2-aminoethoxy-diphenyl borate and SP600125 were from Sigma-Aldrich (St. Louis, MO, USA). To detect the binding of the plant (Griffonia simplicifolia) lectin isolectin B4, ND7/23 cells grown on a cover glass were washed with PBS once and incubated with 10-μg/mL Alexa Fluoro 488-conjugated isolectin B4 (Molecular Probes, Inc., Eugene, OR, USA) in PBS containing 1 mM CaCl2 for 15 min.
Cell Morphology Analysis
Three hours after treatment, micrographs of ND7/23 were randomly acquired using a computer-assisted microscope (TE300; Nikon, Tokyo, Japan) equipped with a CCD digital camera and camera control unit (HV-D28S; Nikon). The total number of cells and the number of cells with neurites longer than the length of the cell body were manually counted from at least four micrographs in a blind manner. The percentage of cells with neurites in total cells was assessed for each treatment.
Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was prepared from ND7/23 cells according to the acid guanidinium thiocyanate-phenol-chloroform method (Chomczynski and Sacchi 1987). cDNA was conducted in a total volume of 20 μL using 4 μg total RNA, 50 units of MuLV-reverse transcriptase (Applied Biosystems, Tokyo, Japan) and 50 pmol random primers and was applied to quantitative PCR using the LightCycler 2.0 (Roche, Tokyo, Japan). Briefly, the given amount of reverse-transcribed cDNA corresponding to 0.1 μg of the original total RNA was used per reaction along with the LightCycler FastStart DNA Master SYBR Green I (Roche) with 1-μM primers. Primers for the target genes tested were designed for substance P precursor (preprotachykinin, PPT) mRNA as ggtgccaacgatgatct (forward) and catcccgtttgcccatt (reverse) and for glyceraldehydes 3-phosphate dehydrogebase (GAPDH) as agcccagaacatcatccctg (forward) and caccaccttcttgatgtcatc (reverse). A standard curve was generatedby real-time PCR of the diluted reverse-transcribed cDNA by serial ten times concentrations. The amounts of sample mRNA were determined as a ratio of the standard curve according to the LightCycler software (Roche) and expressed as normalized values by GAPDH mRNA.
Western Blot Analyses for Phospho-Extracellular Signal-Regulated Kinase and Phospho-p38 MAPK
ND7/23 cells treated with 10 nM PACAP in each 35-mm diameter dish were washed once with phosphate-buffered saline (PBS) and lysed in 100 μL lysis buffer (20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 20 μg/mL leupeptin, 20 μg/mL aprotinin, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 50 nM ocadaic acid, 1 % Nonidet P-40, and 10 % glycerol), and the lysate was centrifuged at 15,000 × g for 10 min at 4°C. The aliquot of the supernatant was electrophoresed through 10 % acrylamide gel and blotted on a nitrocellulose membrane. The membrane was treated with phospho-specific p44/42 MAPK (ERK; Thr202/Tyr204) antibody (1:1000; Cell Signaling, Danvers, MA, USA), p44/42 MAPK (ERK1/2) antibody (1:1000; Cell Signaling), or phospho-specific p38 MAPK (Thr180/Tyr182) antibody (1:1000; Cell Signaling). After washing in tris-buffered saline and tween 20 (TBST), the membrane was treated with horseradish peroxidase-linked donkey anti-rabbit IgG (1:5000; GE Healthcare, Buckinghamshire, UK). After extensive washing in TBST, the membrane was developed with ECL Western Blotting Detection Reagents (GE Healthcare).
Reporter Gene Activity Assay
To observe transcriptional activities, ND7/23 cells were plated 24 h before transfection to 105 cells/mL and transfected with the PPT/pGL3 plasmid using FuGene 6 transfection reagent (Roche). PPT/pGL3 is the construct in which the PPT promoter region spanning −863 to +449 relative to the transcription start site was cloned into pGL3 basic (Promega, Madison, WI, USA) upstream of the firefly luciferase gene and was a kind gift from Dr. Michael J. Bannon (Dept. of Pharmacology, Wayne State University School of Medicine (Calin-Jageman et al. 2006)). At 48 h after transfection, the medium was replaced with 0.5 % FBS-containing medium, and the cells were simulated with drugs and incubated for 6 h. The cells were lysed and luciferase activity was measured using PicaGene (Toyo B-NET Co., Ltd., Tokyo, Japan) and a Luminescencer PSN (AB-2200-R; ATTO, Tokyo, Japan).
Primary Culture of Rat DRG Cells
Rat DRG cells were cultured using a method described previously (Inoue et al. 1999). In brief, DRG isolated from male Wistar adult rats was dispersed using 0.125 % collagenase, 0.25 % trypsin, and mechanical trituration, and cultured in DMEM supplemented with 10 % horse serum, 4 mM glutamine, and penicillin/streptomycin (100 units/mL and 100 μg/mL, respectively). All procedures used in the experiments were approved by the Institutional Animal Care and Use Committee of Fukuyama University, Hiroshima, Japan in accordance with the Guidelines of the United States National Institutes of Health regarding the care and use of animals for experimental procedures.
Data Analysis
Data for the quantification of mRNA are expressed as the mean ± SEM, and mRNA levels were considered to be significantly different from those of the corresponding control using one-way analysis of variance with pairwise comparison by the Bonferroni method when P < 0.05.
Results
Characterization of ND7/23 Cells
ND cell lines were induced by cell fusion of mouse neuroblastoma N18Tg2 cells and DRG neurons from neonatal rats. There are differences among subpopulations of DRG neurons with respect to the cell diameter and biochemical factors present. To characterize the ND7/23 cell line, isolectin B4 (IB4)-binding and the gene expression pattern were tested in this cell line. Undifferentiated ND7/23 cells showed flattened cells attached to the surface of the dish (Fig. 1a, left), some of which had a loosely attached round shape. When incubated with isolectin IB4 conjugated with fluorophore Alexa Fluor 488, the majority of ND7/23 cells could bind IB4 strongly and the others weakly (Fig. 1a, right). When examining the expression of several genes which are known to be present in discrete subpopulations of DRG neurons, mRNAs of TrkA and P2X3 receptors were detected by reverse transcription polymerase chain reaction (RT-PCR). PPT mRNA was also expressed in a small amount, while CGRP mRNA was not expressed. Furthermore, they showed the expressions of PAC1 and VPAC2 receptor mRNA, but not VPAC1 receptor (data not shown).
Morphology of ND7/23 cells. a A phase-contrast micrograph (left) of naïve ND7/23 cells and a fluorescent micrograph (right) of ND7/23 cells labeled with isolectin IB4 conjugated with Alexa Fluor 488. b Phase-contrast micrographs of ND7/23 cells, naïve (C) and treated with 0.1, 1, and 10 nM PACAP for 3 h. c The percentage of cells with neurites longer than the length of the cell body. Cells were exposed to various concentrations of PACAP or VIP for 3 h, and cells were manually counted in at least four microphotographs per preparation. Data are the mean ± SEM (bars) of four independent experiments. Double asterisks indicate P < 0.05 compared with non-treated naïve cells (C). d Neurite outgrowth of primary cultured rat DRG neurons by PACAP. At 12 h after seeding of the dispersed rat DRG, the medium was changed to culture medium containing 0.5 % horse serum, and cultures were maintained for another 12 h with (C) or without (A, B) 10 nM PACAP
ND7/23 cells were treated with PACAP, and changes in cell morphology were examined. Cells were treated with different concentrations of PACAP or VIP maintained in medium containing 0.5 % FBS. PACAP induced neuritogenesis in a dose-dependent manner 3 h after treatment (Fig. 1b). When counting the cell number, PACAP increased the percentage of the number of cells with neurites longer than the length of the cell body for 3 h in a dose-dependent manner and significantly at a dose of 1 nM (Fig. 1c). VIP also induced neuritogenesis and increased the percentage of neurites bearing cells significantly at a dose of 100 nM, which was 100-fold more than that of PACAP (Fig. 1c).
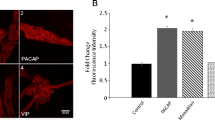
Figure 1d shows examples of primary cultured rat DRG neurons treated with PACAP. At early culture time, when non-treated DRG neuron had few axons (a, b), 10 nM PACAP enhanced neurite outgrowth for 12 h to make highly branched neurites (c).
Expression of PPT mRNA Analyzed by Real-Time RT-PCR
ND7/23 cells were treated with PACAP in 0.5 % serum containing DMEM medium for the indicated hours. While non-treated ND7/23 cells had a small amount of PPT mRNA, 3-h treatment with 10 nM PACAP-38 induced the expression of PPT mRNA significantly (Fig. 2b). PPT mRNA levels in ND7/23 cells treated with PACAP for the indicated hours were analyzed by quantitative RT-PCR (Fig. 2a). A significant amount of PPT mRNA appeared after 1 h of treatment, increased, reached the maximal level at 3 h, and decreased gradually but significantly more than that in non-treated cells to 24 h. PPT mRNA levels were also increased by 1 mM dibutyryl-cyclic AMP (dbcAMP) in a similar manner to PACAP.
Expression of PPT mRNA in ND7/23 cells treated with PACAP. a PPT mRNA levels in cells exposed to PACAP. Total RNA of cells treated with 10 nM PACAP (black circle) or 1 mM dbcAMP (white circle) for the indicated hours was analyzed by quantitative real-time RT-PCR using SYBR Green. PPT mRNA levels were normalized to the corresponding GAPDH mRNA levels. Data are expressed as a percentage of that in non-treated control cells and the mean ± SEM (bars) of three independent experiments. Double asterisk indicate P < 0.05 compared with that of non-treated control cells. b Aliquots of RT-PCR products for PPT mRNA (upper) and GAPDH mRNA (lower) using total RNA from the control (C) and 3-h PACAP-treated (PACAP) cells were electrophoresed with a 100 base-pair DNA ladder as molecular markers (M) on 2 % agarose gel
Dose Dependency of PACAP or VIP
PPT mRNA was analyzed in ND7/23 cells treated with various concentrations of PACAP and VIP (Fig. 3). PACAP increased PPT mRNA levels in a concentration-dependent manner and, at 1 nM, increased the PPT mRNA level significantly by 3.5 times, while VIP could also increase the PPT mRNA level significantly at a concentration of 100 nM, being less potent than PACAP.
Dose-dependent effects of PACAP on PPT mRNA levels in ND7/23 cells. Total RNA of cells treated with 0.1–100 nM PACAP or 1–100 nM VIP was analyzed by quantitative real-time RT-PCR using SYBR Green. Data are expressed as a percentage of that in non-treated control cells and the mean ± SEM (bars) of three independent experiments. Double asterisks indicate P < 0.05 compared with non-treated control cells
Effects of Inhibitors of Several Kinases
The involvement of intracellular signal pathways in the effects of PACAP on PPT mRNA expression was investigated. PPT mRNA levels in ND7/23 cells treated with PACAP in the presence of several inhibitors were analyzed by quantitative RT-PCR (Fig. 4a). The protein kinase-A (PKA) inhibitor KT5720 (KT) at a concentration of 2 μM inhibited the PACAP-induced expression significantly, but 5 μM of the phospholipase C (PLC) inhibitor U73122 (U), 1 μM of the protein kinase-C (PKC) inhibitor bisindolylmaleimide I (Bis), and 100 μM of the inositol-1,4,5-triphosphate (IP3) receptor antagonist 2-aminoethoxy-diphenyl borate did not. Thus, the PKA pathway but not PKC, PLC, or the IP3-involving pathway might be involved in the PPT-mRNA expressing pathway by PACAP.
Involvement of several kinases in PACAP-induced increase of PPT mRNA levels in ND7/23 cells. a, b Effects of inhibitors of several kinases on 10 nM PACAP-induced increase of PPT mRNA levels in ND7/23 cells. Data are expressed as a percentage of that in non-treated control cells and the mean ± SEM (bars) of three independent experiments. Double asterisks indicate P < 0.05 compared with non-treated control cells. c Activation of ERK and p38 MAP kinases induced by PACAP in ND7/23 cells. The cells were treated with 10 nM PACAP in medium containing 0.5 % fetal bovine serum for the indicated time. The lysate of the cells was immunoblotted with phospho-specific ERK antibody, ERK antibody, or phospho-specific-p38 MAPK antibody
The effects of MAPKs on PACAP effects were investigated (Fig. 4b). The increasing effects of PACAP on PPT mRNA were inhibited partially and significantly by the ERK inhibitor U0126 and the p-38-MAPK (p38) inhibitor SB203580 (SB), but not by the c-Jun N-terminal kinase (JNK) inhibitor SP600125 (SP). Thus, the ERK and p38 pathways, but not the JNK pathway, might be involved in the PPT-mRNA expressing pathway by PACAP. No inhibitor affected the PPT mRNA levels when used alone. As shown in Fig. 4c, PACAP stimulated MAPK activities in ND7/23 cells; it stimulated ERK activity 30 min after the addition of 10 nM PACAP and p38 MAPK activity at 15 min.
Luciferase Activities
To investigate the mechanisms of the increase in PPT mRNA levels by PACAP, promoter activities of the PPT gene were analyzed (Fig. 5). ND7/23 cells were transiently transfected with a PPT promoter luciferase reporter construct (−865/+449-pGL3/PPT) using FuGene 6 and treated with 10 nM PACAP in 0.5 % serum containing DMEM for 6 h. A significant increase in PPT promote-driven luciferase activity was observed by 1, 10, and 100 nM PACAP. The increase of luciferase activities was also observed when treated by 1 mM dbcAMP and 10 μM forskolin (data not shown). Thus, the PACAP-induced increase of PPT mRNA levels is therefore likely the result of an increase in PPT gene transcription activities mediated by the pathway involving cAMP.
PPT promoter-luciferase reporter construct activity evoked by PACAP in ND7/23 cells. ND7/23 cells were transfected with PPT promoter-reporter construct pGL3basic/PPT and treated with the indicated concentrations of PACAP for 6 h. Data are expressed as a percentage of that in non-treated control cells (C) and the mean ± SEM (bars) of three independent 3–10 experiments. Double asterisks indicate P < 0.05 compared with non-treated control cells
PPT mRNA Expression in Primary Cultured Rat DRG Cells
To verify the physiological significance of the effects of PACAP, PPT mRNA in primary cultured rat DRG cells was analyzed (Fig. 6). Primary cultured DRG cells were prepared from adult rats and maintained in DMEM supplemented with 10 % horse serum without nerve growth factor (NGF). A primary culture of DRG cells from adult rats can survive in the absence of NGF. On the fifth day of culture, the cells were treated with drugs in DMEM without serum supplementation. PACAP induced the expression of PPT mRNA in primary cultured DRG cells at 10 nM. In addition, 1 mM dbcAMP and 30 ng/mL NGF could induce the expression of PPT.
Expression of PPT mRNA in primary cultured rat DRG cells. Total RNA in DRG cells treated with 10 nM PACAP, 1 mM dbcAMP, or 30 ng/mL NGF for 24 h was analyzed by quantitative real-time RT-PCR using SYBR Green. PPT mRNA levels were normalized to the corresponding GAPDH mRNA levels. Data are expressed as a percentage of that in non-treated control cells and the mean ± SEM (bars) of four independent experiments. Double asterisks indicate P < 0.05 compared with non-treated control cells
Discussion
PACAP is present in the subpopulation of small to medium DRG neurons and may play a physiological role as a transmitter or neuromodulator in sensory C-fibers. Interestingly, it is well known that the expression of PACAP in DRG neurons is upregulated by nerve injury (Jongsma et al. 2000; Pettersson et al. 2004; Boeshore et al. 2004) or inflammation (Zhang et al. 1998; Jongsma et al. 2003), representing its possible adaptive responses to restrict damage and to promote the survival and recovery of the peripheral neurons (reviewed by Waschek 2002; Somogyvari-Vigh and Reglodi 2004). PACAP is a well-documented neurotrophic factor regulating neuronal survival, differentiation, axon growth and growth cone attraction (Falluel-Morel et al. 2005; Monaghan et al. 2008; Scharf et al. 2008). In this report, we show that PACAP could increase SP precursor mRNA in differentiated DRG neuronal cell line ND7/23 cells and primary cultured adult rat DRG cells.
The ND7/23 cell line is a hybrid cell line derived from neonatal rat DRG neurons fused with mouse neuroblastoma N18Tg2 and expresses some properties of primary sensory neurons (Wood et al. 1990). ND7/23 cells could be immunostained by the antibody of a specific neuronal marker, βIII-tubulin (data not shown) and labeled with fluorescent IB4 (Fig. 1a). Most small-diameter DRG neurons can be divided into at least two subpopulations (Stucky and Lewin 1999): peptidergic neurons, which have SP and calcitonin gene-related peptide, and non-peptidergic neurons, which bind the plant lectin IB4. In RT-PCR analyses, naïve ND7/23 cells had mRNA of ATP receptor P2X3 but not CGRP. P2X3 receptors are preferentially expressed on the non-peptidergic small diameter DRG neurons (Burnstock 2000). While we observed a small amount of PPT mRNA in naïve ND7/23 cells, Suburo et al. (1992) could not detect immunoreactivity against substance P by radioimmunoassay. Thus, ND7/23 cells might have the characteristics similar to non-peptidergic small-diameter sensory neurons.
ND7/23 cells can be induced to differentiate when they are incubated with NGF or dbcAMP. The differentiation changes were characterized by neurite outgrowth and by topological arrangements of specific molecular compositions, which were similar to those produced by DRG neurons (Wood et al. 1990; Suburo et al. 1992). These changes may also reflect neuronal plasticity or regeneration unique to sensory neurons after nerve injury or inflammational nerve degeneration (Wu et al. 2008). PACAP could be involved in differentiated changes of ND7/23 cells, such as axon elongation, suggesting that it might contribute to neuronal plasticity or regeneration by being increased under such conditions as nerve injury or inflammation.
Although the molecular and cellular mechanisms of PPT gene expression have been reported to be involved in several cAMP-dependent pathways (Bannon et al. 1991; Adler and Walker 2000; Calin-Jageman et al. 2006), the physiological regulatory mechanisms governing PPT gene expression in injured or inflamed tissue are not well understood. Under such a condition, PACAP might stimulate primary sensory neurons to acquire the property of a plastic phenotypic switch, so that a subpopulation of non-peptidergic sensory neurons will express SP, thereby enhancing neuronal transmission.
PACAP increased PPT mRNA levels in ND7/23 cells for 1 h and continued to increase significantly for 24 h. The effects of PACAP were dependent on its concentration. VIP could mimic PACAP both in neurite outgrowth and in PPT gene expression but with less potency; thus, these phenomena might be mediated through the PAC1 receptor subtype, which needs further detailed experiments for confirmation. Since PACAP activates cAMP production through PACAP receptors, a membrane-permeable cAMP analog dbcAMP was used in ND7/23 cells. dbcAMP could increase PPT mRNA levels similarly to PACAP. Thus, the increasing effect of PACAP on PPT mRNA involved adenylate cyclase activity and produced cAMP-activated protein kinase A since it was blocked by protein kinase A inhibitor KT5720. The neurotrophic or c-fos gene-expressing effects of PACAP on cerebellar granule cells have been reported to involve the protein kinase A-activated pathway (Kienlen et al. 1997; Vaudry et al. 1998). Interestingly, Ma (2010) recently reported that prostaglandin E2 induces the synthesis of substance P in a protein kinase A-dependent manner in cultured sensory ganglion explants through EP1 and EP4 receptors, the latter of which couples to adenylate cyclase. Thus, substance P is synthesized via protein kinase A involving pathways in DRG cells under inflamed conditions.
The MAPK signaling pathways involved in the neurotrophic effects of PACAP have a well-documented role in cell proliferation and differentiation (Villalba et al. 1997; Obara et al. 2007; Ster et al. 2007; Monaghan et al. 2008). PACAP-induced PPT mRNA was partially but significantly decreased by the ERK1/2 inhibitor U0126 and by the p38 inhibitor SB203580, but not by the JNK inhibitor SP600125, indicating that PACAP activated ERK and p38 kinases to increase PPT mRNA levels in ND7/23 cells, the involvement of which is also shown as an increase of phospho-ERK and phospho-p38 MAPK by PACAP, as shown in Fig. 4c. Human neuroblastoma cells, SH-SY5Y cells, similar to ND7/23 cells, differentiate into a more neuronal phenotype by PACAP (Monaghan et al. 2008). Differentiation could be detected by the observation of neuritogenesis and the expressions of neuronal markers, the anti-apoptosis protein Bcl-2 and the growth cone protein GAP-43. The action of PACAP may be mediated through three subtypes of GTP-protein coupled receptors, PAC1, VPAC1, and VPAC2 receptors. SH-SY5Y cells as well as ND7/23 cells expressed PAC1 and VPAC2 receptors, and PACAP induced the differentiation of SH-SY5Y cells through the PAC1 receptor, which is coupled with adenylate cyclase and phospholipase C. Gene expressions by PACAP were through cAMP-dependent but protein kinase A-independent pathways, and the expression of Bcl-2 was inhibited by the inhibitor of p38 kinase and GAP-43 by the inhibitor of ERK kinase. On the other hand, the cAMP-dependent and protein kinase A-independent signaling pathway involved in the neuritogenesis action of PACAP has been reported to be linked with the ERK activation pathway in PC12 cells (Ravni et al. 2008). Thus, the intracellular signaling mechanisms involved in the neurotrophic and differentiating actions of PACAP seem distinct from cell types and the phenomena detected. Furthermore, interestingly, a potent PAC1 receptor antagonist PACAP6-38 behaved as an agonist in rat sensory neurons where a presently unknown receptor or splice variant might exist for PACAP on DRG cells (Reglodi et al. 2008). The receptor subtypes and precise intracellular signaling mechanisms by PACAP in ND7/23 cells remain to be determined.
ND7/23 cells were transfected with PPT promoter-reporter construct (−863/+449) and treated with various concentrations of PACAP for 6 h. We observed a more than 3-fold increase in PPT promoter-driven luciferase activity in response to more than 1 nM PACAP. Therefore, the increase of PACAP-induced PPT mRNA is likely to be the result of an increase in PPT gene transcriptional activity, which may be enhanced by cAMP and adenylate cyclase mechanisms.
In this report, we have shown that PACAP could increase SP precursor mRNA through transcriptional activity, which is dependent on protein kinase A activities and on ERK and p38 MAP kinases in a differentiated DRG neuronal cell line ND7/23 cells, suggesting that the neurotrophic action of PACAP may function to rescue damaged neurons or to switch the neuronal phenotype during sensory nerve injury or inflammation.
References
Adler JE, Walker PD (2000) Cyclic AMP regulates substance P expression in developing and mature spinal sensory neurons. J Neurosci Res 59:624–631
Arimura A, Somogyvari-Vigh A, Weill C, Fiore RC, Tatsuno I, Bay V, Brenneman DE (1994) PACAP functions as a neurotrophic factor. Ann NY Acad Sci USA 739:228–243
Bannon MJ, Haverstick DM, Shibata K, Poosch MS (1991) Preprotachykinin gene expression in the forebrain: regulation by dopamine. Ann N Y Acad Sci 632:31–37
Boeshore KL, Schreiber RC, Vaccariello SA, Sachs HH, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE (2004) Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J Neurobiol 59:216–235
Burnstock G (2000) P2X receptors in sensory neurons. Brit J Anaesth 84:476–488
Calin-Jageman IE, Wang J, Bannon MJ (2006) Regulation of the preprotachykinin-I gene promoter through a protein kinase A-dependent, cyclic AMP response element-binding protein-independent mechanism. J Neurochem 97:255–264
Calza L, Pozza M, Zanni M, Manzini CU, Manzini E, Hokfelt T (1998) Peptide plasticity in primary sensory neurons and spinal cord during adjuvant-induced arthritis in the rat: an immunocytochemical and in situ hybridization study. Neuroscience 82:575–589
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Falluel-Morel A, Vaudry D, Aubert N, Galas L, Benard M, Basille M, Fontaine M, Fournier A, Vaudry H, Gonzaiez BJ (2005) Pituitary adenylate cyclase-activating polypeptide prevents the effects of ceramides on migration, neurite outgrowth, and cytoskeleton remodeling. Proc Natl Acad Sci U S A 102:2637–2642
Fernandes ES, Schmidhuber SM, Brain SD (2009) Sensory-nerve-derived neuropeptides: possible therapeutic targets. Handb Exp Pharmacol 194:393–416
Grider JR (2003) Interleukin-1β selectively increases substance P release and augments the ascending phase of the peristaltic reflex. Neurogastroenterol Motil 15:607–615
Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, Nakata Y (1999) Interleukin-1β induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem 68:128–133
Jongsma H, Danielsen N, Sundler F, Kanje M (2000) Alteration of PACAP distribution and PACAP receptor binding in the rat sensory nervous system following sciatic nerve transection. Brain Research 853:186–196
Jongsma WH, Pettersson LM, Verge VM, Danielsen N (2003) Effect of anti-nerve growth factor treatment on pituitary adenylate cyclase activating polypeptide expression in adult sensory neurons exposed to adjuvant induced inflammation. Neuroscience 120:325–331
Kienlen CP, Crochemore C, Rene F, Monnier D, Koch B, Loeffler JP (1997) PACAP type I receptor activation promotes cerebellar neuron survival through the cAMP/PKA signaling pathway. DNA Cell Biol 16:323–333
Krause JE, Chirqwin JM, Carter MS, Xu ZS, Hershey AD (1987) Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc Natl Acad Sci USA 84:881–885
Lioudyno M, Skoglosa Y, Takei N, Lindholm D (1998) Pituitary adenylate cyclase-activating polypeptide (PACAP) protects dorsal root ganglion neurons from death and induces calcitonin gene-related peptide (CGRP) immunoreactivity in vitro. J Neurosci Res 51:243–256
Ma W (2010) Chronic prostaglandin E2 treatment induces the synthesis of the pain-related peptide substance P and calcitonin gene-related peptide in cultured sensory ganglion explants. J Neurochem 115:363–372
Monaghan TK, Mackenzie CJ, Plevin R, Lutz EM (2008) PACAP-38 induces neuronal differentiation of human SH-SY5Y neuroblastoma cells via cAMP-mediated activation of ERK and p38 MAP kinases. J Neurochem 104:74–88
Neumann S, Doubell TP, Leslie T, Woolf CJ (1996) Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 384:360–364
Nielsen KM, Chaverra M, Hapner SJ, Nelson BR, Todd V, Zigmond RE, Lefcort F (2004) PACAP promotes sensory neuron differentiation: blockade by neurotrophic factors. Mol Cell Neurosci 25:629–641
O’Byme EM, Blancuzzi V, Wilson D, Wong M, Peppard J, Simke JP, Jeng AY (1991) Effects of indomethacin, triamcinolone, and dexamethasone on recombinant human interleukin-1-induced substance P and prostaglandin E2 levels in rabbit knee joints. Agents Actions 34:46–48
Obara Y, Horgan AM, Stork PJ (2007) The requirement of Ras and Rap 1 for the activation of ERKs by cAMP, PACAP, and KCl in cerebellar granule cells. J Neurochem 101:470–482
Pettersson LM, Dahlin LB, Danielsen N (2004) Changes in expression of PACAP in rat sensory neurons in response to sciatic nerve compression. Eur J Neurosci 20:1838–1848
Ravni A, Vaudry D, Gerdin MJ, Elden MV, Falluel-Morel A, Gonzalez BJ, Vaudry H, Elden LE (2008) A cAMP-dependent, protein kinase A-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Mol Pharmacol 73:1688–1708
Reglodi D, Borzsei R, Bagoly T, Boronkai A, Racz B, Tamas A, Kiss P, Horvath G, Brubel R, Nemeth J, Toth G, Helyes Z (2008) Agonistic behavior of PACAP6-38 on sensory nerve terminals and cytotrophoblast cells. J Mol Neurosci 36:270–278
Richardson JD, Vasko MR (2002) Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther 302:839–845
Scharf E, May V, Braas KM, Shutz KC, Mao-Draayer Y (2008) Pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) regulate murine neural progenitor cell survival, proliferation, and differentiation. J Mol Neurosci 36:79–88
Somogyvari-Vigh A, Reglodi D (2004) Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr Pharm Des 10:2861–2889
Ster J, De Bock F, Guerineau NC, Janossy A, Barrere-Lemaire S, Bos JL, Bockaert J, Fagnl L (2007) Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2 + -dependent K + channels in cerebellar neurons. Proc Natl Acad Sci U S A 104:2519–2524
Stucky CL, Lewin GR (1999) Isolectin B4-positive and -negative nociceptors are functionally distinct. J Neurosci 19:6497–6505
Suburo AM, Wheatley SC, Horn DA, Gibson SJ, Jahn R, Fischer-Colbrie R, Wood JN, Latchman DS, Polak JM (1992) Intracellular redistribution of neuropeptides and secretory proteins during differentiation of neuronal cell lines. Neuroscience 46:881–889
Vaudry D, Gonzalez BJ, Basille M, Anouar Y, Fournier A, Vaudry H (1998) Pituitary adenylate cyclase-activating polypeptide stimulates both c-fos gene expression and cell survival in rat cerebeller granule neurons through activation of the protein kinase A pathway. Neuroscience 84:801–812
Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61:283–357
Villalba M, Bockaert J, Journot L (1997) Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci 17:83–90
Waschek JA (2002) Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev Neurosci 24:14–23
Wood JN, Bevan SJ, Coote PR et al (1990) Novel cell lines display properties of nociceptive sensory neurons. Proc R Soc Lond B 241:187–194
Wu D, Huang W, Richardson PM, Priestley JV, Liu M (2008) TRPC4 in rat dorsal root ganglion neurons is increased after nerve injury and is necessary for neurite outgrowth. J Biol Chem 283:416–426
Zhang Y, Danielsen N, Sundler F, Mulder H (1998) Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. Neuroreport 9:2833–2836
Acknowledgments
We thank Dr. M. J. Bannon (Dept. of Pharmacology, Wayne State University School of Medicine) for the generous gift of the PPT/pGL3 plasmid. This work was supported by the Strategic Support Project of Research Infrastructure Formation for Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inoue, A., Ohnishi, M., Fukutomi, C. et al. Protein Kinase A-Dependent Substance P Expression by Pituitary Adenylate Cyclase-Activating Polypeptide in Rat Sensory Neuronal Cell Line ND7/23 Cells. J Mol Neurosci 48, 541–549 (2012). https://doi.org/10.1007/s12031-012-9747-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-012-9747-z