Abstract
Pituitary adenylate cyclase activating polypeptide (Adcyap1; PACAP) is a well-studied neural and endocrine pleiotropic peptide important in development and the homeostatic regulation of many physiological systems. Accordingly, PACAP and its cognate PAC1 receptor (Adcyap1r1) are localized in many central nervous system (CNS) regions and widely distributed in the peripheral nervous system (PNS). PACAP has been identified in a population of small nociceptive neurons in the dorsal root ganglion (DRG) targeting many peripheral tissues. PACAP-immunoreactive preganglionic sympathetic and parasympathetic nerve fibers densely innervate a variety of autonomic and enteric ganglia. Furthermore, a small fraction of the autonomic and enteric postganglionic neurons also endogenously express PACAP. Notably, PACAP belongs to a cohort of neuroplasticity peptides, and PNS insult invariably induces PACAP expression in the affected neurons. Axotomy, nerve crush, inflammation, and neural tissue explant paradigms can dramatically induce neuronal PACAP transcripts, immunoreactivity, content, and cell numbers. This plasticity in PACAP expression has been best appreciated in studies using the PACAP-EGFP transgenic mouse line. The mechanisms driving PACAP expression after insults are not well understood, but likely reflect the aggregate effects of cytokine/inflammatory activation signals at the injury site and an abrogation of retrograde signaling from the target tissues. The few comparative studies suggest that the mechanistic signatures for peptide induction may differ among the various neural systems. This induction of PACAP expression in physiological insults has been implicated to participate in fiber regeneration and anti-inflammatory responses, but PACAP neuroplasticity may also have broader roles in maintaining neurocircuit homeostasis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- PACAP
- PAC1 receptor
- Sensory
- Dorsal root ganglion (DRG)
- Autonomic
- Sympathetic
- Parasympathetic
- Superior cervical ganglion (SCG)
- Cardiac ganglion
- Phenotypic plasticity
- Injury
- Inflammation
Introduction
Many studies have comprehensively examined the distribution and expression of pituitary adenylate cyclase activating polypeptide (Adcyap1; PACAP) in the central nervous system. These surveys can be accessed in a number of excellent primary investigations and reviews [1–4], and from inspection of the Allen Brain Atlas (http://www.brain-map.org/). There are fewer critical studies of PACAP expression and plasticity in the peripheral nervous system and this chapter aims to provide an annotated overview of that literature supplemented with primary data from our laboratories.
In examining the publications reporting PACAP distribution, we have been mindful of several issues. For many peptidergic systems, including PACAP, the immunocytochemical localizations often identified only neuronal fibers, varicosities, and terminals, without significant signal detection in neuronal soma. These localization patterns likely reflected the preferential antibody binding to the fully post-translationally processed mature peptides en route to the terminals, rather than the peptide precursor protein molecules in the cell bodies. Consequently, the origins of tissue PACAP fibers were sometimes misinterpreted until clarification by in situ hybridization of neuronal PACAP transcripts. Finally, while the endogenous expression of PACAP in many neuronal tissues is unequivocal, some of the early studies were performed in colchicine-treated animals. Though colchicine disrupted microtubule polymerization and blocked axonal vesicular transport, facilitating peptide immunocytochemical localization in the cell soma, colchicine likely instilled cellular stress- and injury-induced responses. The resulting induction of PACAP expression may have altered distribution estimates from both immunocytochemistry and in situ hybridization analyses. Hence the sections below will not contain exhaustive information but attempt to generalize consensus data from the many studies. From the many investigations, PACAP has been shown to be widely distributed in sensory ganglia, participating in nociception, and autonomic/enteric neural systems, regulating diverse functions including cardiac and respiratory output, stress responses, gastrointestinal secretion and motility, and urogenital activities. With a number of other peptides, PACAP also demonstrates neurophenotypic plasticity in responses to a variety of physiological challenges.
Sensory Systems
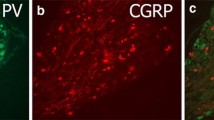
PACAP has long been appreciated to be a sensory peptide. Under normal basal conditions, PACAP immunoreactivity and transcripts were identified in a subpopulation (approximately 10 %) of dorsal root ganglion (DRG) neurons, which appeared to represent predominantly small caliber unmyelinated C-nociceptors [5–8]. PACAP was largely co-localized with substance P- and calcitonin gene-related peptide (CGRP)-expressing sensory neurons which has been corroborated in recent single cell RNA sequencing studies [9]. However, the overall fraction of neurons expressing PACAP was considerably smaller than that for either substance P or CGRP [6, 10]. In the spinal cord, the PACAPergic DRG central fibers were localized predominantly to nociceptive targets in laminae I and II of the dorsal horn [5, 11]. Further, sensory neuron degeneration upon capsaicin treatments diminished PACAP immunoreactivity in both central and peripheral axons, and decreased PACAP content in parallel with CGRP depletion, further supporting PACAP expression in CGRP-containing nociceptive C-fibers [5]. As in DRG, PACAP immunoreactivity and/or transcripts were also identified in subpopulations of trigeminal and nodose ganglia neurons with CGRP [5, 6, 12, 13]. Accordingly, sensory fibers co-expressing PACAP and CGRP have been shown to be widely distributed in peripheral tissues including skin, cerebral vessels, pineal gland, eye, submandibular salivary ducts, tongue, trachea, larynx, urinary bladder and urogenital organs, and the gastrointestinal tract [5, 14–18].
Autonomic Nervous System
Sympathetic Nervous System
As observed in sensory ganglia, PACAP immunoreactivity and precursor protein transcripts were identified in autonomic ganglia and fibers during late embryonic development (E14–E18) [19]. Immunocytochemical processing for PACAP in the superior cervical ganglion (SCG) at the head of the paravertebral sympathetic trunk revealed intense staining of dense fiber networks circumscribing the postganglionic neurons, suggesting the presence of PACAP in sympathetic preganglionic neurons and fibers [20]. Although the sources of the SCG fibers containing PACAP were initially unclear, in situ hybridization coupled with retrograde fluorogold tracing from the sympathetic trunk demonstrated unequivocally that sympathetic preganglionic PACAP-expressing neurons in the intermediolateral cell column of the thoracic cord project to sympathetic ganglia [19, 21, 22]. As nearly all of the SCG postganglionic neurons express PAC1 receptors and PACAP potently and efficaciously stimulated SCG catecholamine and neuropeptide Y (NPY) secretion [20, 23, 24], PACAP represents one of the preganglionic noncholinergic regulators of sympathetic function. Preganglionic PACAP fibers were also found to innervate stellate, celiac, and mesenteric ganglia [25, 26]. In good agreement, PACAP was identified in preganglionic abdominal splanchnic nerves that target the adrenal medulla for epinephrine release [27, 28]. In aggregate, the high levels of PACAP expression in sympathetic systems implicate roles in stress responses, which has been supported in physiological and transgenic animal studies.
A small population of postganglionic sympathetic ganglia neurons also express PACAP endogenously under normal physiological states [20, 29]. Although PACAP immunoreactivity was detected in developing SCG neurons, and PACAP transcript and peptide levels were reported in adult SCG by quantitative PCR and radioimmunoassay, respectively, SCG neurons with endogenous PACAP identified by in situ hybridization analyses represented only a very small fraction of the total population (1–3 %). To obviate non-neuronal sources of SCG PACAP production, highly enriched SCG neurons free of contaminating background cells were maintained in serum-free cultures. Even under these conditions, SCG PACAP transcripts and peptides were readily measured, supporting endogenous neuronal SCG PACAP synthesis. But as in many neuronal systems, endogenous PACAP expression exhibits plasticity and can be dramatically induced under different physiological challenges (see below).
Parasympathetic Nervous System
PACAP also participates in parasympathetic function. For these investigations, the accessibility of the guinea pig cardiac ganglia model has been invaluable for detailed cellular, molecular, and electrophysiological studies. As for SCG, immunocytochemical inspection of cardiac ganglia whole mount preparations demonstrated dense pericellular PACAP networks surrounding the postganglionic neurons [30]. The PACAP-immunoreactive fibers degenerated upon culture of cardiac ganglion explants demonstrating their extrinsic nature. These PACAP-containing fibers were co-localized with choline-acetyltransferase (ChAT)-immunoreactivity, implicating vagal preganglionic origins, but not with either NPY- or substance P-immunoreactivity, which served as markers for sympathetic and sensory fibers, respectively. Furthermore, in the brainstem, in situ hybridization analyses identified PACAP transcript expression in neurons of the nucleus ambiguus and dorsal motor nucleus of the vagus. Hence, despite PACAP identification in nodose sensory ganglia, these results in aggregate demonstrated that PACAP innervation of guinea pig cardiac ganglia neurons was largely via vagal preganglionic parasympathetic fibers. These observations implicated vagal PACAP regulation of cardiac output and function [31–33], and inferred PACAP vagal regulation of other visceral parasympathetic ganglia in respiratory, gastric, pancreatic, and gastrointestinal physiology. PACAP fiber networks have also been described in the cranial ciliary, sphenopalatine, submandibular, and otic parasympathetic ganglia, which were consistent with PACAP/PAC1 receptor-specific effects in the head and neck.
Similar to sympathetic ganglia, a small population of parasympathetic cardiac ganglion neurons (4 %) expressed PACAP endogenously. Intrinsic PACAP was also identified in all four cranial parasympathetic ganglia by immunocytochemistry and in situ hybridization [12, 19]. Noteworthy, unlike sensory or other autonomic ganglia where endogenous PACAP-expressing neurons represented a small fraction of the population, nearly all of the postganglionic parasympathetic neurons in the otic and sphenopalatine ganglia expressed PACAP under normal baseline conditions [12]. The functional implications of prevalent postganglionic parasympathetic innervation of head and neck structures are unclear but recently, sphenopalatine PACAP fiber innervation of the middle meningeal artery has been implicated in migraine.
Mixed Sympathetic and Parasympathetic Ganglion
The major pelvic ganglion (MPG) is a mixed parasympathetic and sympathetic ganglion identified by a composite of cholinergic and catecholaminergic neurons, respectively, that provide major autonomic input to urogenital organs and the lower bowel. Although low levels of MPG PACAP transcripts were identified in normal control tissues by PCR, no PACAP-immunoreactive fibers or neurons were identified in the ganglion [34]. This contrasted with the prevalent expression of vasoactive intestinal peptide (VIP) neurons and fibers in MPG parasympathetic cholinergic neurons. However, unlike VIP in which the levels appeared relatively stable, PACAP expression was induced in some MPG neurons following experimental manipulations.
Enteric Nervous System
The enteric nervous system of the gastrointestinal tract is composed of ganglionated fiber networks in the myenteric plexus and the submucosal plexus. PACAP-immunoreactive fibers were identified in both myenteric and submucosal plexi, often co-localized with CGRP and other sensory peptides implicating extrinsic PACAP origins from DRG or vagal projections. However, capsaicin-induced neurodegeneration or extrinsic denervation only diminished gut PACAP content and immunoreactive fibers, implicating some endogenous PACAP production from intrinsic sources. Accordingly, both immunocytochemistry and in situ hybridization studies identified endogenous PACAP expression in the myenteric plexus throughout the gastrointestinal tract and in the submucosal plexus of the small and large intestine [15].
PACAP Neuroplasticity
Many neuronal systems exhibit phenotypic neuroplasticity under a variety of experimental paradigms in vitro and in vivo that can sometimes result in rather dramatic changes in neurotransmitter and neuropeptide expression. These neurophenotypic plasticity responses are important in development and in adult tissues to maintain neurocircuit homeostasis, which can impact a broad range of activities including behavior [35–38]. For peripheral transmitters, the perturbations can alter the relative levels of tissue catecholaminergic- versus cholinergic-producing neurons [39, 40]. For neuropeptides, the physiological challenges in neuronal injuries or inflammation can reduce the levels of some peptides, but dramatically induce the expression of others from levels that might have otherwise appeared negligible or absent [41, 42]. VIP and PACAP fall into the latter category, and are invariably highly induced in sensory, autonomic, and motor neurons. The plasticity in PACAP expression is illustrated in the following few examples.
Sensory Neurons
The dynamics in DRG PACAP expression have been well described. As noted earlier, approximately 10 % of DRG neurons express PACAP immunoreactivity and transcripts, mostly in small (<35 μm diameter) C-fiber nociceptors. A variety of nerve injury (transection, crush, or chronic constriction injury) and inflammation (adjuvant, cyclophosphamide) models dramatically increased DRG PACAP transcripts, immunoreactivity, and neurons in a time-dependent manner [7, 8, 11, 43–45]. From the small population of DRG PACAP neurons under baseline conditions, the increase in PACAP-expressing DRG neurons after sciatic nerve transection, for example, could be detected within a day after physiological challenge, reaching 50 % after 3 days, and a maximal sustained peak of more than 75 % after 10 days [7]. Notably, the DRG populations demonstrating PACAP induction appeared to depend on the nature of the injury. The increase in DRG PACAP neurons after sciatic nerve transection largely reflected heightened PACAP expression in large DRG neurons (>35 μm diameter), which was accompanied by a modest PACAP reduction in the small caliber DRG neuronal population (<35 μm diameter) [7, 11]. These observations were mirrored by increased PACAP-immunoreactive fiber density in the deep laminae (large DRG neuronal projections) of the spinal cord dorsal horn and decreased central PACAP fibers to the superficial layers (small C-fiber nociceptor projections). The induction of DRG PACAP-expressing neurons following inflammatory insult was confined to the small-sized neuronal population [46] with corresponding increases in PACAP-immunoreactive fiber staining in the superficial layers of the dorsal horn [45], whereas nerve compression increased PACAP levels in both small and large DRG neuronal populations [43]. Transection of the masseteric nerve produced a similar temporal increase in PACAP expression in the trigeminal ganglion [47].
Sympathetic Neurons
Injury to other peripheral neuronal tissues elicited the same PACAP induction responses. From the 1 to 3 % of postganglionic sympathetic SCG neurons that express PACAP under basal states, axotomy of the internal and/or external carotid nerves [20] or placing SCG ganglia in explant cultures augmented the SCG PACAP neuronal population to nearly 40 % (>10-fold increase) which was matched by a 30- to 50-fold increase in SCG PACAP transcript levels and a 30-fold augmentation of ganglionic PACAP peptide content (Fig. 33.1). Similar peptide induction responses were produced following chemical sympathectomy with 6-hydroxydopamine [20, 48] or nerve growth factor (NGF) neutralizing antisera treatments [49]. By contrast, the induction of PACAP and other peptides was far lower following SCG deafferentation or decentralization injury, after transection of sympathetic preganglionic fibers in the sympathetic trunk, from damage to the few sympathetic postganglionic axons that backtracked into the sympathetic chain [20, 50] (Fig. 33.1).
Superior cervical ganglion (SCG) axotomy increases PACAP transcript expression. Adult male rat SCGs were axotomized by transection of the internal and external carotid nerves (ICN and ECN, respectively) or decentralized (Dcx) by transection of the sympathetic trunk (schematic in inset). Control SCGs were from unoperated animals and sham ganglia were surgically exposed but untouched. Following 7 days postsurgical recovery, the rats were euthanized by decapitation and the harvested SCG immediately frozen on dry ice. (A) The SCGs were homogenized in 5N acetic acid with protease inhibitors; the extracts were lyophilized and reconstituted in assay buffer for PACAP38 radioimmunoassay [29]. Axotomy increased SCG PACAP content nearly 30-fold; PACAP induction following decentralization was more modest (threefold). Data expressed either as mean fmol/ganglion (left axis) or fold change from control (right axis) ± SEM. n = 5 per group. (B) SCG RNA was also prepared for Northern analysis as previously described [29]. Axotomy dramatically induced SCG PACAP mRNA levels. The blots were exposed to film with intensifying screens for either 143 h (left panel) or 120 h (right panel), demonstrating PACAP mRNA levels in control SCG and relative induction of the 2.2 and 0.9 kb PACAP transcripts after axotomy. Each lane represents RNA from an individual ganglion
Parasympathetic Neurons
Placing postganglionic parasympathetic cardiac ganglia in explant cultures for 2–4 days changed the number of PACAP-expressing neurons from 1 to 4 % to a maximal increase of 25–35 %, in parallel with a 15-fold induction in PACAP precursor protein transcripts [30, 51]. Compared to other peripheral ganglia and peptides, in which axotomy or tissue explantation can produce double-digit fold changes, the PACAP transcript changes in the mixed autonomic major pelvic ganglia were smaller (<5 %) in explants and appeared absent following transection of the cavernous nerve [52].
PACAP Neuroplasticity in PACAP-EGFP Mice
The recent availability of a PACAP-EGFP transgenic mouse line has allowed studies of PACAP neuroplasticity with high resolution. In this model, expression of the EGFP cassette, inserted upstream of the PACAP ATG initiation codon and under PACAP gene (Adcyap1) promoter control, has allowed detailed central and peripheral nervous system PACAP regulation and induction studies under a variety of physiological paradigms (Fig. 33.2). Under baseline conditions, native PACAP-EGFP fluorescence was low or absent in a variety of peripheral tissues. Placing the SCG and MPG into explant cultures also dramatically induced PACAP-EGFP expression (Fig. 33.2C–F); the number of MPG PACAP-expressing neurons appeared greater than levels indicated in previous PCR analyses. Comparable to previous studies, chronic constriction injury by partial sciatic nerve ligation dramatically induced the number of PACAP-expressing neurons in the ipsilateral DRG, compared to the contralateral sham control ganglion (Fig. 33.2A, B); the increases in DRG PACAP expression were reflected by increased PACAP-EGFP fiber fluorescence in the sciatic nerve, proximal to the ligation, and in the DRG central axons to the ipsilateral dorsal horn (Fig. 33.2G). Similar to previous studies, the dense injury-induced PACAP-immunoreactive fibers were prevalent in the ipsilateral gracile fasciculus and deeper laminae (II–IV) of the dorsal horn (Fig. 33.2G) which implicated preferential PACAP induction in the large (>35 μm diameter) DRG neurons. There were no apparent changes in the number of second order PACAP-EGFP neurons in the superficial (lamina I) layer of the dorsal horn compared to the contralateral sham control side. In agreement with sciatic nerve transection results [22], chronic constriction injury also increased PACAP transcript expression in ventral horn motor neurons (Fig. 33.2G). These studies demonstrate the utility of PACAP-EGFP mice to unambiguously identify induced PACAPergic neurons and pathways under different physiological states.
Peripheral ganglia PACAP expression in transgenic PACAP-EGFP mice is induced after nerve injury or explantation. PACAP-EGFP mice received unilateral partial sciatic nerve ligation for chronic constriction injury (CCI, 14 days). The mice were deeply anesthetized and euthanized by 4 % paraformaldehyde perfusion fixation, and tissue cryosections were prepared for native EGFP fluorescence microscopy. (A, B) Compared to sham control L4 dorsal root ganglion (DRG, A), CCI induced dramatically the number of PACAP-EGFP neurons in the ipsilateral DRG (B). The increase in DRG PACAP-EGFP was reflected in the increased PACAP fiber density in the ipsilateral dorsal horn (DH) and gracile fasciculus (GF, panel G, right half of spinal cord). Notably, CCI also induced PACAP-EGFP expression in motor neurons of the ventral horn (VH). (C, D) Compared to control SCGs which were frozen immediately after harvest for cryosectioning (C), explantation of intact PACAP-EGFP SCG in NGF-free medium for 24 h increased PACAP expression (D). (E, F) Similarly, explant culture of the mixed sympathetic/parasympathetic major pelvic ganglion (MPG) induced PACAP-EGFP expression (F) compared to control ganglion (E)
Mechanisms of PACAP Neuroplasticity
The regulatory mechanisms underlying PACAP expression and injury-induced plasticity are not completely understood. As in other peptidergic systems, multiple factors and signaling mechanisms undoubtedly participate in the endogenous baseline and induced levels of PACAP expression.
Neuronal Activity, Trophic Factors, and Transcriptional Regulators of PACAP Expression
Neuronal activity and the resulting changes in calcium influx can regulate neuronal transmitter and peptide identity and respecification [36, 37]. The expression of galanin, VIP, substance P, and PACAP, for example, can be dramatically induced upon depolarization of cultured neurons [29, 53, 54]. The PACAP mRNA in nervous tissues is typically 2.2 kb in size and interestingly, depolarization not only augmented the 2.2 kb PACAP transcript but induced the expression of a smaller 0.9 kb form which appeared to reflect the use of a variant 3' UTR hexamer signal sequence for alternative transcript polyadenylation [55]. SCG axotomy similarly produced a shortened form of the PACAP transcript (Fig. 33.1). The short PACAP transcript variant appeared to demonstrate enhanced stability, but whether the ~1 kb deletion sequence also harbors transcriptional regulatory elements and/or impacts PACAP mRNA folding to regulate expression is currently unclear.
A variety of other factors influence PACAP expression. The glass bottom boat class of bone morphogenetic proteins (BMP), especially BMP6, can suppress neuronal PACAP expression, but reciprocally upregulate VIP levels [56]. The effects of neurotrophins on PACAP expression are complex. Signaling by NGF through the high affinity TrkA receptor can stimulate PC12 and DRG nociceptor PACAP expression [46, 57, 58] but repress SCG PACAP levels (see below). Neurotrophin-3 (NT-3) appears to suppress PACAP expression in a subpopulation of DRG neurons following injury [57]. The PACAP gene has two neural-restrictive silencer-like elements (NRSLE) [59, 60], suggesting that it can be repressed by RE-1 silencing transcriptional factor (REST). Other regulators include the gp130 cytokines [61] (see below), thyroid transcriptional factor-1 (TTF-1) [62], and T-cell leukemia homeobox protein 1/3 (Tlx1/3) [63]. The roles of Tlx1/3 are notable as Tlx1/3 coordinates PACAP and glutaminergic neuronal identities.
Activation Mechanisms in Injury/Inflammation-Induced PACAP Expression
Roles for activation and repressive retrograde signals have been postulated in models regulating neurophenotypic plasticity elicited by injury or inflammation. Each model was championed at various times, but both mechanisms undoubtedly contribute to the plasticity process. The activation model has emphasized the roles of the neuropoietic cytokines, including leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), interleukin-6 (IL-6), interleukin-11 (IL-11), oncostatin M, and cardiotrophin-1, with the canonical gp130 receptor—JAK/STAT signaling pathway in neuropeptide induction. This process has been well reviewed [61]. SCG axotomy increased ganglia LIF and IL-6 expression in the supporting satellite and Schwann cells without effects on SCG gp130 transcript levels [64, 65]. Furthermore, conditioned medium stimulation of sympathetic culture VIP expression was blocked by preabsorption with anti-LIF [66], and SCG axotomy of LIF−/− animals attenuated VIP, galanin, and neurokinin A expression [67]. However, in vivo SCG exposure to LIF or axotomy of the facial motor nucleus in LIF−/− or IL-6−/− mice had no effects on galanin or VIP/PACAP induction, respectively [49, 68]. In accord, direct application of LIF, CNTF, or IL-6 to primary sympathetic cultures had no or only modest (<2-fold) effects on PACAP expression. These results implicated roles for other gp130 cytokines; however to obviate the testing of each potential mediator, a transgenic model was used in which gp130fl/fl animals were crossed with dopamine-beta-hydroxylase (DBH)-cre mice to delete gp130 from catecholaminergic neurons, including gp130 receptors in the SCG. Axotomy of the gp130DBHcre mouse SCG failed to demonstrate injury-induced VIP or PACAP expression [65], but paradoxically, cholecystokinin transcript levels were still augmented despite the previous demonstration of regulation by LIF. The cytokines may not be derived solely from endogenous ganglia non-neuronal cells. In injuries, immune cells can release a variety of cytokines and mediators generating an inflammatory milieu that can influence neuronal function and phenotypic responses [69, 70]. In support of this concept, axotomy of the facial motor nucleus failed to induce PACAP expression in the immunodeficient SCID mouse; however, VIP induction was not affected. The abrogated PACAP injury response in the SCID mouse could be reversed upon infusion of splenocytes, consistent with roles for inflammatory agents [68]. These studies in aggregate suggest that cytokines and inflammatory factors, from endogenous ganglia or exogenous immune cell origins, can participate in injury-induced peptidergic plasticity.
Abrogation of Retrograde Mechanisms in Injury/Inflammation-Mediated PACAP Expression
Other studies have suggested additional modes of peptide induction in injury. Using the SCG as example, numerous studies have shown that only axotomy of the internal or external carotid nerves are able to dramatically increase SCG VIP, PACAP, and galanin expression; SCG decentralization, as described above, produced only modest changes from axotomy of the few postganglionic SCG fibers traveling in the sympathetic trunk. If the SCG peptidergic responses were dependent on satellite/Schwann cells or immune responses, it is unclear how the cellular mediators would distinguish the two forms of injury. Further, it is unclear how long distance axonal or terminal damage can generate responses back at the soma. As examples, mid-thigh sciatic nerve contusions, catecholaminergic nerve terminal injuries with 6-hydroxydopamine, or TRPV1-mediated sensory nerve terminal damage with capsaicin or the agonist resiniferatoxin dramatically induces peptide expression and plasticity—yet how do the corresponding SCG or DRG neuronal cell bodies recognize the injuries to mount plasticity responses? One mechanism relates to neuronal connectivity with the cognate peripheral target tissues and the retrograde transport of target-derived neurotrophic factors. The retrograde transport of NGF via TrkA receptors at sympathetic nerve terminals back to the soma has been well studied to generate survival signals via CREB signaling [71–74]. In congruence, immunosympathectomy with NGF neutralizing antisera to unoperated SCG recapitulated many of the peptide induction responses observed in axotomy [49]. These observations suggest that retrograde target tissue signaling represents a repressive neurotrophic signal and that axotomy, in relieving that neurotrophic support, activates a stress signal for PACAP induction.
Our own PACAP studies with sympathetic neuronal cultures have supported many of these observations. Primary sympathetic neurons, free of contaminating support cells, can be cultured in defined medium supplemented with NGF. The low levels of endogenous PACAP expression under these culture conditions could be induced over eightfold upon NGF withdrawal in a rapid time-dependent manner, and suppressed again upon the reintroduction of NGF into the culture medium (Fig. 33.3). The levels of SCG PACAP expression were exquisitely sensitive to NGF concentration and could be blocked with NGF neutralizing antisera or with the Trk receptor signaling inhibitor K252a (Fig. 33.4A). NGF/TrkA signaling takes place not only at the plasma membrane but continues in signaling endosomes upon endocytosis. Hence, the blockade of either clathrin- or caveolae-mediated internalization mechanisms also stimulated PACAP transcript expression (Fig. 33.4A). The roles of NGF/TrkA retrograde signaling were tested in compartmentalized Campenot chambers in which the SCG soma were separated from the axons, and in this model the withdrawal of NGF from the axonal compartment alone was sufficient to induce PACAP transcript expression in the cell bodies (Fig. 33.4C–D). NGF withdrawal placed the cultures into modes of cellular stress as reflected by an increase in neuronal JNK phosphorylation; suppression of JNK activation with the inhibitor SP600125 completely blocked the induction in PACAP expression during NGF withdrawal (Fig. 33.4B). By contrast, NGF had a lesser effect on VIP expression; withdrawal of cultured sympathetic neuron NGF augmented VIP transcript levels approximately 50 %. These VIP levels were markedly different from those after SCG axotomy in which VIP induction was typically equal or greater than that for PACAP. Consistent with other studies, cultured SCG neuron treatment with LIF, CNTF, or IL-6 had no effects on PACAP expression.
NGF withdrawal from SCG cultures induces neuronal PACAP expression. Primary neonatal rat SCGs were enzymatically dissociated and seeded into 24-well culture plates; the cultures were treated with arabinoside cytosine to remove background cells and maintained in defined serum-free medium containing 50 ng/ml NGF as previously described [29]. (A) After 7 days in vitro, NGF was withdrawn from the sympathetic cultures and the cells were harvested at the times shown for RNA extraction and semi-quantitative PCR assessments of PACAP transcript levels (left panel). After 24 h in NGF-free medium, NGF was returned back to the replicate plates and the cultures harvested at the times shown after growth factor add-back (right panel). NGF withdrawal increased SCG culture PACAP transcript expression in a time-dependent manner; conversely NGF add-back gradually diminished PACAP transcript levels over 12 h. (B) SCG neurons were cultured in 50 ng/ml NGF and after 7 days, the defined medium was replaced with fresh medium containing the indicated NGF concentrations; the neurons were maintained in culture for an additional 12 h before harvesting for PCR analyses. Decreasing NGF concentrations increased culture PACAP transcript levels in a concentration-dependent manner. Each lane represents an individual replicate culture well. (C) SCG cultures were maintained in NGF-supplemented (+NGF) or NGF-free (-NGF) defined medium for 24 h before fixation and processing for PACAP immunocytochemistry using a Cy3-conjugated secondary antibody. NGF withdrawal increased the number of PACAP-immunoreactive neurons in the SCG cultures
Abrogation of retrograde NGF signaling and JNK activation increases SCG PACAP expression. (A) SCG neurons were cultured in defined medium containing 50 ng/ml NGF for 7 days before addition of the neurotrophin Trk receptor tyrosine kinase inhibitor K252a (100 nM) or NGF neutralizing antibody (nAb, 1 μg/ml) for 24 h. Replicate cultures were also treated with endocytosis inhibitors chlorpromazine (CPZ, 10 μg/ml, clathrin endocytosis inhibitor), monodansylcadaverine (MDC, 200 μM, clathrin endocytosis inhibitor), or filipin (2 μg/ml, caveolae endocytosis inhibitor). Blockade of NGF/Trk signaling and endocytosis/trafficking induced SCG neuronal PACAP transcript expression examined by PCR analyses. (B) NGF withdrawal from sympathetic cultures stimulated JNK activation [76]. To assess the roles of JNK/c-jun signaling in PACAP expression, SCG cultures were withdrawn from NGF, and treated simultaneously with the JNK inhibitor SP600125 or an unrelated p38 MAPK inhibitor SB203580. NGF removal induced culture PACAP transcripts (Vehicle) compared to control NGF-supplemented cultures (Control). The addition of the JNK inhibitor to the NGF-free cultures blocked PACAP induction; the unrelated p38 MAPK inhibitor had no effects on PACAP induction. (C) Schematic of SCG Campenot compartmentalized culture preparation. The SCG neurons were seeded onto a 1 mm wide compartment (Soma, left) and the axons were allowed to tunnel through a vacuum grease-sealed teflon barrier to reach the NGF-saturated axonal compartment (Axons, right). Hence, the SCG neuronal somas were compartmentalized from the axons. Dotted lines depict the width of the teflon barrier. (D) Sympathetic neuron somal PACAP mRNA levels from Campenot cultures maintained with NGF (50 ng/ml) in the axonal compartment (+NGF) were compared to those in which NGF was withdrawn from the axonal compartment for 24 h (-NGF). The neuronal soma compartments contained 10 ng/ml NGF (minimal survival dose) in all conditions. NGF withdrawal from the axonal compartment and resulting abrogation of NGF/TrkA retrograde neurotrophin signaling were sufficient to induce neuronal PACAP transcript expression
Despite the apparent roles of NGF in regulation of SCG PACAP levels, NGF may not be the principal retrograde neuroplasticity signal for all peptides or neuronal systems. Neonatal sympathetic neurons are sensitive to NGF levels for trophic support, and accordingly the cultures are more responsive to cellular stress following neurotrophin withdrawal to initiate phenotypic responses. Adult sympathetic neurons may be more resilient to changes in NGF levels, and the relative contribution of abrogated retrograde NGF/TrkA signaling to other signals (i.e., inflammatory cytokines) may be different. Notably, one study demonstrated that the concurrent addition of anti-NGF and LIF to unoperated SCG induced peptide expression levels beyond those of anti-NGF alone, suggesting that dual activation signals and loss of target tissue factors are synergistic for neuropeptide plasticity [49]. Unlike sympathetic neurons, neonatal DRG neurons can be maintained in culture without NGF and by contrast, NGF treatments facilitated DRG PACAP expression in vitro, which appeared consistent with the stimulatory effects of NGF on DRG PACAP in vivo [46, 57, 75]. Indeed, NGF-induced DRG peptidergic plasticity has been associated with small caliber C-nociceptor hyperalgesia in tissue inflammation [75]. These results suggest NGF has different mechanisms of action on neuropeptide plasticity in different tissues; NGF suppresses PACAP expression in sympathetic neurons through neurotrophic signaling, but augments PACAP levels in DRG sensory neurons. For DRG and other autonomic tissues, the abrogation of different retrograde target tissue factors, in combination with activation mechanisms, may be important for axotomy-induced peptidergic plasticity. Described earlier, DRG PACAP expression is sensitive to NT-3/TrkC signaling [57]. In agreement, the induction of PACAP expression in guinea pig cardiac ganglion explants was not suppressed with NGF treatments but by the glial cell line-derived neurotrophic factor neurturin, implicating glial-derived neurotrophic factor (GDNF) receptor alpha2 (GFRalpha2) signaling in the regulation of cardiac neuron PACAP expression [51]. These observations suggest that there may not be a unifying mechanism underlying neurotransmitter/neuropeptide phenotypic plasticity. Rather, these studies in aggregate illustrate that neuropeptidergic plasticity mechanisms are complex; there are different mechanisms underlying injury- and inflammation-induced neurophenotypic plasticity, and the induction of each peptide may have a unique mechanistic signature. Accordingly, the totality of the peripheral nervous system peptide plasticity responses to the many physiological challenges represents an aggregate of these mechanisms.
Functional Roles of PACAP in Neuropeptidergic Plasticity
The induction of specific peptides in neuroplasticity has long been suggested to represent a reprogramming of cellular functions from neurotransmission to neuroprotection and regeneration. Many of the induced peptidergic systems, including PACAP, can engage neurotrophic signaling pathways [2, 76]; however, unequivocal evidence for these functions has proven somewhat elusive. Sciatic nerve transection in galanin−/− mice delayed nerve regeneration as assessed by toe spreading [77]. Axotomy of the facial nerve in PACAP−/− mice had no apparent effects on neuronal survival and demonstrated modest impairments in nerve regeneration, but enhanced inflammatory responses at the injury site [78]. Among other potential roles, these studies implicated injury-induced PACAP expression for anti-inflammatory processes.
However, injury- and inflammation-induced neuropeptidergic plasticity may also be viewed broadly as intrinsic neuronal mechanisms to maintain neural system homeostasis [36]. The balance of excitatory and inhibitory neurocircuit signaling has little margin for error, which is essential for proper activities in developing and mature networks. Under system perturbations such as stress, injury, or disease, the abilities of subpopulations of neurons to undergo neurochemical dynamics and plasticity may be a means of maintaining system homeostatic responsiveness. Congruently, PACAP signaling has clear roles in neuronal excitability [32, 79–81]. Noteworthy, JNK/c-jun regulation of the homeobox gene tlx3 may coordinate PACAP and glutaminergic phenotypic identities in some neuronal systems [63, 82], and PACAP can modulate glutaminergic signaling by regulating cell surface NMDA and mGlu receptor expression. Hence, one potential function of DRG PACAP induction in injury and inflammation may be the initiation of adaptive mechanisms to maintain homeostatic signaling and responsiveness in the dorsal horn. Whether PACAP neuroplasticity has comparable homeostatic functions in other neuronal systems remains to be studied.
Conclusions
PACAP is widely distributed in sensory, autonomic, and enteric neurons. The population of endogenous PACAP-expressing neurons in these ganglia can be increased dramatically from the low number under basal conditions in parallel with heightened levels of PACAP transcripts, immunoreactivity, and content after experimental neuronal injury or inflammation. The mechanisms underlying the neuronal plasticity of PACAP and other peptides have not been fully elucidated, but likely involve multiple signals from the abrogation of retrograde target tissue regulators and an augmentation of stimulatory cytokine/inflammatory factors. The differential induction of PACAP, VIP, and other peptides may require distinct mechanistic signatures for the various neural tissues. Among roles including neurotrophic and anti-inflammatory responses, PACAP neuroplasticity may be an intrinsic mechanism required to maintain neurocircuit homeostasis.
References
Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol. 2002;453:389–417.
Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357.
Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, et al. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol. 1996;371:567–77.
Jaworski DM, Proctor MD. Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Dev Brain Res. 2000;120:27–39.
Moller K, Zhang YZ, Hakanson R, Luts A, Sjolund B, Uddman R, et al. Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence. Neuroscience. 1993;57:725–32.
Mulder H, Uddman R, Moller K, Zhang YZ, Ekblad E, Alumets J, et al. Pituitary adenylate cyclase activating polypeptide expression in sensory neurons. Neuroscience. 1994;63:307–12.
Zhang Q, Shi TJ, Ji RR, Zhang YZ, Sundler F, Hannibal J, et al. Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res. 1995;705:149–58.
Zhang YZ, Hannibal J, Zhao Q, Moller K, Danielsen N, Fahrenkrug J, et al. Pituitary adenylate cyclase activating peptide expression in the rat dorsal root ganglia: up-regulation after peripheral nerve injury. Neuroscience. 1996;74:1099–110.
Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–53.
Noguchi K, Dubner R, De Leon M, Senba E, Ruda MA. Axotomy induces preprotachykinin gene expression in a subpopulation of dorsal root ganglion neurons. J Neurosci Res. 1994;37:596–603.
Jongsma H, Danielsen N, Sundler F, Kanje M. Alteration of PACAP distribution and PACAP receptor binding in the rat sensory nervous system following sciatic nerve transection. Brain Res. 2000;853:186–96.
Mulder H, Uddman R, Moller K, Elsas T, Ekblad E, Alumets J, et al. Pituitary adenylate cyclase activating polypeptide is expressed in autonomic neurons. Regul Pept. 1995;59:121–8.
Rytel L, Palus K, Całka J. Co-expression of PACAP with VIP, SP and CGRP in the porcine nodose ganglion sensory neurons. Anat Histol Embryol. 2015;44:86–91.
Zvarova K, Dunleavy JD, Vizzard MA. Changes in pituitary adenylate cyclase activating polypeptide expression in urinary bladder pathways after spinal cord injury. Exp Neurol. 2005;192:46–59.
Hannibal J, Ekblad E, Mulder H, Sundler F, Fahrenkrug J. Pituitary adenylate cyclase activating polypeptide (PACAP) in the gastrointestinal tract of the rat: distribution and effects of capsaicin or denervation. Cell Tissue Res. 1998;291:65–79.
Edvinsson L, Elsas T, Suzuki N, Shimizu T, Lee TJ. Origin and co-localization of nitric oxide synthase, CGRP, PACAP, and VIP in the cerebral circulation of the rat. Microsc Res Tech. 2001;53:221–8.
Fahrenkrug J, Hannibal J. Pituitary adenylate cyclase activating polypeptide innervation of the rat female reproductive tract and the associated paracervical ganglia: effect of capsaicin. Neuroscience. 1996;73:1049–60.
Wang ZY, Alm P, Hakanson R. Distribution and effects of pituitary adenylate cyclase-activating peptide in the rabbit eye. Neuroscience. 1995;69:297–308.
Nielsen HS, Hannibal J, Fahrenkrug J. Embryonic expression of pituitary adenylate cyclase-activating polypeptide in sensory and autonomic ganglia and in spinal cord of the rat. J Comp Neurol. 1998;394:403–15.
Moller K, Reimer M, Ekblad E, Hannibal J, Fahrenkrug J, Kanje M, et al. The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res. 1997;775:166–82.
Beaudet MM, Braas KM, May V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol. 1998;36:325–36.
Pettersson LM, Heine T, Verge VM, Sundler F, Danielsen N. PACAP mRNA is expressed in rat spinal cord neurons. J Comp Neurol. 2004;471:85–96.
Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem. 1999;274:27702–10.
May V, Braas KM. Pituitary adenylate cyclase-activating polypeptide (PACAP) regulation of sympathetic neuron neuropeptide Y and catecholamine expression. J Neurochem. 1995;65:978–87.
Zalecki M. Localization and neurochemical characteristics of the extrinsic sympathetic neurons projecting to the pylorus in the domestic pig. J Chem Neuroanat. 2012;43:1–13.
Ermilov LG, Schmalz PF, Miller SM, Szurszewski JH. PACAP modulation of the colon-inferior mesenteric ganglion reflex in the guinea pig. J Physiol. 2004;560:231–47.
Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, Eiden LE. PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice. Endocrinology. 2013;154:330–9.
Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, et al. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci U S A. 2002;99:461–6.
Brandenburg CA, May V, Braas KM. Identification of endogenous sympathetic neuron pituitary adenylate cyclase-activating polypeptide (PACAP): depolarization regulates production and secretion through induction of multiple propeptide transcripts. J Neurosci. 1997;17:4045–55.
Calupca MA, Vizzard MA, Parsons RL. Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J Comp Neurol. 2000;423:26–39.
Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci. 1998;18:9766–79.
Tompkins JD, Lawrence YT, Parsons RL. Enhancement of Ih, but not inhibition of IM, is a key mechanism underlying the PACAP-induced increase in excitability of guinea pig intrinsic cardiac neurons. Am J Physiol Regul Integr Comp Physiol. 2009;297:R52–9.
Hoover DB, Tompkins JD, Parsons RL. Differential activation of guinea pig intrinsic cardiac neurons by the PAC1 agonists maxadilan and pituitary adenylate cyclase-activating polypeptide 27 (PACAP27). J Pharmacol Exp Ther. 2009;331:197–203.
Girard BM, Galli JR, Young BA, Vizzard MA, Parsons RL. PACAP expression in explant cultured mouse major pelvic ganglia. J Mol Neurosci. 2010;42:370–7.
Demarque M, Spitzer NC. Neurotransmitter phenotype plasticity: an unexpected mechanism in the toolbox of network activity homeostasis. Dev Neurobiol. 2012;72:22–32.
Spitzer NC. Activity-dependent neurotransmitter respecification. Nat Rev Neurosci. 2012;18:94–106.
Spitzer NC, Borodinsky LN, Root CM. Homeostatic activity-dependent paradigm for neurotransmitter specification. Cell Calcium. 2005;37:417–23.
Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340:449–53.
Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–66.
Walicke PA, Campenot RB, Patterson PH. Determination of transmitter function by neuronal activity. Proc Natl Acad Sci U S A. 1977;74:5767–71.
Zigmond RE, Hyatt-Sachs H, Mohney RP, Schreiber RC, Shadiack AM, Sun Y, et al. Changes in neuropeptide phenotype after axotomy of adult peripheral neurons and the role of leukemia inhibitory factor. Perspect Dev Neurobiol. 1996;4:75–90.
Hokfelt T, Zhang X, Wiesenfeld-Hallin Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994;17:22–30.
Pettersson LM, Dahlin LB, Danielsen N. Changes in expression of PACAP in rat sensory neurons in response to sciatic nerve compression. Eur J Neurosci. 2004;20:1838–48.
Zhang Y, Danielsen N, Sundler F, Mulder H. Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation. Neuroreport. 1998;9:2833–6.
Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000;420:335–48.
Jongsma Wallin H, Pettersson LM, Verge VM, Danielsen N. Effect of anti-nerve growth factor treatment on pituitary adenylate cyclase activating polypeptide expression in adult sensory neurons exposed to adjuvant induced inflammation. Neuroscience. 2003;120:325–31.
Larsen JO, Hannibal J, Knudsen SM, Fahrenkrug J. Expression of pituitary adenylate cyclase-activating polypeptide (PACAP) in the mesencephalic trigeminal nucleus of the rat after transection of the masseteric nerve. Mol Brain Res. 1997;46:109–17.
Hyatt-Sachs H, Bachoo M, Schreiber R, Vaccariello SA, Zigmond RE. Chemical sympathectomy and postganglionic nerve transection produce similar increases in galanin and VIP mRNA but differ in their effects on peptide content. J Neurobiol. 1996;30:543–55.
Shadiack AM, Vaccariello SA, Sun Y, Zigmond RE. Nerve growth factor inhibits sympathetic neurons’ response to an injury cytokine. Proc Natl Acad Sci U S A. 1998;95:7727–30.
Mohney RP, Siegel RE, Zigmond RE. Galanin and vasoactive intestinal peptide messenger RNAs increase following axotomy of adult sympathetic neurons. J Neurobiol. 1994;25:108–18.
Girard BM, Young BA, Buttolph TR, White SL, Parsons RL. Regulation of neuronal pituitary adenylate cyclase-activating polypeptide expression during culture of guinea-pig cardiac ganglia. Neuroscience. 2007;146:584–93.
Girard BM, Galli JR, Vizzard MA, Parsons RL. Galanin expression in the mouse major pelvic ganglia during explant culture and following cavernous nerve transection. J Mol Neurosci. 2012;48:713–20.
Klimaschewski L. Regulation of galanin in rat sympathetic neurons in vitro. Neurosci Lett. 1997;234:87–90.
Sun Y, Rao MS, Landis SC, Zigmond RE. Depolarization increases vasoactive intestinal peptide- and substance P-like immunoreactivities in cultured neonatal and adult sympathetic neurons. J Neurosci. 1992;12:3717–28.
Harakall SA, Brandenburg CA, Gilmartin GA, May V, Braas KM. Induction of multiple pituitary adenylate cyclase activating polypeptide (PACAP) transcripts through alternative cleavage and polyadenylation of proPACAP precursor mRNA. Ann N Y Acad Sci. 1998;865:367–74.
Pavelock KA, Girard BM, Schutz KC, Braas KM, May V. Bone morphogenetic protein down-regulation of neuronal pituitary adenylate cyclase-activating polypeptide and reciprocal effects on vasoactive intestinal peptide expression. J Neurochem. 2007;100:603–16.
Jongsma Wallin H, Danielsen N, Johnston JM, Gratto KA, Karchewski LA, Verge VM. Exogenous NT-3 and NGF differentially modulate PACAP expression in adult sensory neurons, suggesting distinct roles in injury and inflammation. Eur J Neurosci. 2001;14:267–82.
Hashimoto H, Hagihara N, Koga K, Yamamoto K, Shintani N, Tomimoto S, et al. Synergistic induction of pituitary adenylate cyclase-activating polypeptide (PACAP) gene expression by nerve growth factor and PACAP in PC12 cells. J Neurochem. 2000;74:501–7.
Sugawara H, Inoue K, Iwata S, Shimizu T, Yamada K, Mori N, et al. Neural-restrictive silencers in the regulatory mechanism of pituitary adenylate cyclase-activating polypeptide gene expression. Regul Pept. 2004;123:9–14.
Sugawara H, Tominaga A, Inoue K, Takeda Y, Yamada K, Miyata A. Functional characterization of neural-restrictive silencer element in mouse pituitary adenylate cyclase-activating polypeptide (PACAP) gene expression. J Mol Neurosci. 2014;54:526–34.
Zigmond RE. gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front Mol Neurosci. 2012;4:62.
Kim MS, Hur MK, Son YJ, Park JI, Chun SY, D'Elia AV, et al. Regulation of pituitary adenylate cyclase-activating polypeptide gene transcription by TTF-1, a homeodomain-containing transcription factor. J Biol Chem. 2002;277:36863–71.
Guo Z, Zhao C, Huang M, Huang T, Fan M, Xie Z, et al. Tlx1/3 and Ptf1a control the expression of distinct sets of transmitter and peptide receptor genes in the developing dorsal spinal cord. J Neurosci. 2012;32:8509–20.
Banner LR, Patterson PH. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc Natl Acad Sci U S A. 1994;91:7109–13.
Habecker BA, Sachs HH, Rohrer H, Zigmond RE. The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons. Dev Neurobiol. 2009;69:392–400.
Sun Y, Rao MS, Zigmond RE, Landis SC. Regulation of vasoactive intestinal peptide expression in sympathetic neurons in culture and after axotomy: the role of cholinergic differentiation factor/leukemia inhibitory factor. J Neurobiol. 1994;25:415–30.
Rao MS, Sun Y, Escary JL, Perreau J, Tresser S, Patterson PH, et al. Leukemia inhibitory factor mediates an injury response but not a target-directed developmental transmitter switch in sympathetic neurons. Neuron. 1993;11:1175–85.
Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, et al. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–7.
Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32.
Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84.
Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, et al. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell Calcium. 2004;118:243–55.
Ye H, Kuruvilla R, Zweifel LS, Ginty DD. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron. 2003;39:57–68.
Tsui-Pierchala BA, Ginty DD. Characterization of an NGF-P-TrkA retrograde-signaling complex and age-dependent regulation of TrkA phosphorylation in sympathetic neurons. J Neurosci. 1999;19:8207–18.
Riccio A, Pierchala BA, Ciarallo CL, Ginty DD. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277:1097–100.
Woolf CJ. Phenotypic modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:441–8.
May V, Lutz E, MacKenzie C, Schutz KC, Dozark K, Braas KM. Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase and vesicle endocytosis for neuronal survival. J Biol Chem. 2010;285:9749–61.
Holmes FE, Mahoney S, King VR, Bacon A, Kerr NC, Pachnis V, et al. Targeted disruption of the galanin gene reduces the number of sensory neurons and their regenerative capacity. Proc Natl Acad Sci U S A. 2000;97:11563–8.
Armstrong BD, Abad C, Chhith S, Cheung-Lau G, Hajji OE, Nobuta H, et al. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience. 2008;151:63–73.
Merriam LA, Baran CN, Girard BM, Hardwick JC, May V, Parsons RL. Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. J Neurosci. 2013;33:4614–22.
Tompkins JD, Hardwick JC, Locknar SA, Merriam LA, Parsons RL. Ca2+ influx, but not Ca2+ release from internal stores, is required for the PACAP-induced increase in excitability in guinea pig intracardiac neurons. J Neurophysiol. 2006;95:2134–42.
Tompkins JD, Merriam LA, Girard BM, May V, Parsons RL. Nickel suppresses the PACAP-induced increase in guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol. 2015;308:C857–66.
Marek KW, Kurtz LM, Spitzer NC. cJun integrates calcium activity and tlx3 expression to regulate neurotransmitter specification. Nat Neurosci. 2010;13:944–50.
Acknowledgments
We thank Galen Missig, Beatrice M. Girard, Anton Kurtz, and Kristin C. Schutz for technical support in some of the studies. This work was supported by grants NS-01636 (KMB), HD-27468 and MH-096764 (VM), DK-051369, DK-060481 and DK-065989 (MAV), NS-23978 and HL-65481 (RLP), and funds from the University of Vermont (UVM) Center of Biomedical Research Excellence (Neuroscience COBRE, NCRR P30 RR032135/NIGMS P30 GM103498).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Braas, K.M., Parsons, R.L., Vizzard, M.A., Waschek, J.A., May, V. (2016). PACAP Expression and Plasticity in the Peripheral Nervous System. In: Reglodi, D., Tamas, A. (eds) Pituitary Adenylate Cyclase Activating Polypeptide — PACAP. Current Topics in Neurotoxicity, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-319-35135-3_33
Download citation
DOI: https://doi.org/10.1007/978-3-319-35135-3_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-35133-9
Online ISBN: 978-3-319-35135-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)